Abstract
Purpose
Test the feasibility of a planned phase I study of image-guided adaptive radiotherapy in locally advanced lung cancer.
Methods and Materials
Weekly 4D FBCTs of ten lung cancer patients undergoing concurrent radiochemotherapy were used to simulate adaptive radiotherapy: After an initial IMRT plan (0–30 Gy/2 Gy), adaptive replanning was performed on week 2 (30 to 50 Gy/2 Gy) and week 4 scans (50 to 66 Gy/2 Gy) to adjust for volume and shape changes of primary tumors and lymph nodes. Week 2 and 4 clinical target volumes (CTV) were deformably warped from the initial planning scan to adjust for anatomical changes. On week 4 scan a simultaneous integrated volume-adapted boost was created to the shrunken PT with dose increases in five 0.4 Gy steps from 66 Gy to 82 Gy in two scenarios: Plan A. lung isotoxicity and B. normal tissue tolerance. Cumulative dose was assessed by deformably mapping and accumulating biologically equivalent dose normalized to 2 Gy-fractions (EQD2).
Results
The 82 Gy level was achieved in 1/10 patients in scenario A resulting in a 13.4 Gy EQD2 increase and a 22.1% increase in tumor control probability (TCP) compared to the 66 Gy plan. In scenario B, 2 patients reached the 82 Gy level with a 13.9 Gy EQD2 and 23.4% TCP increase.
Conclusions
The tested IGART strategy enabled relevant increases in EQD2 and TCP. Normal tissue was often dose limiting, indicating a need to modify the present study design prior to clinical implementation.
Introduction
Local tumor control affects overall survival in locally advanced non small-cell lung cancer (LA-NSCLC) (1). The ability to increase tumor control by delivering higher dose is limited by normal tissue toxicity and variations in tumor geometry.
The development of image-guided adaptive radiation therapy (IGART) promises improved tumor targeting with smaller margins, allowing for the safe delivery of higher doses than currently feasible. With a limited number of lung IGART trials open for accrual, clinical strategies are not well established, but should likely include reimaging (2), respiration management, adaptive replanning (3) and deformable dose summation. Relevant increases of tumor dose and/or reduction of lung doses were reported from planning studies using during treatment PET (4, 5) and repeated CT imaging (6). The goal of the present plan simulation is to test the feasibility of a planned phase I IGART dose escalation study in LA-NSCLC. The planned study differs from existing literature in the following aspects:
Delivery of a hypofractionated simultaneous integrated boost to the decreased primary tumor volume only without extending treatment time.
Warping of the clinical target volume (CTV) for safe coverage of microscopic disease.
IMRT planning for high dose conformality and plan optimization using relevant normal tissue tolerances.
Materials and Methods
Patient information
On an IRB-approved prospective image acquisition protocol, ten patients with LA-NSCLC underwent weekly 4D fan beam computed tomography (4D FBCT) with audiovisual biofeedback (7), while concomitant radiochemotherapy was delivered per institutional standard of care. Table 1 summarizes patient-specific information.
Table 1.
Patient and tumor characteristics
| Patient | Age (years) |
Tumor stage | PT location |
LN location |
PT volume week 0 (cm3) |
PT volume week 4 (cm3) |
CTV week 0 (cm3) |
Warped CTV week 4 (cm3) |
Lungs-ITV week 0 (cm3) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | T2N2M0 | LUL | L4, L10 | 18 | 10 | 58 | 55 | 2306 |
| 2 | 62 | T2N2M0 | RUL | R4 | 11 | 7 | 70 | 68 | 5671 |
| 3 | 66 | T4N3M0 | RLL | R4, L4, 7 | 312 | 304 | 694 | 745 | 3446 |
| 4 | 67 | T3N2M0 | RUL | R4 | 44 | 29 | 165 | 169 | 4832 |
| 5 | 57 | T3N2M0 | RUL apex | R4 | 31 | 22 | 83 | 84 | 3738 |
| 6 | 51 | T4N2M0 | RLL | 7 | 175 | 113 | 441 | 418 | 6826 |
| 7 | 67 | T1N2M0 | RUL | R2, R4 | 7 | 4 | 33 | 35 | 3223 |
| 8 | 74 | T3N2M0 | LUL apex | L5, L6 | 30 | 25 | 125 | 131 | 4963 |
| 9 | 63 | T3N2M0 | RML | R4, R10 | 11 | 3 | 49 | 46 | 2701 |
| 10 | 68 | Recurrence | RML | R4 | 12 | 4 | 71 | 66 | 1477 |
| Mean | 63 | 65 | 52 | 179 | 182 | 3918 | |||
CTV: clinical target volume; ITV: internal target volume; L: left; LL: lower lobe; LN: lymph node; ML: middle lobe; PT: primary tumor; R: right; UL: upper lobe
Image acquisition and image registration
4D FBCTs were obtained on a 16-slice helical CT scanner (Brilliance Big Bore, Philips Medical Systems, Andover, MA) as respiration-correlated CTs with 10 breathing phases (0 to 90%, phase-based binning) and 3 mm slice thickness. Using normalized cross correlation (Syntegra, Pinnacle 9.1, Philips Medical Systems, Fitchburg, WI), the mid-ventilation phase (30%) from the weekly 4D FBCT was rigidly registered to the corresponding phase on the planning 4D FBCT to align bony anatomy. The registered images were visually inspected and, if necessary, manually adjusted.
