SUMMARY
Nα-terminal acetylation of cellular proteins was recently discovered to create specific degradation signals, termed Ac/N-degrons and targeted by the Ac/N-end rule pathway. We show that Hcn1, a subunit of the APC/C ubiquitin ligase, contains an Ac/N-degron that is repressed by Cut9, another APC/C subunit and the ligand of Hcn1. Cog1, a subunit of the Golgi-associated COG complex, is also shown to contain an Ac/N-degron. Cog2 and Cog3, direct ligands of Cog1, can repress this degron. The subunit decoy technique was used to show that the long-lived endogenous Cog1 is destabilized and destroyed via its activated (unshielded) Ac/N-degron if the total level of Cog1 increased in a cell. Hcn1 and Cog1 are the first examples of protein regulation through the physiologically relevant transitions that shield and unshield natural Ac/N-degrons. This mechanistically straightforward circuit can employ the demonstrated conditionality of Ac/N-degrons to regulate subunit stoichiometries and other aspects of protein quality control.
Keywords: proteolysis, N-degron, ubiquitin, Not4, Cog1, Hcn1
INTRODUCTION
Approximately 90% of human proteins are Nα-terminally acetylated (Nt-acetylated) (Arnesen et al., 2009; Polevoda and Sherman, 2003; Van Damme et al., 2012). Nt-acetylation is apparently irreversible, in contrast to acetylation-deacetylation of internal lysines in cellular proteins (Starheim et al., 2012). Nt-acetylation can occur posttranslationally but the bulk of it is cotranslational, being mediated by ribosome-associated Nt-acetylases (Gautschi et al., 2003; Park and Szostak, 1992).
We discovered that one biological function of Nt-acetylation is the creation of specific degradation signals (degrons) in cellular proteins (Hwang et al., 2010b). Nt-acetylated proteins are targeted by a specific branch of the N-end rule pathway. This pathway recognizes proteins containing N-terminal degradation signals called N-degrons, polyubiquitylates these proteins and thereby causes their degradation by the proteasome (Figure S1A, B). The main determinant of an N-degron is a destabilizing N-terminal residue of a protein. Recognition components of the N-end rule pathway are called N-recognins. In eukaryotes, N-recognins are E3 ubiquitin (Ub) ligases that can target N-degrons. Regulated destruction of specific proteins by the N-end rule pathway mediates a strikingly broad range of biological functions, cited in the legend to Figure S1 (Dougan et al., 2011; Mogk et al., 2007; Tasaki et al., 2012; Varshavsky, 2008, 2011).
In eukaryotes, the N-end rule pathway comprises two branches, the Arg/N-end rule pathway and the Ac/N-end rule pathway (Figure S1A, B). The Arg/N-end rule pathway targets specific unacetylated N-terminal residues. N-terminal Arg, Lys, His, Leu, Phe, Tyr, Trp, and Ile are directly recognized by E3 N-recognins. In addition, N-terminal Asn, Gln, Asp, and Glu (as well as Cys, under some metabolic conditions) are destabilizing owing to their preliminary enzymatic modifications, which include Nt-deamidation and Nt-arginylation (Figure S1B) (Hwang et al., 2010a; Piatkov et al., 2012; Varshavsky, 2011).
In contrast to the Arg/N-end rule pathway, the Ac/N-end rule pathway targets proteins through their Nt-acetylated residues, largely Nt-acetylated Met, Ala, Val, Ser, Thr, or Cys. These degradation signals are called Ac/N-degrons, to distinguish them from other N-degrons. In the first study of these degradation signals, 7 out of 9 natural Nt-acetylated proteins in the yeast Saccharomyces cerevisiae (MATα2, His3, Tbf1, Slk1, Aro8, Hsp104, and Ymr090w) were shown to contain Ac/N-degrons (Hwang et al., 2010b). (Because the assays employed were definitive only in positive cases, the other 2 proteins may also contain Ac/N-degrons.) In addition, Cog1 and Hcn1, the proteins of this study, have also been found to contain Ac/N-degrons, as described below. Given the quasi-random choices of the 11 natural Nt-acetylated proteins examined so far, it is likely that Ac/N-degrons are present in many (possibly most) Nt-acetylated proteins. We suggested that the biological roles of Ac/N-degrons encompass protein quality control, including the regulation of input stoichiometries of subunits in oligomeric proteins (Hwang et al., 2010b; Varshavsky, 2011). In the latter process, a free subunit of a protein complex is short-lived until the subunit becomes a part of the cognate complex.
Consider an oligomeric protein in which a subunit’s Nt-acetylated residue is sterically shielded. The initial destruction, through an Ac/N-degron, of some of this subunit’s newly formed molecules would be halted by formation of the oligomer and the resulting sequestration of the Ac/N-degron. If this shielding occurs through intramolecular folding of a subunit or through homo-oligomeric interactions, such an Ac/N-degron would become repressed by default, i.e., regardless of the level of subunit’s expression, either during translation of the subunit’s polypeptide chain or soon thereafter. In contrast, if the shielding involves hetero-oligomeric interactions, the control of the Ac/N-degron would require a sufficiently low level of subunit expression. Above that level, some molecules of this subunit would remain short-lived, owing to titration of protein ligands that normally sequester the subunit’s Ac/N-degron.
In this mechanism, the regulation of input stoichiometries of subunits in protein complexes involves the degradation of uncomplexed (e.g., overproduced) subunits through their Ac/N-degrons. As mentioned above, this regulation would be relevant largely to subunits in which Ac/N-degrons can be sequestered through hetero-oligomeric interactions, because the shielding of a protein’s Ac/N-degron through homo-oligomeric interactions would occur even if this protein is overproduced. The conditionality of Ac/N-degrons may also mediate, through the same degron-shielding mechanism, the selective destruction of misfolded proteins, those among them that cannot sequester their Ac/N-degrons. Because most Ac/N-degrons are formed cotranslationally, the degradation of a protein by the Ac/N-end rule pathway would occur, in part, concurrently with the synthesis of a nascent polypeptide or shortly after its completion, for example during the cotranslational assembly of protein complexes, or in the context of a polypeptide’s interaction with chaperones (Brandman et al., 2012; Duncan and Mata, 2011; Halbach et al., 2009; Hartl et al., 2011).
The same logic applies to any conditional degradation signal. Indeed, specific internal degrons participate in proteolysis-mediated homeostasis, including the destruction of abnormal proteins (Finley et al., 2012; Fredrickson and Gardner, 2012; Guerriero and Brodsky, 2012; Ravid and Hochstrasser, 2008; Varshavsky, 2011, 2012; Wolf and Stolz, 2012). Note, however, that compared to other degradation signals, Ac/N-degrons are the earliest degrons to form in nascent polypeptide chains. Ac/N-degrons are also likely to be unusual in their high prevalence.
To explore Ac/N-degrons and the Ac/N-end rule pathway, we focused on Cog1 and Hcn1. S. cerevisiae Cog1 is a 48 kDa subunit of the Conserved Oligomeric Golgi (COG) complex, which comprises the subunits Cog1-Cog8, resides in the cytosol, associates with membranes, and participates in Golgi transport (Miller and Ungar, 2012; Sztul and Lupashin, 2009). Cog1 is a bridging subunit between the lobes of the COG complex (Fotso et al., 2005; Lees et al., 2010). Hcn1 (Cdc26) of the yeast Schizosaccharomyces pombe is a 9 kDa subunit of the Anaphase-Promoting Complex/Cyclosome (APC/C) Ub ligase, which mediates the degradation of mitotic regulators and other proteins (Barford, 2011; Hershko, 2010; Pines, 2011).
We show here that both Cog1 and Hcn1 contain Ac/N-degrons. In addition, we identified Not4 (Mot2) as an E3 Ub ligase that targets Cog1. We also show, using the subunit decoy technique, that the Ac/N-degrons of Cog1 and Hcn1 are regulated through interactions between these proteins and their protein ligands. These results constitute the first evidence for the physiologically relevant conditionality of Ac/N-degrons and for the steric sequestration basis of this conditionality. Hcn1 and Cog1 are the first examples of protein regulation through transitions that shield and unshield natural Ac/N-degrons.
