Abstract
Purpose
Hypothalamic GABA signaling has been shown to regulate the hormonal response to hypoglycemia in animals. The hypothalamus is a challenging brain region for magnetic resonance spectroscopy (MRS) due to its small size and central location. To investigate the feasibility of measuring GABA in the hypothalamus in humans, ultra-high field MRS was used.
Methods
GABA levels in the hypothalamus and occipital cortex (control region) were measured in healthy volunteers during euglycemia and hypoglycemia at 7 tesla using short-echo STEAM (TE=8ms, TR=5s).
Results
Hypothalamic GABA levels were quantified with a mean within-session test-retest coefficient of variance of 9%. Relatively high GABA levels were observed in the hypothalamus compared to other brain regions. Hypothalamic GABA levels were 3.5±0.3 µmol/g during euglycemia (glucose 89±6 mg/dL) vs. 3.0±0.4 µmol/g during hypoglycemia (glucose 61±3 mg/dL) (P=0.06, N=7). In the occipital cortex, GABA levels remained constant at 1.4±0.4 vs.1.4±0.3 µmol/g (P=0.3, N=5) as glucose fell from 91±4 to 61±4 mg/dL.
Conclusion
GABA concentration can be quantified in the human hypothalamus and shows a trend towards decrease in response to an acute fall in blood glucose. These methods can be used to further investigate role of GABA signaling in the counter regulatory response to hypoglycemia in humans.
Keywords: Hypothalamus, magnetic resonance spectroscopy, GABA, Hypoglycemia, 7 tesla
Introduction
γ-Aminobutyric acid (GABA) is the primary inhibitory neurotransmitter and is widely distributed throughout the central nervous system. Changes in GABA levels have been implicated in several neurological and psychiatric disorders (1, 2). GABA further appears to be involved in the response of the brain to changes in fuel availability. Namely, recent work in animal models suggested hypothalamic GABA signaling to play an important role in regulating the counterregulatory hormone response to hypoglycemia (3). If blood glucose is reduced to below physiological levels, the nervous system responds with a coordinated hormonal response to recover from hypoglycemia, which involves the sequential secretion of counterregulatory hormones including glucagon, epinephrine, growth hormone and cortisol. Glucose sensing neurons in the hypothalamus, particularly the ventromedial hypothalamic nucleus (VMH), are thought to play an important role in this response (4,5). Thus, local VMH glucopenia causes the release of counterregulatory hormones (6), whereas infusion of glucose into the VMH suppresses their release during systemic hypoglycemia (7). A decrease in the local availability of glucose within the VMH is thought to lower local GABA release, which in turn modulates the magnitude of hypoglycemia induced hormone secretion (8). Increased GABAergic tone within the VMH in rats has been shown to suppress the counterregulatory response to subsequent episodes of hypoglycemia and has been implicated in the development of hypoglycemia associated autonomic failure (HAAF)(3,9). HAAF develops in patients with diabetes who are exposed to recurrent iatrogenic hypoglycemia, therefore understanding the role of hypothalamic GABA in the coordination of the counterregulatory response to hypoglycemia in humans has clinical implications. Implementation and validation of magnetic resonance (MR) methodology that can reliably assess the inhibitory GABA tone in the hypothalamus in humans is desirable to address the role of hypothalamic GABA in coordinating the counterregulatory response to hypoglycemia in both health and disease.
MR spectroscopy (MRS) of GABA in the hypothalamus is challenging due to the small size of this brain region and its location in the center of the brain. The feasibility of acquiring proton spectra from the human hypothalamus was demonstrated previously; however GABA concentrations were not reported in these studies (10, 11). In this study we investigated the feasibility of quantifying hypothalamic GABA levels using ultra-high field MRS, a multi-channel transceiver array coil and local transmit B1 (B1+) shimming (12). We further explored whether hypothalamic GABA levels decrease in response to acute hypoglycemia in healthy volunteers. Based on prior observations in rodents using microdialysis (8), we hypothesized that healthy volunteers will have lower concentrations of GABA in the hypothalamus during hypoglycemia as compared to euglycemia. To insure that any changes seen are specific for the hypothalamus, we also studied the occipital cortex as a control region.