Volumes and deformable CTV propagation
The initial planning scan (week 0), week 2 and week 4 4D FBCTs were manually contoured. Normal tissue structures were defined on the mid-ventilation phase. The gross tumor volume (GTV) consisted of primary tumor (PT) and lymph nodes (LN) (positive according to biopsy or PET) that were contoured on all 10 phases to generate internal target volumes (ITVs). On the initial scan, clinical target volumes (CTVs) were defined as 6 mm and 3 mm expansions of the PT and LN ITVs, respectively. Assuming daily soft tissue-based image guidance, the planning target volume (PTV) was obtained by expansion of both the CTV PT and CTV LN by 5 mm.
To account for shape and volume changes caused by tumor regression, CTV PTs on week 2 and 4 scans were deformably propagated from the initial planning scan using the displacement fields from deformable registration. Deformable image registration was performed using a small deformation, inverse-consistent, linear-elastic algorithm (8) which has previously been benchmarked for co-registering thoracic FBCT images for the purpose of mapping the CTV from one image to another (9). Each weekly mid-ventilation phase was non-rigidly registered to the corresponding phase of the planning 4D FBCT. The fused images, displacement fields, and deformed CTV contours were visually inspected for agreement. PTVs on week 2 and 4 scans were created by 5 mm expansion of the warped CTVs. A boost PTV was created on the week 4 scan defined as the week 4 ITV PT surrounded by a 3 mm margin.
Treatment planning and deformable dose summation
Intensity-modulated radiotherapy (IMRT) planning with coplanar fixed fields was performed on the 30% phase of the initial, week 2 and week 4 4D FBCT (Pinnacle 9.1, Philips Medical Systems, Fitchburg, WI) with the goal to deliver a dose of 66 Gy in 33 fractions to 95% of the PTV volume (D95%= 66 Gy) using multiple normal tissue constraints (Table 2). The comprehensive IGART plan had the following dose components: 0–30 Gy/15 fractions to initial PTV, 30–50 Gy/10 fractions to week 2 PTV and 50–66 Gy/8 fractions to week 4 PTV. In addition, a simultaneous integrated volume-adapted boost (SIVAB) to the boost PTV was delivered integrated in the 8 fractions of the week 4 adaptation. Dose-per-fraction of SIVAB was increased in five 0.4 Gy dose steps between 0 Gy (= no boost) and 2 Gy, resulting in comprehensive IGART doses between 66 and 82 Gy. The boost dose was planned as volumetric-modulated arc therapy (VMAT). Representative dose distributions are shown in Figure 1.
Table 2.
EQD2 values for normal tissue tolerance
| Structure | Physical Dose (Gy) |
Acceptable Volume |
α/β (Gy) | EQD2 |
|---|---|---|---|---|
| Esophagus PRV | 60 | 30% | 3 | 57.6 |
| Esophagus PRV | 34 | 50% | 3 | 27.4 |
| Esophagus PRV | 74 | 0% | 3 | 74 |
| Spinal cord PRV | 50 | 0% | 2 | 44 |
| Heart | 40 | 100% | 3 | 33.7 |
| Heart | 45 | 65% | 3 | 39.3 |
| Heart | 60 | 35% | 3 | 57.6 |
| Heart | 74 | 0% | 3 | 74 |
| Lungs minus ITV | 19 | nMLD | 4 | 14.5 |
| Brachial plexus | 66 | 0% | 2 | 66 |
| Aorta | 74 | 0% | 3 | 74 |
| Trachea and main stem bronchus | 80 | 0% | 3 | 80 |
ITV: internal target volume; nMLD: normalized mean lung dose; EQD2: dose equivalent to 2 Gy dose; PRV: planning organ at risk volume.