Apart from other ramifications of these advances, they also clarify, in hindsight, the cause of the long-held assumption in the field (before the 2010 discovery of Ac/N-degrons) that Nt-acetylation protects intracellular proteins from degradation. Specifically, many previously studied Nt-acetylated proteins appeared to be long-lived, a correct in part but fundamentally incomplete conclusion. This is shown here through a deeper understanding of the conditionally long-lived endogenous Cog1, its reversible interactions with other COG subunits, and the ensuing shielding and unshielding of its Ac/N-degron. The resulting view is two-fold. First, an Ac/N-degron in a protein precludes the targeting of this protein by proteolytic systems (e.g., the Arg/N-end rule pathway) that require the unmodified N-terminus. Second, a molecule of an Ac/N-degron-containing protein that becomes long-lived enters this state once this molecule ceases (through steric shielding) to be targeted by the Ac/N-end rule pathway. Whether this hiatus from being vulnerable to degradation is long-lasting or transient (and recurring) is determined by the in vivo dynamics of the complex that sequesters the protein’s Ac/N-degron.
RESULTS
The Ac/N-Degron of Cog1, a Subunit of the COG Complex
S. cerevisiae Cog1 is Nt-acetylated (Arnesen et al., 2009; Van Damme et al., 2012). The encoded Met-Asp (MD) N-terminal sequence of wild-type (wt) Cog1, termed MD-Cog1wt, implied that its (retained) N-terminal Met is Nt-acetylated by the NatB Nt-acetylase (Figure S2B–D). MD-Cog1wt was expressed in yeast using low copy plasmids, the PCUP1 promoter, and C-terminal epitope tagging.
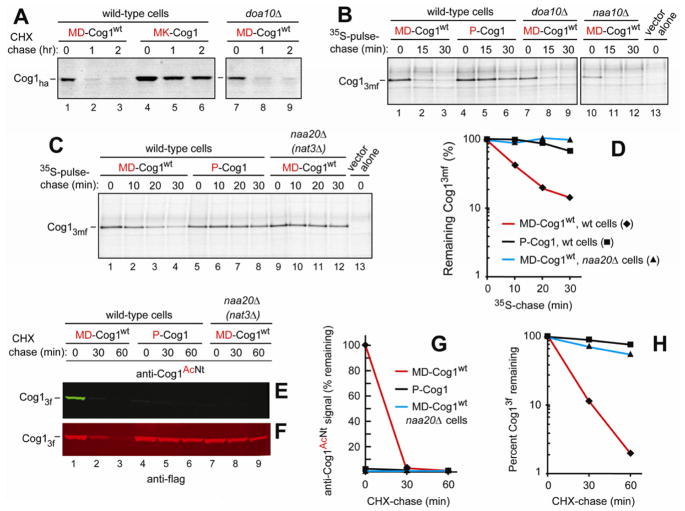
Cycloheximide (CHX)-chases showed that MD-Cog1wt was short-lived in wt cells (t1/2 ≪ 1 hr) (Figure 1A). Our previous work identified the Doa10 E3 Ub ligase as an N-recognin of the Ac/N-end rule pathway (Hwang et al., 2010b). However, MD-Cog1wt remained short-lived in doa10Δ cells (Figures 1A, S3A and S5D, E), prompting the search (described later in the paper) for another N-recognin. In agreement with the presence of an Ac/N-degron in MD-Cog1wt, it was stabilized, including its increased levels at time zero (the beginning of the chase) in naa20Δ (nat3Δ) cells, which lacked the catalytic subunit of the cognate NatB Nt-acetylase (Figures S2D and S3A, C, G). In contrast, the instability of MD-Cog1wt did not require non-cognate Nt-acetylases (Figures S2C, D and S3C).
Figure 1. The Ac/N-degron of Cog1.
(A) Cycloheximide (CHX)-chases were performed at 30°C with wt or doa10Δ S. cerevisiae expressing either wt Cog1, termed MD-Cog1wt, or its MK-Cog1 derivative in which Asp2 was replaced with Lys2. Both proteins were C-terminally ha-tagged. At the indicated times of chase, proteins in cell extracts were fractionated by SDS-PAGE and assayed by immunoblotting with anti-ha antibody.
(B) 35S-pulse-chase with MD-Cog1wt or P-Cog1 in wt, doa10Δ, or naa10Δ (ard1Δ) S. cerevisiae, the latter strain lacking the catalytic subunit of the non-cognate (for MD-Cog1wt) NatA Nt-acetylase (Figure S2). Cog1 proteins were C-terminally tagged with 3 flag epitopes modified to contain a Met residue in each epitope, to increase 35S-Met in Cog1.
(C) Same as in B but another 35S-pulse-chase. It included naa20Δ (nat3Δ) S. cerevisiae lacking the catalytic subunit of the cognate (for MD-Cog1wt) NatB Nt-acetylase (Figure S2).
(D) Quantification of data in C.◆, MD-Cog1wt in wt cells. ▲, MD-Cog1wt in naa20Δ cells. ■, P-Cog1 in wt cells.
(E) Anti-Cog1AcNt antibody specific for Nt-acetylated MD-Cog1wt (see Figure S4A–C) was used for immunoblotting in CHX-chase assays with MD-Cog1wt and P-Cog1 (C-terminally tagged with 3 flag epitopes) in either wt or naa20Δ (nat3Δ) S. cerevisiae.
(F) Same as in E, except that membrane was reprobed with anti-flag antibody.
(G) Quantification of anti-Cog1AcNt-specific immunoblotting patterns in E using a linear scale, with the level of MD-Cog1wt at time zero in wt cells taken as 100%. ◆, MD-Cog1wt in wt cells. ▲, MD-Cog1wt in naa20Δ cells. ■, P-Cog1 in wt cells.
(H) Same as in G but a semi-log plot of the flag-specific Cog1 immunoblotting patterns in F. Same designations as in G. See also Figures S1, S2, S5, and Tables S1 and S2.
In agreement with CHX-chases (which monitor both “young” and “old” protein molecules), the newly formed MD-Cog1wt, analyzed by 35S-pulse-chases, was also short-lived in wt and doa10Δ cells (t1/2 ≈ 10 min) but was nearly completely stabilized in naa20Δ cells lacking the NatB Nt-acetylase (Figures 1B,C, D and S2D).
We also constructed the MK-Cog1D2K and P-Cog1 mutants. In MK-Cog1D2K, wt Asp2 (Figure S2B) was replaced by Lys. In P-Cog1, Pro was inserted before Asp2. The N-terminal sequences of mutant proteins were Met-Lys (MK) and Pro-Asp (PD), respectively, the latter after the cotranslational removal of N-terminal Met. Either N-terminal Pro followed by any residue or N-terminal Met followed by a basic residue are not Nt-acetylated in S. cerevisiae (Figure S2C) (Arnesen et al., 2009; Goetze et al., 2009). In agreement with the presence of an Ac/N-degron in MD-Cog1wt, the non-Nt-acetylatable MK-Cog1D2K and P-Cog1D2P were long-lived in CHX-chases, in addition to their increased levels at the beginning of the chase (Figure 1A and S3B). P-Cog1D2P was also stabilized in 35S-pulse-chases (Figure 1B, C, D).
The degradation of MD-Cog1wt was mediated largely by the proteasome, as shown using either pdr5Δ S. cerevisiae, which allowed for the intracellular retention of MG132, a proteasome inhibitor (Figure S3D, E), or through the use of wt cells made permeable to MG132 by low levels of SDS (Figure S3G). MD-Cog1wt was also examined using the conditional uba1-204 allele of the Uba1 Ub-activating (E1) enzyme (Ghaboosi and Deshaies, 2007). MD-Cog1wt was short-lived in wt and uba1-204 cells at 30°C and remained short-lived in wt cells at the nonpermissive temperature of 37°C (Figure S3H). In contrast, MD-Cog1wt was stabilized at 37°C in uba1-204 cells, including its increased levels at time zero (Figure S3H). Thus, the degradation of MD-Cog1wt requires the Ub-activating enzyme.