Methods
Subjects
Thirteen healthy volunteers were recruited for measurement of GABA concentration in the hypothalamus. Four subjects were studied to assess the within-session test-retest coefficient of variance (CV) of the GABA measurement without intravenous (IV) infusions in the scanner (3F/1M, age 30±5 years and BMI 22±4 kg/m2, mean±SD). Seven subjects successfully completed a euglycemic/hypoglycemic clamp study in the scanner during which GABA levels were obtained from the hypothalamus (3F/4M, age 28±5 years and BMI 23±3 kg/m2). Six subjects were recruited for GABA measurement in the occipital cortex during a euglycemic/hypoglycemic clamp. Five subjects successfully completed the occipital cortex experiment(1M/4F,age 31±4 years and BMI 24±5 kg/m2). Four out of 5 subjects in the occipital cortex study also participated in the hypothalamus study. For subjects who participated in both hypothalamus and occipital cortex experiments, studies were scheduled at least 6 weeks apart. The incomplete clamp studies from 6 subjects in the hypothalamus and 1 in the occipital cortex experiment were excluded due to excessive head movement during the scan. Specifically, data were excluded if spectral linewidths degraded over time during the study and if the localizer showed more than a 2mm change in voxel position in any direction at the end of the scan. Before participation, all subjects provided informed consent as governed by the Institutional Review Board at the University of Minnesota.
Experimental protocol for test-retest assessments in the hypothalamus
To assess the within-session test-retest reproducibility of GABA quantification in the hypothalamus, 5 consecutive spectra were acquired from the hypothalamus in 10 minute blocks.
Experimental protocol for euglycemic/hypoglycemic clamp studies
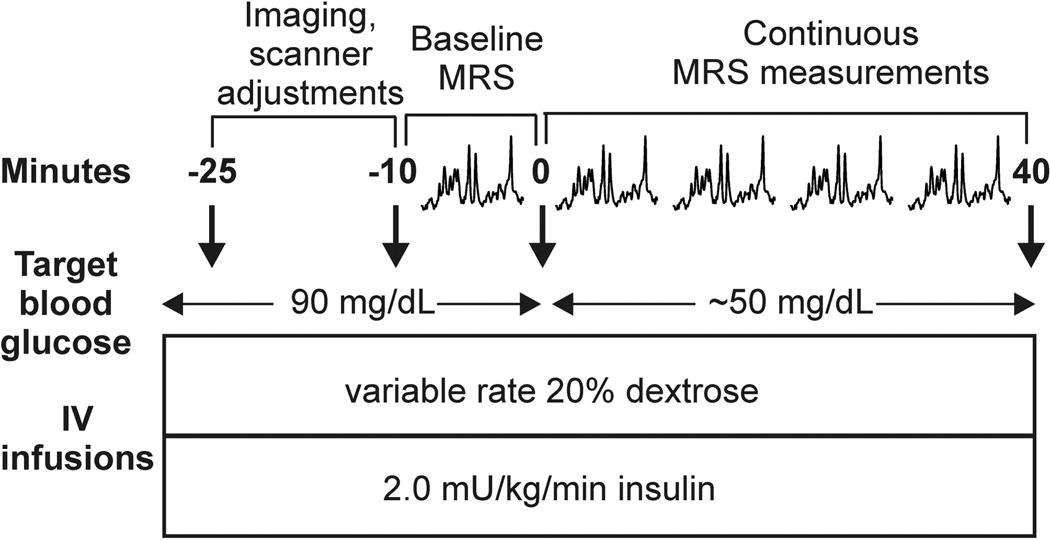
On the day of study, subjects arrived at the Center for Magnetic Resonance Research (CMRR) in the morning after an overnight fast. Upon arrival, an IV catheter was placed antegrade in a forearm for subsequent IV infusions. IV catheters were placed retrograde in one or both lower legs for blood sampling. The leg used for blood sampling was wrapped in heated towels and hot packs to arterialize the venous blood (13). Subjects underwent a 2-step hyperinsulinemic clamp in which insulin was infused at a rate of 2.0 mU/kg/min and potassium phosphate at a rate of 4 mEq/hour. Blood glucose was initially maintained at 90 mg/dL by the IV infusion of 20% dextrose while the MRS data were collected according to the protocol detailed below. After collection of data in the euglycemic condition, blood glucose was allowed to drop to 50 mg/dL by the discontinuation of the dextrose infusion, and then maintained at the hypoglycemic plateau by a variable dextrose infusion (Fig. 1). MRS data were acquired continuously in 10 minute blocks as blood glucose was dropped and clamped around 50 mg/dL. Samples for blood glucose were collected every 5 minutes.