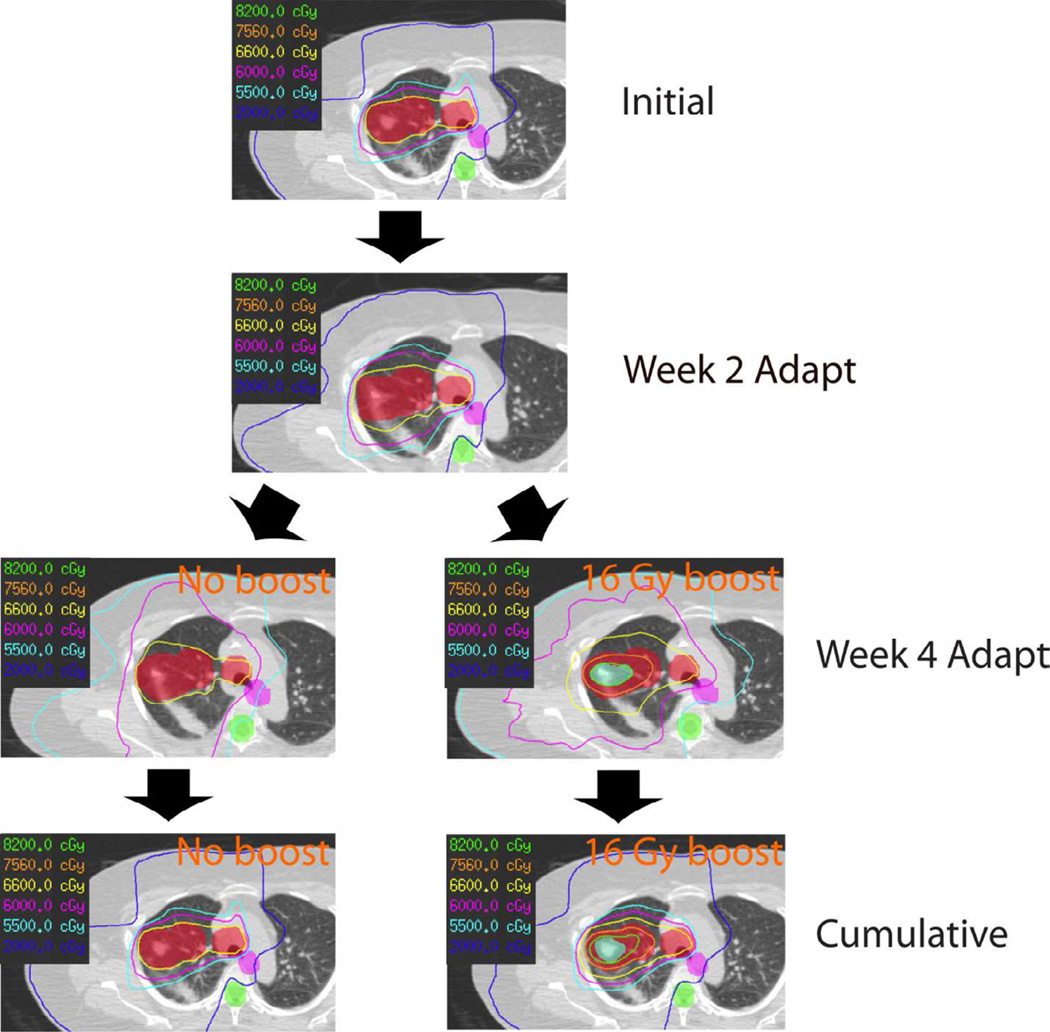
Figure 1.
Example for adapted 66 Gy and 82 Gy boost plans.
The replanning steps and the cumulative dose distribution are shown for a 66 Gy plan (no boost) on the left and for 82 Gy with SIVAB (16 Gy boost) on the right. A 66 Gy plan was created on each CT scan with the cumulative plan consisting of a) 15 fractions based on the initial CT, b) 10 fractions of week 2 CT, and c) 8 fractions of the week 4 CT simultaneous integrated volume-adapted boost plan, adding up to a total dose between 66 Gy (no boost) and 82 Gy (16 Gy boost).