Antibody Specific for Nt-Acetylated Cog1
The Nt-acetylated N-terminal peptide of MD-Cog1wt, Ac-MDEVLPLFRDS, was used to produce a rabbit antibody, termed anti-Cog1AcNt, that recognized the Nt-acetylated peptide but not its unacetylated counterpart (Figure S4A–C). This antibody detected Nt-acetylated MD-Cog1wt in extracts from wt cells either at the beginning of the chase (Figure 1E) or under steady-state conditions (Figure S4B). In contrast, anti-Cog1AcNt detected, at most, trace amounts of MD-Cog1wt in extracts from naa20Δ (nat3Δ) cells lacking the cognate NatB Nt-acetylase (Figure 1E), thereby providing independent (immunological) confirmation of the mass spectrometric evidence (Arnesen et al., 2009; Van Damme et al., 2012) for Nt-acetylation of MD-Cog1wt in wt cells. The same immunoblot re-probed with anti-Flag antibody revealed comparable levels of MD-Cog1wt (C-terminally tagged with 3 flag epitopes) in both wt and naa20Δ cells at the beginning of the chase, thereby confirming the specificity of anti-Cog1AcNt (Figure 1E, F). In agreement with previously described chase assays (Figures 1A–D and S3A–C), MD-Cog1wt was short-lived in wt cells but was stabilized in naa20Δ cells (Figure 1E–H), whereas P-Cog1 was nearly stable under all conditions (Figure 1E, F, H).
Together, CHX-chases, 35S-pulse-chases, and anti-Cog1AcNt results with wt versus mutant S. cerevisiae and with mutants of MD-Cog1wt (Figures 1, S3 and S4) identified this subunit of the COG complex as a short-lived substrate of the Ac/N-end rule pathway.
Cog1 Interactions with Other COG Subunits and Membranes
Cog1 contributes to interactions of the COG complex with the Golgi membrane (Sztul and Lupashin, 2009). In a test that did not distinguish between Golgi and other membranes, cell extracts were fractionated into soluble (“cytosol”) and insoluble (“membrane”) parts. The absence of Nt-acetylation of MD-Cog1wt in naa20Δ cells did not affect its partitioning between membrane and soluble fractions (Figure S4D–E).
Schulman and colleagues showed that the Nt-acetyl moiety can significantly contribute to the affinity between an Nt-acetylated protein and its protein ligand (Monda et al., 2012; Scott et al., 2011). We found that both Cog3ha and Cog4ha could be coimmunoprecipitated (co-IPed) with MD-Cog1wt from both wt and naa20Δ extracts (Figure S4G, H). Thus, Nt-acetylation of MD-Cog1wt is not strictly required for its direct or indirect interactions with Cog3 and Cog4, i.e., a possibly lower affinity of unacetylated MD-Cog1wt (in naa20Δ cells) for its protein ligands was still sufficient for co-IP. We also found that the apparent Mr of the 93-kDa Cog3 was increased by ~8 kDa either in naa20Δ cells or in the absence of overexpressed MD-Cog1wt. Upshifted Cog3 could be co-IPed with MD-Cog1wt (Figure S4G). One interpretation of these results is that Cog3 is monoubiquitylated under the above conditions.
Cog1 Is Targeted by the Not4 (Mot2) E3 Ubiquitin Ligase
The Doa10 E3 N-recognin physically binds to Nt-acetylated polypeptides and contributes to the in vivo degradation of previously examined Nt-acetylated proteins (Hwang et al., 2010b). However, Doa10 was not required for the degradation of MD-Cog1wt (Figures 1A, B, S1A, S3A and S5D). Consistently, MD-Cog1wt was not stabilized in ubc6Δ ubc7Δ cells lacking two cognate E2s of the Doa10 E3 (Figure S5F, G). Null mutants in 9 other E2s also did not stabilize MD-Cog1wt (Figure S5A–C). In addition, MD-Cog1wt remained short-lived in ubr1Δ and san1Δ cells, which lacked, respectively, the E3 N-recognin of the Arg/N-end rule pathway (Figure S1B) and a nuclear E3 that targets misfolded proteins (Fredrickson and Gardner, 2012) (Figure S5D, E). Given these negative results, we examined the stability of MD-Cog1wt in a not4Δ mutant, because the Not4 (Mot2) E3 Ub ligase interacts, in particular, with a ribosome-bound chaperone called the Nascent Polypeptide-Associated Complex (NAC) (Collart et al., 2013).
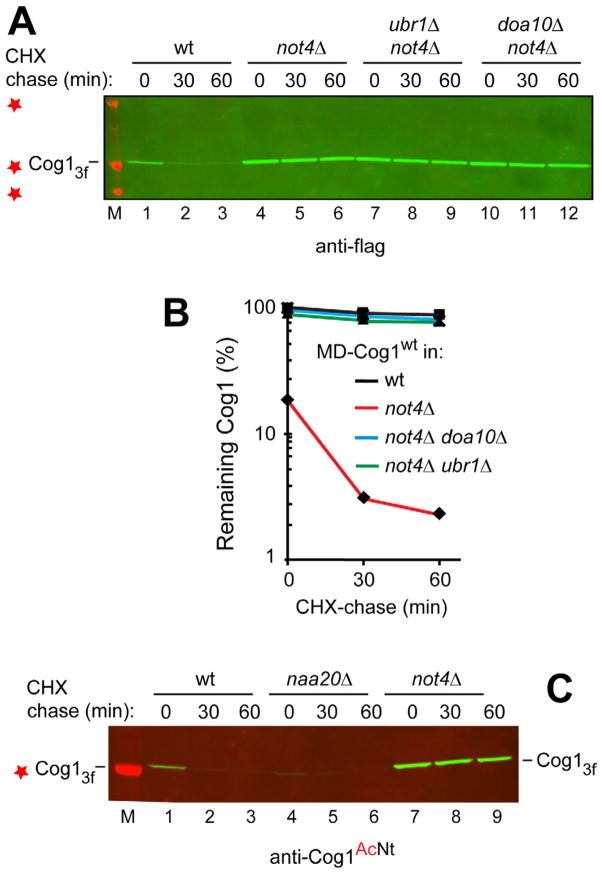
Remarkably, MD-Cog1wt was strongly stabilized in not4Δ cells, including its increased levels at the beginning of the chase (Figure 2A, B). In addition, the observed stabilization of MD-Cog1wt in the absence of Not4 was not significantly affected in not4Δ doa10Δ and not4Δ ubr1Δ double mutants that also lacked either Doa10, an N-recognin of the Ac/N-end rule pathway, or Ubr1, the N-recognin of the Arg/N-end rule pathway (Figures 6A, B and S1A, B). The same results (dependence of the MD-Cog1wt degradation on the presence of Not4) were obtained in CHX-chase assays in which MD-Cog1wt was detected by immunoblotting with anti-Cog1AcNt antibody, which selectively recognized Nt-acetylated MD-Cog1wt (Figure 2C). The discovery that Not4 is the putative second N-recognin of the Ac/N-end rule pathway opens up a separate direction of studies (see Discussion).
Figure 2. Stabilization of Cog1 in S. cerevisiae Lacking the Not4 E3 Ubiquitin Ligase.
(A) CHX-chases with yeast expressing MD-Cog1wt C-terminally tagged with three flag epitopes. Lane M and red stars, Mr markers of 37, 50, and 100 kDa, respectively. MD-Cog1wt in wt yeast (lanes 1–3), and in not4Δ (lanes 4–6), not4Δ ubr1Δ (lanes 7–9), and not4Δ doa10Δ mutants (lanes 10–12).
(B) Quantification of immunoblots in H, with the level of MD-Cog1wt in not4Δ cells at the beginning of chase taken as 100%. ■, MD-Cog1wt C in not4Δ cells (black curve). ◆, MD-Cog1wt in wt cells (red curve).▲, MD-Cog1wt in not4Δ ubr1Δ cells (green curve). X,MD-Cog1wt in not4Δ doa10Δ cells (blue curve).
(C) Same as in A but an independent CHX-chase, and immunoblotting with anti-Cog1AcNt antibody specific for Nt-acetylated MD-Cog1wt. Lane M and red star, an Mr marker of 50 kDa. MD-Cog1wt in wt yeast (lanes 1–3), and in naat20Δ (nat3Δ) (lanes 4–6), and not4Δ mutants lanes 7–9). See also Figure S5.
Figure 6. Conditionality of Ac/N-degrons.
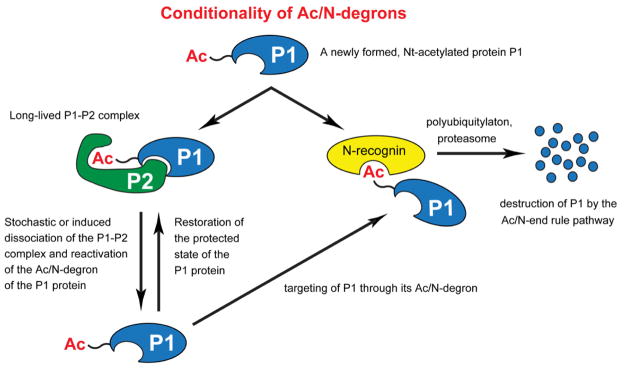
This diagram summarizes the functional understanding of the dynamics of Nt-acetylated proteins vis-à-vis the Ac/N-end rule pathway attained in the present study, in conjunction with results that initially revealed the Ac/N-end rule pathway (Hwang et al., 2010b). See also Figure S1.