Figure 1.
Experimental protocol for euglycemic/hypoglycemic clamp studies during which GABA levels were obtained from the hypothalamus or occipital cortex.
Laboratory analyses
Plasma glucose concentration was measured in duplicates during the scanning session using an Analox machine (Analox Instruments, Lunenburg, MA). Blood samples obtained for measurement of counterregulatory hormones were sent to Vanderbilt Diabetes Research and Training Center core laboratory for analysis. Plasma epinephrine and norepinephrine were measured by high-performance liquid chromatography (Dionex, formerly ESA, Inc.). Plasma growth hormone and cortisol were measured by radioimmunoassay (Diagnostic Products Corporation, Inc). Plasma glucagon was measured by radioimmunoassay (modified Millipore, Merck).
MR protocol
MR experiments were performed using a 7T, 90-cm horizontal bore magnet (Magnex) equipped with a Siemens console. For data acquisition from the hypothalamus, a 16-channel transmit/receive transmission line array head coil was used (14). Destructive B1+ interferences in the volume-of-interest (VOI) were reduced by localized B1+ shimming as described before (12). For data acquisition from the occipital cortex, an elliptical quadrature half-volume transceiver was used as described previously (15). Images acquired with a 1-mm isotropic resolution MPRAGE sequence (repetition time TR = 3 s, inversion time TI = 1.5 s, echo time TE = 3.67 ms, 192 partition-encode steps, 256 phase-encode steps, 256 data points in the read direction, nominal flip angle = 6°, total acquisition time = 6 min 58 s) were used for the selection of the VOI. All first- and second-order shims were adjusted using FASTMAP with echo-planar imaging readout (16). Spectra were acquired from the hypothalamus (13 × 12 × 10 mm3) and occipital cortex (22 × 22 × 22 mm3) with a short-echo stimulated echo acquisition mode (STEAM) sequence (TE = 8 ms, TR = 5 s, mixing time TM = 32 ms, in blocks of 128 transients) with variable power RF pulses with optimized relaxation delays (VAPOR) water suppression and outer volume saturation (12, 17). Symmetric sinc RF pulses (1.5 ms, 5.9 kHz bandwidth) were used for STEAM localization and required a peak B1 of 34 μT, which was achieved with ~5.3 kW peak power(~330 W per channel) at the array head coil together with local B1+ shimming (12) in the hypothalamus (Fig. 2c). Unsuppressed water spectra acquired from the same VOI were used to remove residual eddy current effects and to reconstruct the phased array spectra obtained with the 16-channel coil (18). Single-shot data were saved during acquisition. When the signal-to-noise ratio (SNR) of single-shot data was sufficient (i.e. in the occipital cortex), individual FIDs were frequency and phase corrected prior to summation. When the SNR was not sufficient (in the hypothalamus), data were first averaged over 16 scans, then corrected for frequency variations and summed. Localizer images were repeated at the end of the scanning session to assess gross motion.