Colorwash red: planning target volume (PTV) based on the respective initial, week 2 and week 4 CT scan, colorwash blue: PTV boost, colorwash purple: planning organ at risk volume (PRV) esophagus, colorwash green: PRV spinal cord.
The details of cumulative dose calculation are included in Appendix A. Biologically equivalent doses normalized to 2 Gy fractions (EQD2) were calculated for PT, LN and normal tissue. Table 2 lists α/β-values for normal tissue structures with no repopulation assumed.
Two adaptive planning scenarios were considered:
Plan A: IGART with SIVAB at lung isotoxicity: Dose escalation was performed until the normalized mean lung dose (nMLD) of the adapted 66 Gy plan was exceeded by more than 1 Gy.
Plan B: IGART with SIVAB at normal tissue tolerance: Dose escalation was performed until any of the limiting normal tissue EQD2s was reached.
In addition to adaptive planning, accumulated EQD2 was calculated for the non-adaptive scenario, presuming delivery of the initial plan for all fractions (= unadapted 66 Gy plan).
Data analysis
The principal goal of this study was to analyze the resulting composite plans for their ability to increase SIVAB dose at the specified dose levels for Plan A and B scenarios. First, tumor regression characteristics of the PT, LN and CTV PT were evaluated. Second, to analyze the effect of adaptation itself without dose change, differences in EQD2 and tumor control probability (TCP) between unadapted and adapted 66 Gy plans were evaluated. Third, the maximum tolerated doses were recorded for Plan A and B and EQD2s of ITV and CTV PT and LN analyzed. For both Plan A and B, achievable doses were compared for lung only as dose limiting structure and for any normal tissue structure being dose limiting. TCPs for PT and LN were calculated according to Appendix B.
Statistics
Differences in EQD2s and TCPs between the planning scenarios, and differences of PT and LN volume reduction were analyzed for significance using a Wilcoxon signed rank test assuming significance for p < 0.05.
Results
Tumor regression
The PT / LN volumes on week 0 scan measured, on average, 65 cm3 (range 7 – 312 cm3) / 5 cm3 (range 1 – 8 cm3). In week 4 these volumes were reduced to on average 52 cm3 (3–304 cm3) / 2 cm3 (range 1 – 6 cm3) resulting in a mean reduction by 39% and 51% (p=0.09). The mean week 0 CTV was comparable to the warped week 4 CTV (179 cm3 (range 33 – 694 cm3) versus 184 cm3 (range 35 – 745 cm3)), see Table 1. The mean respiration-related motion of the PT centroid measured on the initial planning CT was 3 mm (range 0 – 9 mm).
Adapted versus unadapted 66 Gy plans
The difference between mean EQD210 for D95% of ITV and CTV of PT and LN, and for normal tissue for unadapted and adapted 66 Gy plans was less than 1 Gy (Table 3 and 4). Patient 3 (PT volume 312 cm3) was excluded from dose escalation, as a 66 Gy plan could not be achieved without exceeding lung tolerance.
Table 3.
Comparison of mean EQD2 for tumor and selected normal tissues of unadapted and adapted 66 Gy plans.
| Unadapted 66 Gy (SD) | Adapted 66 Gy (SD) | p-values | |
|---|---|---|---|
| D95% CTV PT | 53.5 (1.1) | 54.3 (1.1) | p<0.01 |
| D95% ITV PT | 53.7 (1.5) | 54.3 (1.3) | p>0.05 |
| D95% CTV LN | 53.4 (1.3) | 53.3 (2.2) | p>0.05 |
| D95% ITV LN | 53.7 (1.8) | 54.1 (1.6) | p>0.05 |
| Lungs mean | 12.6 (2.6) | 12.6 (2.7) | p>0.05 |
| Heart mean | 5.7 (6.8) | 5.7 (6.8) | p>0.05 |
| Esophagus mean | 14.7 (6.3) | 15.0 (6.1) | p>0.05 |
| Spinal cord max | 16.3 (4.2) | 16.5 (3.2) | p>0.05 |
| Aorta max | 67.2 (3.6) | 67.4 (4.1) | p>0.05 |
| Airways max | 71.0 (2.4) | 71.6 (2.2) | p>0.05 |
CTV: clinical target volume; D95% dose in 95% of the volume; ITV: internal target volume; NTD: EQD2: dose equivalent to 2 Gy dose.