Short-Lived Cog1 Is Stabilized by Coexpression of Cog2-Cog4
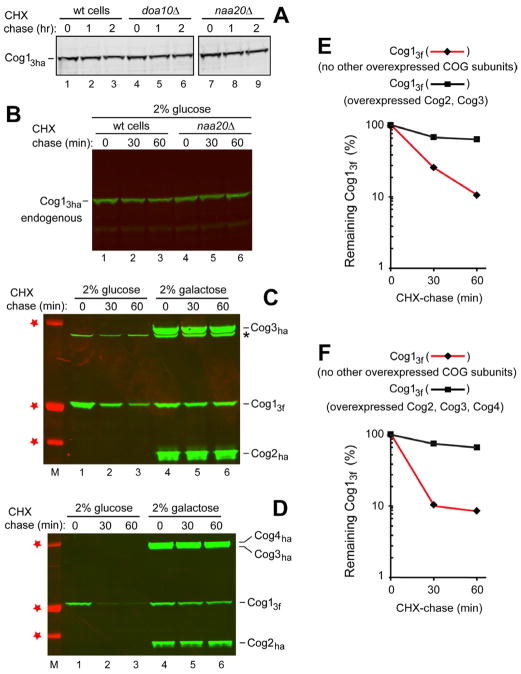
Both the conjectured conditionality of Ac/N-degrons (Hwang et al., 2010b) and electron microscopy of the yeast Cog1-Cog4 subcomplex (Figure S1D) (Lees et al., 2010) suggested that the Ac/N-degron of MD-Cog1wt may be at least partially sequestered in the subcomplex. Cog2 and Cog3 are direct binding ligands of MD-Cog1wt (Sztul and Lupashin, 2009). We asked whether the short-lived, moderately overexpressed (from the PCUP1 promoter on a low copy plasmid) could be stabilized by expressing, from the galactose-inducible PGAL1-10 promoter on a high copy plasmid, the COG subunits Cog2, Cog3 and Cog4. Indeed, the short-lived was strongly stabilized by coexpression of either Cog2 and Cog3 or of Cog2, Cog3 and Cog4 (Figure 3C–F). We conclude that the Ac/N-degron of MD-Cog1wt is conditional in a physiologically relevant manner (see Discussion).
Figure 3. Stabilization of Overexpressed, Short-Lived Cog1 by Coexpressed Cog2-Cog4.
(A) CHX-chases with endogenous (C-terminally tagged with 3 ha epitopes) expressed from the chromosomal COG1 locus and the native PCOG1 promoter in wt, doa10Δ, and naa20Δ (nat3Δ) cells.
(B) Same as in A but an independent CHX-chase. S. cerevisiae (in 2% glucose) expressing endogenous and carrying a plasmid that could express the decoy but only in the presence of galactose. Lanes 4–6, same as lanes 1–3 but in naa20Δ cells.
(C) Stabilization of overexpressed MD-Cog1wt (C-terminally tagged with 3 flag epitopes) by coexpressed Cog2 and Cog3. Lane M and red stars, Mr markers of 37, 50, and 100 kDa, respectively. Lanes 1–3, wt S. cerevisiae in 2% glucose, expressing MD-Cog1wt from the PCUP1 promoter on a low copy plasmid and carrying a high copy plasmid that expressed, only in galactose, both Cog2 and Cog3 (C-terminally tagged with ha) from the bidirectional PGAL1/10 promoter. Lanes 4–6, same as lanes 1–3 but with cells in 2% galactose. Asterisk on the right indicates a protein crossreacting with anti-ha.
(D) Same as in C but cells also carried a second high copy plasmid expressing Cog4 (C-terminally tagged with ha) from the PGAL1/10 promoter.
(E) Quantification of data in C for MD-Cog1wt. ◆, MD-Cog1wt in cells that did not coexpress other COG subunits. ■, MD-Cog1wt in cells that coexpressed (in galactose) Cog2 and Cog3.
(F) Quantification of data in D for MD-Cog1wt. ◆, MD-Cog1wt in cells that did not coexpress other COG subunits. ■, MD-Cog1wt in cells that coexpressed (in galactose) Cog2-Cog4. See also Figure S1.
Endogenous Cog1 Is Long-Lived If No Other Cog1 Is Coexpressed In a Cell
To address the degradation of endogenous Cog1, we replaced, through in vivo recombination, the chromosomal COG1 with an otherwise identical DNA segment that expressed the C-terminally triply ha-tagged MD-Cog1wt ( ) from the endogenous PCOG1 promoter. Remarkably, in cells expressing solely the endogenous , it was much more stable than the triply flag-tagge overexpressed from the PCUP1 promoter. Specifically, less than 10% of the endogenous was degraded during 2 hrs of CHX-chase, in contrast to degradation of the bulk of overexpressed (Figure 3A vs., for example, Figure 1A, F). In addition, the metabolic stability of endogenous remained unaltered in naa20Δ cells lacking the cognate NatB Nt-acetylase, in contrast to a strong stabilization of the (short-lived) overexpressed in the absence of NatB (Figure 3A, B vs. Figures 1C, S3A, and S3C). The reason for this striking difference in stability between the endogenous Cog1 and overexpressed Cog1 was identified through the subunit decoy technique, as described below.
Subunit Decoy Technique Reveals the Cause of Stability of Endogenous Cog1
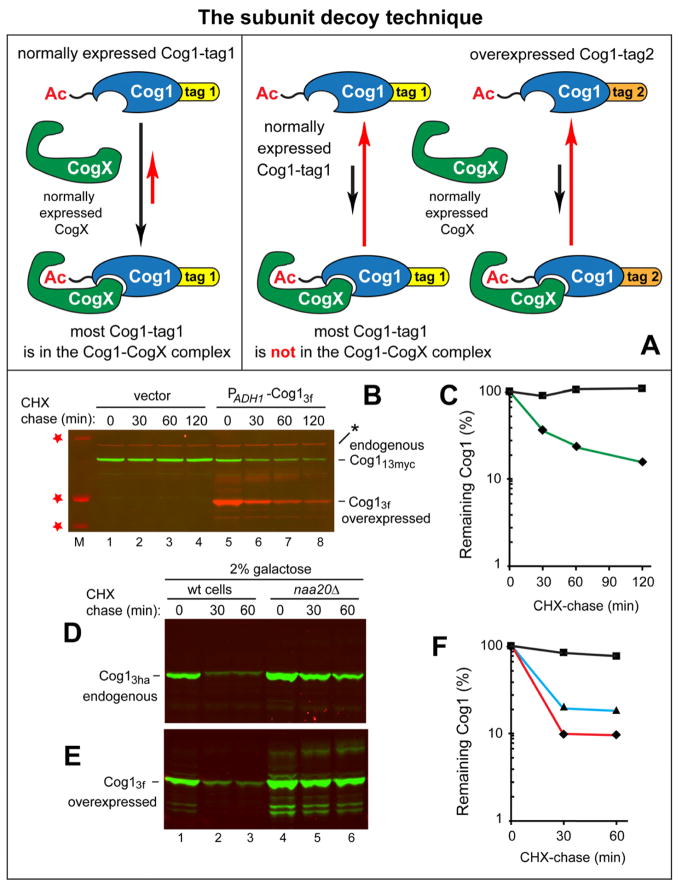
The results described so far suggested that the stability of endogenous MD-Cog1wt (Figure 3A) stems from sequestration of its Ac/N-degron by other COG subunits during the assembly of the degradation-resistant COG complex. We addressed this possibility by an approach termed the subunit decoy technique. First, a C-terminally epitope-tagged protein of interest is expressed at its endogenous level from its native chromosomal location and transcriptional promoter. Second, the same protein but C-terminally tagged with a different epitope is also expressed in these cells, as described in Figure 4A.
Figure 4. Subunit Decoy Technique and the Cause of Stability of Endogenous Cog1.