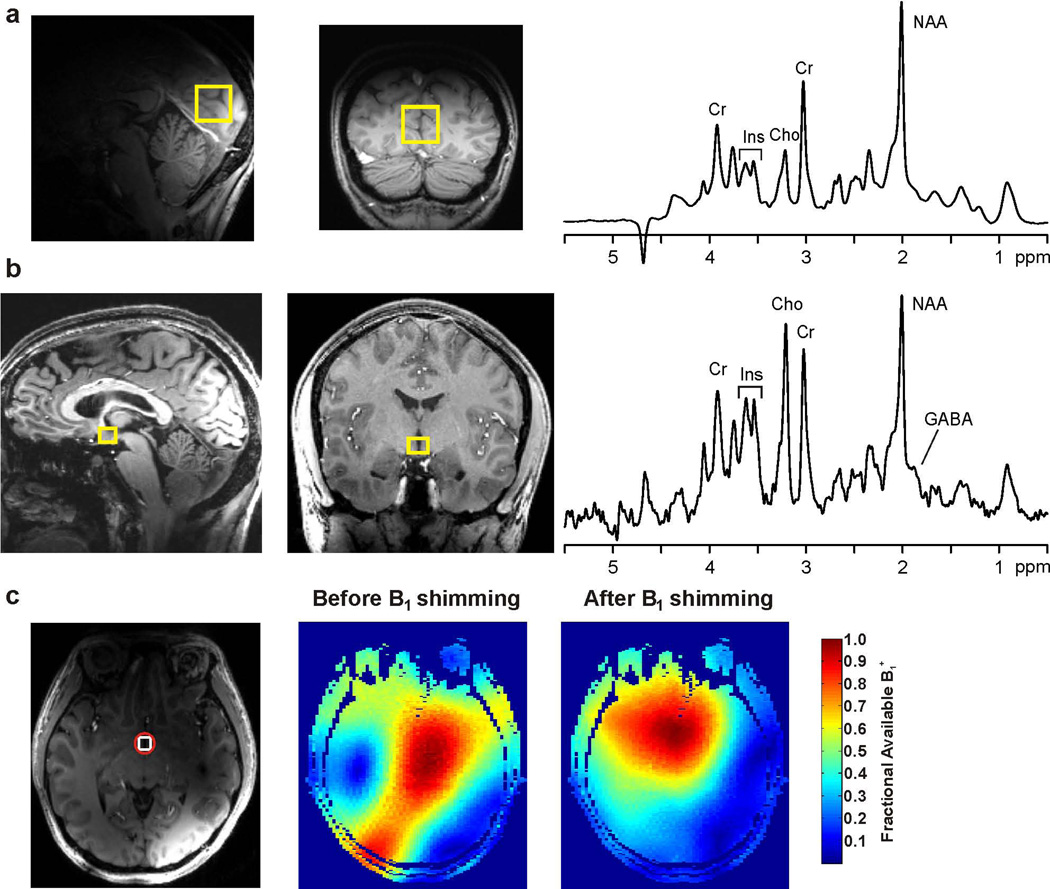
Figure 2.
Spectral quality obtained at 7T from the occipital cortex (a) and hypothalamus (b) and B1+ shimming results in the hypothalamus of a healthy volunteer. 1H MR spectra (right in a and b) were obtained with STEAM (TR = 5 s, TE = 8 ms, TM = 32 ms, 128 transients) from the volumes-of-interest shown on T1-weighted mid-sagittal and coronal images (left in a and b). The hypothalamus spectrum was acquired with a 16-channel transceive array coil, while the occipital cortex spectrum was acquired with a quadrature half-volume transceiver. The FIDs were multiplied with a Gaussian function (σ = 0.08 s) prior to Fourier transformation. The marked resonances are: N-acetylaspartate, creatine, choline, myo-inositol and GABA. (c) The region-of-interest (red circle) used for B1+ shimming for the hypothalamus VOI (white square) is shown on a transverse T1-weighted image together with maps of the fraction of available B1+ before and after B1+ shimming.
Metabolite quantification
Metabolites were quantified using LCModel (19). The model spectra of alanine (Ala), aspartate (Asp), ascorbate/vitamin C (Asc), glycerophosphocholine (GPC), phosphocholine (PC), creatine (Cr), phosphocreatine (PCr), GABA, glucose (Glc), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (myo-Ins), lactate (Lac), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphoethanolamine (PE), scyllo-inositol (scyllo-Ins) and taurine (Tau) were generated based on previously reported chemical shifts and coupling constants (20,21). Macromolecule spectra were acquired from the occipital cortex of 5 volunteers using an inversion recovery sequence (TR = 2 s, TI = 0.680 s) (22). Concentrations were not corrected for T1 and T2 effects because long TR and ultra-short TE values were used. Metabolites quantified with Cramér-Rao lower bounds (CRLB, estimated error of the metabolite quantification) > 50% were classified as not detected, as recommended by the LC Model manual (23). If the correlation between two metabolites was consistently high (correlation coefficient < −0.5) in a given region, their sum was used for analysis, such as Glc + Tau, NAA + NAAG (tNAA, total NAA), Cr + PCr (tCr, total creatine), GPC + PC (tCho, total choline). Due to unavoidable and substantial contributions of cerebrospinal fluid (CSF) to the hypothalamus VOI at the midline, metabolite concentrations obtained from this VOI were corrected for CSF by using the fractional CSF content of the voxel obtained by segmenting the MPRAGE images with SPM8.