Table 4.
Mean TCPs (%) for plan A and B
| Plans | CTV PT 1% (SD) |
ITV PT 100% (SD) |
CTV LN 1% (SD) |
ITV LN 100% (SD) |
|
|---|---|---|---|---|---|
| 1. Unadapted 66 Gy | 97.2 (0.9) | 20.9 (12.8) | 98.5 (0.4) | 42.0 (13.2) | |
| 2. Adapted 66 Gy | 97.3 (0.9) | 22.5 (13.5) | 98.5 (0.5) | 42.0 (13.2) | |
| p-values 1. versus 2. | <0.01 | <0.005 | > 0.05 | > 0.05 | |
| Plan A | |||||
| 3. not exceeding any normal tissue tolerance | 98.0 (1.3) | 44.6 (28.3) | 98.5 (0.5) | 43.2 (15.0) | |
| p-values 2. versus 3. | <0.01 | < 0.01 | > 0.05 | > 0.05 | |
| Plan B | |||||
| 4. not exceeding any normal tissue tolerance | 98.2 (1.3) | 45.9 (28.0) | 98.6 (0.4) | 45.9 (14.3) | |
| p-values 2. versus 4. | < 0.01 | < 0.01 | > 0.05 | <0.01 | |
CTV: clinical target volume; ITV: internal target volume; LN: lymph nodes; PT: primary tumor; SD: standard deviation; TCP: tumor control probability.
Dose escalation with lung isotoxicity
At lung isotoxicity, the following dose steps were reached: 82 Gy in 4, 72.4 Gy in 1, 69.2 Gy in 3, 66 Gy in 1 patient. While keeping lung isotoxicity the following tolerances were exceeded: Heart in three patients (2×72.4 Gy, 1×75.6 Gy), esophagus in one patient at 69.2 Gy, aorta in 1 patient at 72.4 Gy, airways in 4 patients (3×72.4 Gy, 1×75.6 Gy). Only one patient reached the 82 Gy dose step without exceeding any normal tissue tolerance at lung isotoxicity.
Average EQD210 of ITV and CTV PT were significantly increased by 17.7 Gy (range 7.7–27.3 Gy) and 10.1 Gy (range 2.4–17.4 Gy), whereas ITV and CTV LN showed no relevant change compared to the adapted 66 Gy plan (Table 5). Adding dose via SIVAB required downscaling of the monitor units of the adapted 66 Gy plan to keep the required lung isotoxicity. This resulted in slightly reduced dose to CTV LN. Respecting all normal tissue limitations reduced the achievable mean ITV PT dose increase to 13.4 Gy (range 0–27.3 Gy). Dose escalation resulted in a significantly improved mean TCP of the ITV PT by absolute 22.1% (range 0–39.2%). Mean TCP change was less than 1% for CTV PT and LN, and 1.2% increase for ITV LN (Table 4).
Table 5.
Mean EQD210 for D95% of CTV and ITV for A lung isotoxic and B to normal tissue tolerance plans.
| Plans | CTV PT (SD) |
ITV PT (SD) |
CTV LN (SD) |
ITV LN (SD) |
|
|---|---|---|---|---|---|
| Plan A | |||||
| 1. | EQD210 at max dose step without increasing mean lung dose | 64.4 (6.3) | 72.0 (8.3) | 52.7 (2.8) | 54.9 (3.6) |
| 2. | 1. and not exceeding any normal tissue tolerance | 61.6 (7.1) | 67.7 (11.6) | 52.9 (2.7) | 54.4 (3.4) |
| p-values 2. versus 66 Gy | <0.01 | <0.01 | >0.05 | >0.05 | |
| Plan B | |||||
| 3. | EQD210 at max dose step without exceeding lung tolerance | 66.1 (7.5) | 72.2 (10.1) | 54.1 (2.5) | 55.7 (3.1) |
| 4. | 3. and not exceeding any normal tissue tolerance | 63.4 (7.7) | 68.2 (11.1) | 54.1 (2.5) | 55.6 (3.0) |
| p-values 4. versus 66 Gy | <0.01 | <0.01 | <0.025 | <0.025 | |
CTV: clinical target volume; D95% dose in 95% of the volume; ITV: internal target volume; LN: lymph nodes; EQD210 dose equivalent to 2 Gy dose for α/β=10 Gy; PT: primary tumor; SD: standard deviation.