(A) The subunit decoy technique. “CogX” denotes a Cog1-interacting COG subunit (Cog2 or Cog3) that can shield the Ac/N-degron of Cog1. “Normally expressed” refers to levels of expression from endogenous promoters and chromosomal loci. The normally expressed Cog1-tag1 bears a C-terminal tag denoted as “tag1”, whereas the otherwise identical but overexpressed Cog1-tag2 decoy bears a different C-terminal tag (“tag2”). In the absence of decoy, the bulk of (normally expressed) Cog1-tag1 would occur as a CogX-Cog1-tag1 complex in which the Ac/N-degron of Cog1 is largely sequestered. By contrast, in the presence of overexpressed Cog1-tag2 decoy, the bulk of both Cog1-tag1 and Cog1-tag2 would not be in the complex with CogX (i.e., their Ac/N-degron would be active), given relatively low levels of a (normally expressed) CogX “shielding” protein.
(B) Lane M and red stars, Mr markers of 37, 50, and 100 kDa, respectively. Lanes 1–4, stability of endogenous (C-terminally tagged with 13 myc epitopes) in the absence of the decoy (C-terminally tagged with 3 flag epitopes). CHX-chase with expressed from the chromosomal COG1 locus and the native PCOG1 promoter in wt cells in the presence of vector alone. Lanes 5–8, same as lanes 1–4 but cells carried a plasmid that expressed the decoy from the PADH1 promoter. An asterisk denotes a protein crossreacting with anti-flag.
(C) Quantification of data in B. ◆, endogenous in wt cells that did not express the decoy. ■, endogenous in wt cells that expressed .
(D) Same as in C but with wt and naa20Δ cells expressing the endogenous in 2% galactose, i.e., in the presence of the coexpressed decoy. Immunoblotting with anti-ha, specific for .
(E) Same as in D but also probed (in a parallel immunoblot) with anti-flag, specific for .
(F) Quantification of data in D, E. ◆, endogenous in wt cells grown in 2% glucose. ■, endogenous in wt cells grown in 2% galactose, i.e., in the presence of the decoy. ▲, decoy. See also Figures S4 and S5.
Cells expressing wt levels of (C-terminally tagged with 13 myc epitopes) from the chromosomal COG1 locus were transformed either with a vector alone or with a plasmid expressing the otherwise identical (C-terminally tagged with 3 flag epitopes) from the constitutive PADH1 promoter (Figure 4B). Remarkably, whereas the endogenous was stable in the absence of ectopically expressed , the same endogenous became a short-lived protein in the presence of the (also short-lived) (Figures 3A, B and 4B, C). In another design of the subunit decoy experiment, cells expressing wt levels of (C-terminally tagged with 3 ha epitopes) from the chromosomal COG1 locus were transformed with a plasmid expressing the otherwise identical from the galactose-inducible PGAL1 promoter. In glucose medium, where was not expressed, the endogenous was long-lived (t1/2 ≫ 1 hr), as evidenced by its stability in a CHX-chase and the absence of a significant change in naa20Δ cells lacking the cognate NatB Nt-acetylase (Figure 3B). In striking contrast, the same endogenous became short-lived (t1/2 ≪ 30 min) in the presence of galactose, which induced (Figure 4D–F). As described in Discussion, these technically robust results are likely to be of general significance (i.e., far beyond the subject of Cog1), because the conditional sequestration model of Ac/N-degrons (see Introduction) predicts the observed destabilization of endogenous MD-Cog1wt in the presence of additional Cog1 molecules in a cell.
The Ac/N-Degron of Hcn1, a Subunit of the APC/C Ubiquitin Ligase
In the crystal structure, by Barford and colleagues, of the complex between the APC/C subunits Hcn1 and Cut9 of S. pombe (Zhang et al., 2010), the Nt-acetylated Met residue of Hcn1 was found to be enclosed within a chamber of the folded Cut9 subunit (Figure S1C). Zhang et al. (2010) suggested that this configuration might be the first structurally explicit example of the sequestration of Ac/N-degrons proposed by Hwang et al. (2010b).
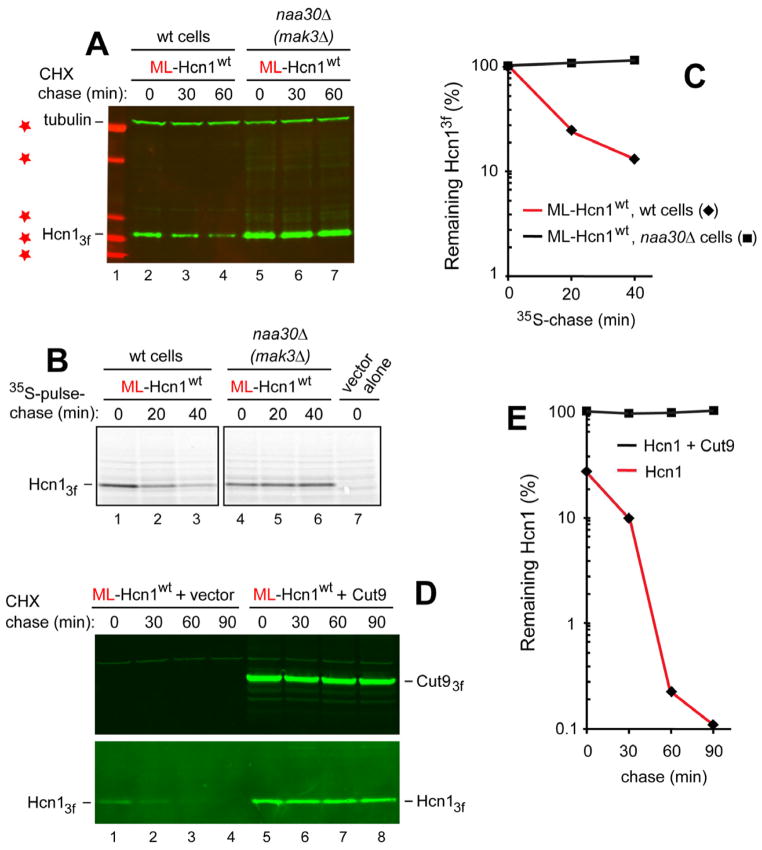
We addressed this possibility as follows. The encoded Met-Leu (ML) N-terminal sequence of the 9-kDa wt Hcn1, termed ML-Hcn1wt, implied that its (retained) N-terminal Met was Nt-acetylated by the NatC Nt-acetylase (Figure S2C). ML-Hcn1wt was C-terminally tagged with 3 flag epitopes and expressed in S. cerevisiae from the PCUP1 promoter. As determined using both CHX-chases and 35S-pulse-chases, ML-Hcn1wt was indeed short-lived in wt cells, with t1/2 < 40 min in the CHX-chase (Figure 5A) and t1/2 of ~10 min in the 35S-pulse-chase (Figure 5B, C). In contrast, ML-Hcn1wt was virtually completely stabilized in naa30Δ (mak3Δ) cells lacking the catalytic subunit of the cognate NatC Nt-acetylase (Figure 5A–C). We conclude that the degradation of ML-Hcn1wt is mediated by its Ac/N-degron.
Figure 5. Hcn1 and Repression of Its Ac/N-degron by Cut9.
(A) CHX-chases in wt or naa30Δ (mak3Δ) S. cerevisiae expressing the wt S. pombe Hcn1, termed ML-Hcn1wt, C-terminally tagged with 3 flag epitopes. naa30Δ cells lacked the catalytic subunit of the cognate NatC Nt-acetylase (Figure S2). Lane 1 and red stars, Mr markers of 10, 15, 20, 37, and 50 kDa, respectively.
(B) 35S-pulse-chase with ML-Hcn1wt in wt and naa30Δ (mak3Δ) S. cerevisiae. Lane 7, vector alone.
(C) Quantification of data in B. ◆, ML-Hcn1wt in wt cells. ■, ML-Hcn1wt in naa30Δ cells.
(D) Lanes 1–4, CHX-chase with wt cells in 2% galactose (and without methionine) that expressed ML-Hcn1wt from the methionine-repressible PMET25 promoter on a low copy plasmid and carried a vector alone (no Cut9 expression). Note the metabolic instability of ML-Hcn1wt (lower panel). Lanes 5–8, same as lanes 1–4 but with a low copy plasmid (instead of control vector) expressing Cut9 from the galactose-inducible PGAL1 promoter, with both ML-Hcn1wt and Cut9 C-terminally tagged with 3 flag epitopes. Note the metabolic stabilization of ML-Hcn1wt(lower panel), including a strong increase of its level at the beginning of the chase.