Statistical analysis
Within-session test-retest reliability of the GABA concentrations in the hypothalamus (during normoglycemia, with no infusions) is summarized within-person coefficient of variation (CV). For the clamp studies, GABA levels obtained during the last 10 minute block of euglycemia, and during the first 10 minute block after the participant’s glucose fell below 70 mg/dL, were used for analysis. The full time course during the hypoglycemic clamp was not utilized since the last 1 or 2 data points were not available in 3 of the 7 volunteers who completed the hypothalamus experiment. For each region separately, GABA concentrations are presented as mean ± SD by experimental condition. While our a priori hypothesis was that hypothalamic GABA would fall with falling glucose, due to the small sample size we chose to conservatively use a two-sided (rather than one-sided) paired t-test to compare between the euglycemic and hypoglycemic conditions in the hypothalamus as well as in the occipital cortex. As exploratory analyses, other neurochemical measuments and counterregulatory hormones were compared between the euglycemic and hypoglycemic conditions with two-sided paired t-tests. A P-value of <0.05 indicated significant difference.
Results
Representative 1H MR spectra obtained at 7T from the hypothalamus and occipital cortex of healthy volunteers are shown in Figure 2. Despite the small size and central location of the hypothalamic VOI close to air-tissue interfaces, good quality spectra (acceptable SNR, excellent linewidths and water suppression, free of unwanted coherences) were obtained from this brain region. B1 shimming ensured that a B1+ of at least 34 µT was available for the hypothalamus VOI, which was confirmed directly by calibration of the RF pulses in STEAM. For example in the volunteer shown in Figure 2c, the fraction of available B1+ in the region-of-interest selected around the hypothalamus VOI was 58% before and 94% after B1 shimming, and the predicted relative transmit efficiency (12) was 1.6. The spectral quality allowed the quantification of 8 neurochemicals (tNAA, tCr, tCho, myo-Ins, Glu, Gln, GABA, Glc+Tau) in the hypothalamus with mean CRLB ≤ 20% from spectra averaged over 10 minutes (128 transients) (Table 1). Among these, GABA was quantified with mean CRLB of 15%. The mean within-session test-retest CV of the GABA measurement in 4 subjects who were studied during normoglycemia was 9% (range 3%–13%). In the occipital cortex, GABA was quantified with mean CRLB of 10% from spectra averaged over 10 minutes. GABA concentration under euglycemia was 2–3 fold higher in the hypothalamus than in the occipital cortex (3.5±0.3 μmol/g vs. 1.4±0.3 μmol/g).
Table 1.
Metabolite concentrations (mean ± SD) determined by LCModel fitting of spectra from the hypothalamus under the euglycemic and hypoglycemic conditions.