Dose escalation to normal tissue tolerance
Six patients reached the 82 Gy dose step without exceeding lung tolerance alone. However, heart tolerance was exceeded in 2 patients at 72.4 Gy and 75.6 Gy dose level, esophagus tolerance in 1 patient for all dose steps, aorta tolerance in two patients (72.4 Gy and 78.8 Gy) and airways in 4 patients (3×72.4 Gy, 1×75.6 Gy). The 82 Gy dose step was reached in only 2 patients without exceeding any normal tissue tolerance. Both patients had peripheral PT location. EQD210 of ITV PT and CTV PT increased by 17.9 Gy and 11.8 Gy with keeping lung tolerance alone. When keeping any normal tissue tolerance, significant mean EQD210 increases of ITV PT by 13.9 Gy (range 0–23.4 Gy) and CTV PT by 9.1 Gy (range 0–18.7 Gy) were achieved (Table 5). The increased dose resulted in a mean absolute TCP improvement of the ITV PT by 23.4% (range 0–43.6%) compared to the 66 Gy plan. Mean TCP change was less than 1% for CTV PT and LN, and 3.9% increase for ITV LN (Table 4).
Discussion
Image-guided adaptive radiotherapy presents a promising methodology to improve the therapeutic ratio through more accurate tumor targeting and improved normal tissue sparing.
During treatment PET/CT has been used in 2 studies for boost definition using conventional fractionation and 3D conformal planning (4, 5). During-treatment adaptation allowed dose escalation by 30–102 Gy in the study by Feng et al. using lung isotoxicity (4). A smaller increase from 66 Gy to a mean dose of 74 Gy was observed by Gillham et al. at normal tissue tolerance (5).
Guckenberger et al. (6) simulated normofractionated dose escalation using 3D conformal radiotherapy, adaptation to geometrical tumor changes, and deformable image and dose registration. The present study used a similar framework, but included repeated assessment of tumor motion, as well as macroscopic and microscopic tumor volume changes, and used IMRT planning with SIVAB.
In Guckenberger’s study a mean 7 Gy GTV dose increase was achieved with two CT-based adaptations without exceeding lung and spinal cord isotoxicity, whereas with the present methodology PT EQD210 was increased by an average 13 Gy taking into account multiple normal tissue dose limitations. At higher radiation doses structures such as airways and esophagus become a concern (12, 13), which were accounted for in the present study.
The present study concept increased boost dose only to the PT, which is the location at the highest risk of tumor recurrence (14). RTOG 9410 showed that primary tumors are at a near 3-fold risk for first recurrence compared to lymph nodes (15). Also, prolonged treatment times originating from higher dose application with conventional fractionation were avoided using SIVAB, hereby compensating for accelerated repopulation (16). Adaptive treatment strategies carry the risk of underdosing microscopic disease by adjusting treatment fields to shrinking tumors and ignoring the potentially continuing presence of tumor cells in their position relative to the initial anatomy (11, 17). As has been demonstrated by Hugo et al. (9), the volume and position of assumed microscopic disease vary during radiotherapy, although to a lesser degree than the macroscopic tumor. In the current study, the initial CTV volume was preserved through warping, ensuring the prescribed dose coverage even in the presence of shape and volume changes of the macroscopic tumor.
Variations in the daily range of respiratory motion are usually small and were assessed in the repeated CTs in this study. Using biofeedback and comparing the ITV motion envelopes to the daily CBCT information will limit excessive variations during daily set-up. Higher frequency of adaptive replanning is expected to allow even larger dose increase, however, in the clinical setting would require additional technical development with automation of several processes including contouring, deformable registration, replanning and quality assurance of all steps.
The present study, performed on a representative set of locally advanced lung cancer patients, demonstrated overall appropriateness of the planned IGART trial. Simulation of the planned study allowed us to test new technical aspects of deformable image registration and dose summation including CTV warping in a clinical scenario. Also, this simulation study enabled assessment of achievable dose increase with tumor shrinkage and identification of potential normal tissue risks. Estimation of required patient numbers for the clinical trial through determination of the expected outcome benefit became feasible.