(E) Quantification of data in D. ◆, ML-Hcn1wt in the absence of co-expressed Cut9. ■, ML-Hcn1wt in the presence of co-expressed Cut9.
See also Figure S1.
Coexpression of Cut9 with Hcn1 Represses the Ac/N-degron of Hcn1
Given the sequestration of the Nt-acetylated Met of ML-Hcn1wt in the cleft of Cut9 (Figure S1C) and the demonstrated degradation of ML-Hcn1wt (in the absence of Cut9) by the Ac/N-end rule pathway (Figure 5A–C), we asked whether coexpression of Cut9 would inhibit the degradation of ML-Hcn1wt. Several independent CHX-chase assays have shown that the short-lived ML-Hcn1wt was virtually completely stabilized by Cut9 upon its overexpression from the galactose-inducible PGAL1 promoter (Figure 5D, E). These results were analogous to the metabolic stabilization of the short-lived MD-Cog1wt by its coexpressed ligands Cog2-Cog4 (Figure 3C–F), with the added advantage of knowing that the observed inhibition of the degradation of ML-Hcn1wt by Cut9 (Figure 5D, E) can be visualized and understood at atomic resolution, owing to the crystal structure of the Hcn1-Cut9 complex (Figure S1C) (Zhang et al., 2010).
DISCUSSION
This study extended key ramifications of the discovery of Ac/N-degrons and the Ac/N-end rule pathway (Figure S1A) (Hwang et al., 2010b). We proposed that the functions of Ac/N-degrons are based on their conditionality and encompass the control of protein quality, including the regulation of input stoichiometries of subunits in oligomeric protein complexes (Hwang et al., 2010b; Varshavsky, 2011). Until now, these were merely suggestions about possible steric sequestration and biological functions of Ac/N-degrons.
To address these functions, we chose two unrelated natural Nt-acetylated proteins, Cog1 (MD-Cog1wt) and Hcn1 (ML-Hcn1wt), described in Introduction. CHX-chase and pulse-chase experiments with MD-Cog1wt and its mutants expressed in wt or mutant S. cerevisiae, as well as the development of an antibody, anti-Cog1AcNt, that selectively recognized Nt-acetylated MD-Cog1wt, identified this subunit of the COG complex as a short-lived substrate of the Ac/N-end rule pathway (Figures 1 and S3–S5). Experiments with ML-Hcn1wt also showed it to be an unstable protein targeted through its Ac/N-degron (Figure 5).
Through a different line of inquiry in this study, we discovered that at least Cog1 and also, presumably, other proteins containing Ac/N-degrons are targeted for degradation by the Not4 (Mot2) E3 Ub ligase (Figure 2). The Not4 E3 is a part of the large (>1 MDa) Ccr4-Not complex. It is present in the nucleus and cytosol, and interacts, in particular, with a ribosome-bound NAC chaperone (Collart et al., 2013). The apparent preference of distinct E3 N-recognins of the Ac/N-end rule pathway such as Doa10 and Not4 for specific (possibly overlapping) classes of substrates containing Ac/N-degrons remains to be understood. One possibility is that different N-recognins may differ in their requirements for determinants of Ac/N-degrons that are additional to the Nt-acetyl group of a substrate protein. Such determinants may include, for example, the location and/or flexibility of a substrate’s internal Lys residue(s) that has to be polyubiquitylated by an E3-associated E2 enzyme (Varshavsky, 2011). Despite the clarity of molecular genetic evidence for the involvement of Not4 in the degradation of Cog1 (Figure 2), a comprehensive identification of Not4 as an N-recognin of the Ac/N-end rule pathway would still require, among other things, a demonstration that Not4 specifically binds to Nt-acetylated polypeptides. Being outside the main focus of the present work (see Introduction), this exciting advance is being developed further in a separate study.
ML-Hcn1wt is an S. pombe protein and was, therefore, not expected to have ligands in S. cerevisiae that might shield its Nt-acetylated Met and thereby repress its Ac/N-degron. The disposition was different with S. cerevisiae MD-Cog1wt, in that some newly formed molecules of overexpressed MD-Cog1wt were expected to associate with Cog1-binding subunits of the COG complex and thereby repress, through steric shielding, the Ac/N-degron of MD-Cog1wt. However, a majority of newly formed molecules of overexpressed MD-Cog1wt would be unable to experience such a fate, as they would “run out” of cognate COG protein ligands to bind to. If so, overexpressed MD-Cog1wt and ML-Hcn1wt would be expected to be stabilized by coexpression of their cognate protein ligands.
This prediction was shown to be correct. A short-lived, overexpressed MD-Cog1wt became a long-lived protein when Cog2 and Cog3 (direct ligands of Cog1) or Cog2, Cog3 and Cog4 were coexpressed with MD-Cog1wt (Figure 3C–F). Analogously, the short-lived ML-Hcn1wt was stabilized upon coexpression of its cognate ligand Cut9 (Figure 5D, E). Thus, the Ac/N-degrons of MD-Cog1wt and ML-Hcn1wt are conditional in a physiologically relevant manner, as they can be repressed through the previously demonstrated interactions between these proteins and their ligands.
Further analyses utilized an approach we termed the subunit decoy technique. In this method, a C-terminally epitope-tagged protein is expressed at its endogenous (wt) level in the absence or presence of the same protein that is alternatively tagged and overexpressed (Figure 4A). Several studies over the last two decades reported that an overexpressed protein could diminish the expression of its endogenous counterpart, and that this repression could be posttranscriptional, i.e., it involved changes in either translation or protein degradation, through degrons that remained unknown (Chen and Archer, 2005).
When the C-terminally ha-tagged was expressed from its chromosomal locus at wt levels and in the absence of other versions of Cog1 in the cell, it was found to be long-lived, in striking contrast to overexpressed MD-Cog1wt, the bulk of which was degraded through the Ac/N-degron (Figures 1A and 3A). The subunit decoy technique revealed the mechanism of this phenomenon in the context of Ac/N-degrons. Specifically, when the long-lived endogenous was expressed in the presence of the otherwise identical but flag-tagged and overexpressed decoy, the previously stable became short-lived (Figure 4). The conditional sequestration model of Ac/N-degrons (see Introduction) predicts this outcome. Specifically, endogenous (i.e., sufficiently low) expression levels of would be comparable to endogenous levels of the protective subunits of the COG complex such as Cog2 and Cog3 (Figure 3). As a result, most (though not necessarily all) molecules of newly formed escape the degradation by the Ac/N-end rule pathway through the formation of complexes with COG subunits that shield the Ac/N-degron of . In contrast, the “additional” expression of the flag-tagged decoy results in total levels of MD-Cog1wt that outstrip the supply of endogenous Cog1-binding subunits of the COG complex. Consequently, most newly formed molecules of ha-tagged are no longer forming degradation-resistant COG-based complexes and therefore remain short-lived, alongside the also short-lived molecules of the flag-tagged decoy (Figure 4).
The subunit decoy experiments produced yet another insight. It was the bulk (not just a small subset) of the endogenous that was destabilized by the coexpression of the decoy (Figure 4). In other words, this destabilization of endogenous included its older molecules that were the long-lived components of the COG complex, before the expression of the decoy. These findings strongly suggested that the COG complex is dynamic in vivo, with dissociations-reassociations of COG subunits that repeatedly unshield (activate) and later sequester the Ac/N-degron of . The fact that endogenous is relatively long-lived in the absence of the decoy (Figure 3A, B) implies that the probability of destruction of upon a single cycle of its transient (and rapidly reversed) dissociation from the COG complex is low. However, when the COG complex dissociates in the presence of coexpressed decoy, the latter, being in excess, would prevent, through direct competition, an efficacious re-incorporation of into the reassembled COG complex, thereby greatly increasing the probability of destruction of the “orphaned” (competed out) by the Ac/N-end rule pathway, as was observed in these experiments (Figure 4).