Conclusion
The present study indicates a significant potential for dose increase with IGART. Limitations were identified suggesting modifications in patient selection and achievable dose intensification prior to opening the planned phase I study.
Supplementary Material
Summary.
In a virtual clinical trial the feasibility of dose intensification with IGART for locally advanced lung cancer was tested using simultaneous integrated boost, and deformable image and dose summation including non-rigid CTV warping. The results show the ability to safely increase the biologically equivalent-to-2 Gy dose (EQD2) by 13.4 Gy /13.9 Gy and the expected tumor control probability (TCP) by 22.1% / 23.4 % at lung isotoxicity / normal tissue tolerance, respectively.
Acknowledgement
The authors thank Drs. Nuzhat Jan and Alfredo Urdaneta for contouring support; Dr. Paul Keall for grant support, review of the manuscript and for making the audiovisual biofeedback system available; Dr. Mihaela Rosu for support with the audiovisual biofeedback system and 4D FBCT acquisition.
This work has been supported by NIH grants P01CA116602 and P30CA016059.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
None of the authors has any actual or potential conflicts of interest.
References
- 1.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 2.Britton KR, Starkschall G, Liu H, et al. Consequences of anatomic changes and respiratory motion on radiation dose distributions in conformal radiotherapy for locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phy. 2009;73:94–102. doi: 10.1016/j.ijrobp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Spoelstra FO, Pantarotto JR, Van Sörnsen de Koste JR, et al. Role of adaptive radiotherapy during concomitant chemoradiotherapy for lung cancer: analysis of data from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2009;75:1092–1097. doi: 10.1016/j.ijrobp.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Feng M, Kong FM, Gross M, et al. Using fluorodeoyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys. 2009;73:1228–1234. doi: 10.1016/j.ijrobp.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillham C, Zips D, Pönisch F, et al. Additional PET/CT in week 5–6 of radiotherapy for patients with stage III non-small cell lung cancer as a means of dose escalation planning? Radiother Oncol. 2008;88:335–341. doi: 10.1016/j.radonc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Guckenberger M, Wilbert J, Richter A, et al. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:901–908. doi: 10.1016/j.ijrobp.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Venkat RB, Sawant A, Suh Y, et al. Development and preliminary evaluation of a prototype audiovisual biofeedback device incorporating a patient-specific guiding waveform. Phys Med Biol. 2008;53:197–208. doi: 10.1088/0031-9155/53/11/N01. [DOI] [PubMed] [Google Scholar]
- 8.Christensen GE, Song JH, Lu W, et al. Tracking lung tissue motion and expansion/compression with inverse consistent image registration and spirometry. Med Phys. 2007;34:2155–2163. doi: 10.1118/1.2731029. [DOI] [PubMed] [Google Scholar]
- 9.Hugo GD, Weiss E, Badawi A, et al. Localization accuracy of the clinical target volume during image-guided radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:560–567. doi: 10.1016/j.ijrobp.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Baardwijk A, Bosmans G, Bentzen SM, et al. Radiation dose prescription for non-small-cell lung cancer according to normal tissue dose constraints: an in silico clinical trial. Int J Radiat Oncol Biol Phys. 2008;71:1103–1110. doi: 10.1016/j.ijrobp.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Guckenberger M, Richter A, Wilbert J, et al. Adaptive radiotherapy for locally advanced non-small-cell lung cancer does not underdose the microscopic disease and has the potential to increase tumor control. Int J Radiat Oncol Biol Phys. 2011;81:e275–e282. doi: 10.1016/j.ijrobp.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 12.Lievens Y, Nulens A, Gaber MA, et al. Intensity-modulated radiotherapy for locally advanced non-small-cell lung cancer: a dose-escalation planning study. Int J Radiat Oncol Biol Phys. 2011;80:306–313. doi: 10.1016/j.ijrobp.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):70–76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willner J, Baier K, Caragiani E, et al. Dose, volume, and tumor control prediction in primary radiotherapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:382–389. doi: 10.1016/s0360-3016(01)01823-5. [DOI] [PubMed] [Google Scholar]
- 15.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machtay M, Hsu C, Komaki R, et al. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys. 2005;63:667–671. doi: 10.1016/j.ijrobp.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Sonke J, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol. 2010;20:94–106. doi: 10.1016/j.semradonc.2009.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.