This deeper understanding (Figures 1, 2–4, and 6) led to a clarifying reinterpretation of statements, in a number of studies over the last two decades, that most Nt-acetylated proteins were long-lived in vivo. Experimental support of such statements stemmed primarily from assays that would not be expected to detect a transient degradation of a protein of interest. Given the logic of the deeper understanding produced with Cog1 in the present work, we conclude that perception, in earlier studies, about the in vivo stability of Nt-acetylated proteins was correct in part but fundamentally incomplete at least for some and possibly for many of these proteins. Specifically, the targeting, by the Ac/N-end rule pathway, of an Ac/N-degron-containing protein such as Cog1 is (reversibly) prevented once Cog1 becomes a part of the COG complex. Although this complex is stable enough for in vitro studies (Sztul and Lupashin, 2009), it is clearly dynamic in vivo (Figure 4 and discussion above), similarly to many other protein complexes (Barford, 2011; Hartl et al., 2011). Thus, an Ac/N-degron of an “intermittently sheltered” protein molecule can become available for proteolytic targeting repeatedly during the lifetime of this molecule in a cell.
Because the bulk of Nt-acetylation is cotranslational, i.e., it involves nascent proteins, it is possible (we suggest it is likely) that every nascent and/or newly formed protein that acquires an Ac/N-degron is targeted for destruction by the Ac/N-end rule pathway through this degron, with a varying (from protein to protein) but nearly always non-zero probability of degradation. This initial vulnerability results in a fraction of each protein’s molecules being destroyed cotranslationally or nearly so. Previous work introduced the in vivo Ub sandwich technique and employed it to demonstrate the existence and significant extent of the cotranslational degradation of nascent proteins that contained N-degrons of the Arg/N-end rule pathway (Turner and Varshavsky, 2000). It should now be possible to measure, using analogous methods, the extent of initial (including cotranslational) degradation of specific Nt-acetylated proteins by the Ac/N-end rule pathway.
The degradation of a nascent or newly formed protein that contains an Ac/N-degron is a part of quality control in that the degradation ceases if and when this degron is shielded from the Ac/N-end rule pathway. For example, during the assembly of the COG complex the initially active Ac/N-degron of Cog1 would be (reversibly) sequestered through its interactions with specific COG subunits such as Cog2 and Cog3 (Figure 3), thereby yielding a degradation-resistant intermediate that coalesces with other COG subunits to yield the COG complex. Because some protein complexes assemble largely cotranslationally and some largely posttranslationally, and because many proteins undergo chaperone-assisted folding before they can productively encounter their cognate protein ligands (Hartl et al., 2011), specific routes through which a nascent protein bearing an Ac/N-degron would sequester it from the Ac/N-end rule pathway are expected to vary from one protein to another. The speed of sequestration is expected to vary as well, even for normal proteins in unstressed cells. Consequently, the extent of the initial (and usually transient) destruction of a protein that accompanies the cotranslational formation of its Ac/N-degron is expected to vary significantly among different proteins.
There are, fundamentally, three routes to the steric shielding of an Ac/N-degron: through intramolecular folding of a polypeptide containing this degron; through homo-oligomeric interactions among identical protein subunits; and through hetero-oligomeric interactions in which an Ac/N-degron of one subunit of a complex is shielded by another protein that interacts with this subunit (Figures 6 and S1C). The sequestration of an Ac/N-degron that occurs through intramolecular folding or homo-oligomeric interactions is not “saturable”, i.e., Ac/N-degrons of this class would become extinguished regardless of whether a protein containing such a degron is expressed normally or overexpressed. In contrast, if the shielding involves hetero-oligomeric interactions, the self-regulation of Ac/N-degron would require a sufficiently low level of subunit expression. Above that level, some subunit molecules would remain short-lived, owing to titration of protein ligands that can sequester the subunit’s Ac/N-degron. Such is the case with both Cog1 and Hcn1 of the present study, as their Ac/N-degrons are repressed through hetero-oligomeric interactions (Figures 3–6 and S1C, D).
The mechanics of Ac/N-degrons can underlie both the input stoichiometries of specific subunits in protein complexes and the selective destruction of misfolded proteins, those among them that cannot sequester their Ac/N-degrons rapidly enough. In the present study, Cog1 vis-à-vis the COG complex and Hcn1 vis-à-vis the APC/C cyclosome (Figures 6 and S1C, D) are likely examples of the physiological control of input stoichiometries through the Ac/N-end rule pathway. It is unknown, so far, whether Ac/N-degrons play important roles in the selective elimination of misfolded or otherwise abnormal proteins. It should be also noted that the function of the Nt-acetyl group as a key determinant of Ac/N-degrons is compatible with other known aspects of Nt-acetylation, including its contributions to protein-membrane interactions, to physical affinities between specific proteins, and to the negative regulation of protein translocation into the endoplasmic reticulum (ER) (Starheim et al., 2012).
The apparent irreversibility of Nt-acetylation (see Introduction) is curious because it is likely that evolution could have readily produced an Nt-deacetylase, given the existence of efficacious enzymes that deacetylate acetylated internal lysines in many proteins. The absence of Nt-deacetylases suggests an adaptive value of retaining an Ac/N-degron in a protein molecule that acquired it, most likely because this degron can become active (unshielded) repeatedly during the molecule’s sojourn in a cell, as discussed above in the context of Cog1 and the COG complex.
One setting of likely physiological relevance vis-à-vis Ac/N-degrons is aneuploidy, in which the chromosome number in a cell is not an exact multiple of the haploid number. Physiological defects in aneuploid cells are caused, at least in part, by maladaptive molar ratios of newly formed proteins in such cells, given their deviations from normal gene dosages. This may account for the hypersensitivity of aneuploid cells to proteasome inhibitors and for other manifestations of proteotoxic stress in such cells (Oromendia et al., 2012). Now that the conditionality of Ac/N-degrons is no longer a conjecture (Figures 4–6), the relevance of the Ac/N-end rule pathway to protection from stress caused by aneuploidy can be explored through approaches described in the present work.
The ER-embedded transmembrane Doa10 E3 Ub ligase is a previously identified N-recognin of the Ac/N-end rule pathway (Figure S1A) (Hwang et al., 2010b). Either “soluble” or transmembrane proteins that enter the ER can be retrotranslocated to the cytosol and destroyed if these proteins are recognized by the quality control system termed ER-Associated Degradation (ERAD) (Guerriero and Brodsky, 2012; Wolf and Stolz, 2012). The Doa10 E3 contributes to the targeting of proteins through ERAD, in addition to being an N-recognin of the Ac/N-end rule pathway. Consequently, Ac/N-degrons and the Ac/N-end rule pathway may also contribute to ERAD, a verifiable proposition.
We conclude by noting that the physiologically relevant conditionality of Ac/N-degrons that has been demonstrated and analyzed in the present study is fundamentally analogous to conditional properties of other degradation signals, including internal degrons. These aspects of internal degrons, while clearly present and probably critical to their physiology (Ravid and Hochstrasser, 2008), are largely unexplored.
EXPERIMENTAL PROCEDURES
Yeast Strains, Plasmids, and Genetic Techniques
Tables S1 and S2 describe S. cerevisiae strains and plasmids. Standard techniques were employed for strain and plasmid construction.
Cycloheximide-Chase and Pulse-Chase Assays
They were carried out largely as described (Hwang et al., 2010b). The Odyssey near-infrared scanner (Li-Cor) was used for immunoblotting analyses and quantification.
Antibody Specific for Nt-Acetylated Cog1
The Nt-acetylated peptide AcMDEVLPLFRDSC and its unacetylated counterpart MDEVLPLFRDSC were synthesized by Abgent (San Diego, CA). Production of the anti-Cog1AcNt antibody is described in Supplemental Experimental Procedures.
Coimmunoprecipitation of Cog1 with Cog2-Cog4
S. cerevisiae coexpressing flag-tagged MD-Cog1wt and either Cog3 or Cog4 (ha-tagged) were lysed, and clarified extracts were subjected to co-IP using anti-flag magnetic beads.
Supplementary Material
HIGHLIGHTS.
N-terminal acetylation of cellular proteins can create specific Ac/N-degrons.
Hcn1 and Cog1 are shown to contain specific Ac/N-degrons.
Ac/N-degrons of Cog1 and Hcn1 are repressed by their ligands Cog2/3 and Cut9.
Conditionality of Ac/N-degrons can mediate the control of protein stoichiometries.
Acknowledgments
We thank R. Deshaies and K. Gould for gifts of plasmids. We are grateful to the present and former members of the Varshavsky laboratory for their assistance and advice. This work was supported by grants to C.-S.H. from the National Research Foundation of Korea (NRF-2011-0021975) and the Korea Healthcare technology R&D Project (A111324), and to A.V. from the National Institutes of Health (DK039520, GM031530 and GM085371).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, Figures S1-S5, and Tables S1 and S2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast to humans. Proc Natl Acad Sci USA. 2009;106:8157–8162. doi: 10.1073/pnas.0901931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford D. Structure, function and mechanism of the anaphase promoting complex (APC/C) Q Rev Biophys. 2011;44:153–190. doi: 10.1017/S0033583510000259. [DOI] [PubMed] [Google Scholar]
- Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S, King D, Shen PS, Weibezahn J, et al. A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell. 2012;151:1042–1054. doi: 10.1016/j.cell.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Archer T. Regulting SWI/SNF subunit levels via protein-protein interactions and proteasomal degradation: BAF155 and BAF170 limit expression of BAF57. Mol Cell Biol. 2005;25:9016–9027. doi: 10.1128/MCB.25.20.9016-9027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart M, Panasenko O, Nikolaev S. The Not3/5 subunit of the Ccr4-Not complex: A central regulator of gene expression that integrates signals between the cytoplasm and the nucleus in eukaryotic cells. Cell Signal. 2012;25:743–751. doi: 10.1016/j.cellsig.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Dougan DA, Micevski D, Truscott KN. The N-end rule pathway: from recognition by N-recognins to destruction by AAA+ proteases. Biochim Biophys Acta. 2011;1823:83–91. doi: 10.1016/j.bbamcr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Duncan CD, Mata J. Widespread cotranslational formation of protein complexes. PLoS Genet. 2011;7:e1002398. doi: 10.1371/journal.pgen.1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Ulrich HD, Sommer T, Kaiser P. The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics. 2012;192:319–360. doi: 10.1534/genetics.112.140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotso P, Koryakina Y, Pavliv O, Tsiomenko AB, Lupashin VV. Cog1p plays a central role in the organization of the yeast conserved oligomeric Golgi complex. J Biol Chem. 2005;280:27613–27623. doi: 10.1074/jbc.M504597200. [DOI] [PubMed] [Google Scholar]
- Fredrickson EK, Gardner RG. Selective destruction of abnormal proteins by ubiquitin-mediated protein quality control degradation. Semin Cell Dev Biol. 2012;23:530–537. doi: 10.1016/j.semcdb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi M, Just S, Mun A, Ross S, Rücknagel P, Dubaquié Y, Ehrenhofer-Murray A, Rospert S. The yeast N-alpha-acetyltransferase NatA is quantitatively anchored to the ribosome and interacts with nascent polypeptides. Mol Cell Biol. 2003;23:7403–7414. doi: 10.1128/MCB.23.20.7403-7414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaboosi N, Deshaies RJ. A conditional yeast E1 mutant blocks the ubiquitin- proteasome pathway and reveals a role for ubiquitin conjugates in targeting Rad23 to the proteasome. Mol Biol Cell. 2007;18:1953–1963. doi: 10.1091/mbc.E06-10-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetze S, Qeli E, Mosimann C, Staes A, Gerrits B, Roschitzki B, Mohanty S, Niederer EM, Laczko E, Timmerman E, et al. Identification and functional characterization of N-terminally acetylated proteins in Drosophila melanogaster. PLoS Biol. 2009;7:e1000236. doi: 10.1371/journal.pbio.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92:537–576. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbach A, Zhang H, Wengi A, Jablonska Z, Gruber IM, Halbeisen RE, Dehé PM, Kemmeren P, Holstege F, Géli V, et al. Cotranslational assembly of the yeast SET1C histone methyltransferase complex. EMBO J. 2009;28:2959–2570. doi: 10.1038/emboj.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and homeostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Hershko A. From rabbit reticulocytes to clam oocytes: in search of the system that targets mitotic cyclins for degradation. Mol Biol Cell. 2010;15:1645–1647. doi: 10.1091/mbc.E09-07-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CS, Shemorry A, Varshavsky A. The N-end rule pathway is mediated by a complex of the RING-type Ubr1 and HECT-type Ufd4 ubiquitin ligases. Nat Cell Biol. 2010a;12:1177–1185. doi: 10.1038/ncb2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CS, Shemorry A, Varshavsky A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science. 2010b;327:973–977. doi: 10.1126/science.1183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JA, Yip CK, Walz T, Houghton FM. Molecular organization of the COG vesicle tethering complex. Nat Struct Mol Biol. 2010;11:1292–1298. doi: 10.1038/nsmb.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VJ, Ungar D. Re’COG’nition at the Golgi. Traffic. 2012;13:891–897. doi: 10.1111/j.1600-0854.2012.01338.x. [DOI] [PubMed] [Google Scholar]
- Mogk A, Schmidt R, Bukau B. The N-end rule pathway of regulated proteolysis: prokaryotic and eukaryotic strategies. Trends Cell Biol. 2007;17:165–172. doi: 10.1016/j.tcb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Monda JK, Scott DC, Miller DJ, Lydeard J, King D, Harper JW, Bennett EJ, Schulman BA. Structural conservation of distinctive N-terminal acetylation-dependent interactions across a family of mammalian NEDD8 ligation enzymes. Structure. 2012;21:1–12. doi: 10.1016/j.str.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oromendia AB, Dodgson SE, Amon A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012;26:2696–2708. doi: 10.1101/gad.207407.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EC, Szostak JW. ARD1 and NAT1 proteins form a complex that has N- terminal acetyltransferase activity. EMBO J. 1992;11:2087–2093. doi: 10.1002/j.1460-2075.1992.tb05267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatkov KI, Brower CS, Varshavsky A. The N-end rule pathway counteracts cell death by destroying proapoptotic protein fragments. Proc Natl Acad Sci USA. 2012;109:E1839–E1847. doi: 10.1073/pnas.1207786109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. Cubism and the cell cycle: the many faces of the APC/C. Nat Rev Mol Cell Biol. 2011;12:427–438. doi: 10.1038/nrm3132. [DOI] [PubMed] [Google Scholar]
- Polevoda B, Sherman F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J Mol Biol. 2003;325:595–622. doi: 10.1016/s0022-2836(02)01269-x. [DOI] [PubMed] [Google Scholar]
- Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin- proteasome system. Nat Rev Mol Cell Biol. 2008;9:679–689. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DC, Monda JK, Bennett EJ, Harper JW, Schulman BA. N-terminal acetylation acts as an avidity enhancer within an interconnected multiprotein complex. Science. 2011;334:674–678. doi: 10.1126/science.1209307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starheim KK, Gevaert K, Arnesen T. Protein N-terminal acettyltransferases: when the start matters. Trends Biochem Sci. 2012;37:152–161. doi: 10.1016/j.tibs.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Sztul E, Lupashin V. Role of vesicle tethering factors in the ER-Golgi membrane traffic. FEBS Lett. 2009;583:3770–3783. doi: 10.1016/j.febslet.2009.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki TS, Sriram SM, Park KS, Kwon YT. The N-end rule pathway. Annu Rev Biochem. 2012;81:261–289. doi: 10.1146/annurev-biochem-051710-093308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GC, Varshavsky A. Detecting and measuring cotranslational protein degradation in vivo. Science. 2000;289:2117–2120. doi: 10.1126/science.289.5487.2117. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Lasac M, Polevoda B, Gazquezc C, Elosegui-Artolae A, Kim DS, De Juan-Pardoe E, Demeyera K, Holef K, Larreac E, et al. N-terminal acetylome analyses and functional insights of the N-terminal acetyltransferase NatB. Proc Natl Acad Sci USA. 2012;109:12449–12454. doi: 10.1073/pnas.1210303109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. Discovery of cellular regulation by protein degradation. J Biol Chem. 2008;283:34469–34489. doi: 10.1074/jbc.X800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule pathway and regulation by proteolysis. Prot Sci. 2011;20:1298–1345. doi: 10.1002/pro.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. The ubiquitin system, an immense realm. Annu Rev Biochem. 2012;81:167–176. doi: 10.1146/annurev-biochem-051910-094049. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Stolz A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochim Biophys Acta. 2012;1823:117–124. doi: 10.1016/j.bbamcr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Kulkarni K, Hanrahan SJ, Thompson AJ, Barford D. The APC/C subunit Cdc16/Cut9 is a contiguous tetratricopeptide repeat superhelix with a homodimer interface. EMBO J. 2010;29:3733–3744. doi: 10.1038/emboj.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.