| Concentration (µmol/g) |
|||
|---|---|---|---|
| Euglycemia | Hypoglycemia | P | |
| GABA | 3.5±0.3 | 3.0±0.4 | 0.06 |
| Gln | 3.2±0.8 | 3.1±0.5 | 0.7 |
| Glu | 8.7±0.9 | 8.1±1.3 | 0.24 |
| GSH | 2.0±1.0 | 1.9±-0.6 | 0.75 |
| Myo-Ins | 14.3±2.1 | 13.2±3.0 | 0.03* |
| scyllo-Ins | 0.8±0.4 | 0.7±0.3 | 0.33 |
| tNAA | 12.4±1.8 | 12.2±1.6 | 0.48 |
| tCho | 4.0±0.4 | 3.8±0.7 | 0.17 |
| tCr | 11.2±0.9 | 10.5±1.4 | 0.08 |
| Glc+Tau | 4.0±1.1 | 3.3±1.0 | 0.002* |
Metabolites that were significantly different between the euglycemic and hypoglycemic conditions are marked with *P <0.05 (two-sided paired t-test)
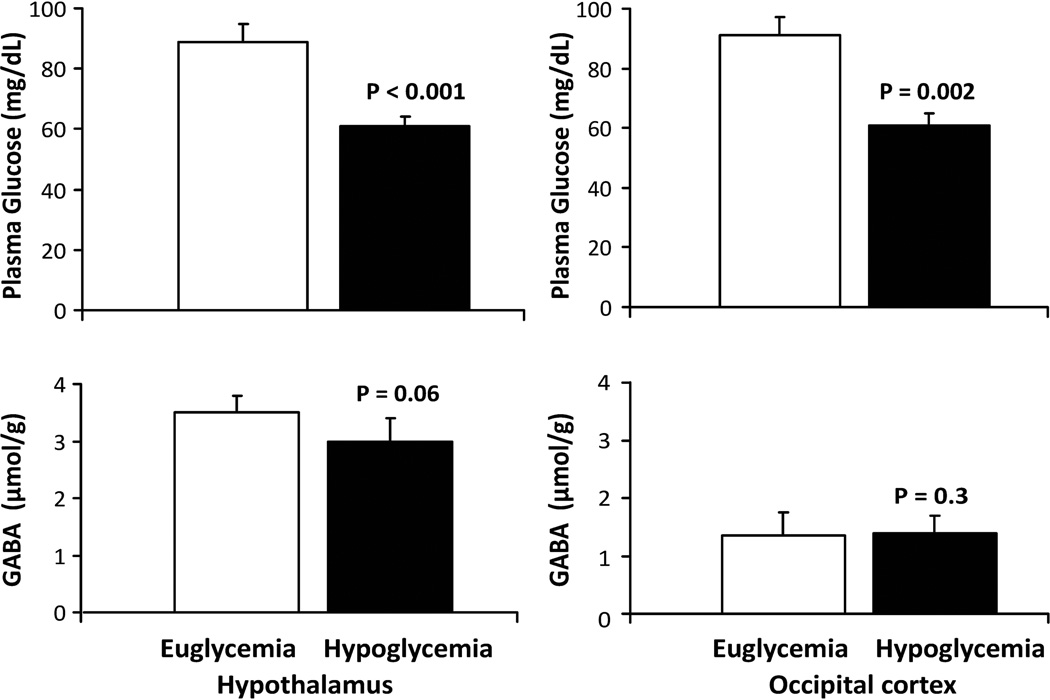
Seven subjects successfully completed the two step hyperinsulinemic clamp without moving their head and displacing the hypothalamic voxel at least until the end of the first 10 minute data acquisition during hypoglycemia. In these subjects, blood glucose dropped from 89±6 mg/dL during euglycemia to 61±3 mg/dL (P < 0.0001, Fig. 3) as hypothalamic GABA concentration changed from 3.5±0.3 to 3.0±0.4 µmol/g (P = 0.06, Fig. 3) during the first 10 minutes of hypoglycemia. Figure 4 demonstrates the stability of the spectral and LC Model fitting quality in the euglycemic vs. hypoglycemic states in one subject in whom the average GABA response (~0.5 µmol/g) was observed. This 12% decrease in GABA was greater than the 9% test-retest CV determined for GABA measurement during normoglycemia. Glucose + taurine (P = 0.002) and myo-inositol (P = 0.03) also decreased during hypoglycemia, while other metabolites did not change during hypoglycemia as compared to euglycemia (Table 1). In the occipital cortex, as blood glucose dropped from 91±6 mg/dL at baseline to 61±4 mg/dL (P = 0.001), GABA concentration remained stable (1.4±0.3 µmol/g vs. 1.4±0.4 µmol/grespectively, P = 0.3, Fig. 3). Peak epinephreine, glucagon, growth hormone, norepinephrine and cortisol levels were significantly higher during hypoglycemia as compared to euglycemia during both hypothalamus and occipital cortex studies (Table 2), demonstrating that sufficient hypoglycemia was attained to stimulate a counterregulatory response.
Figure 3.
Mean hypothalamic and occipital cortex GABA concentrations and plasma glucose levels during euglycemia (white bars) and hypoglycemia (black bars). Glucose and GABA levels obtained during the last 10 minute block of euglycemia and during the first 10 minute block after the participant’s glucose fell below 70 mg/dL are shown. Error bars are SD.
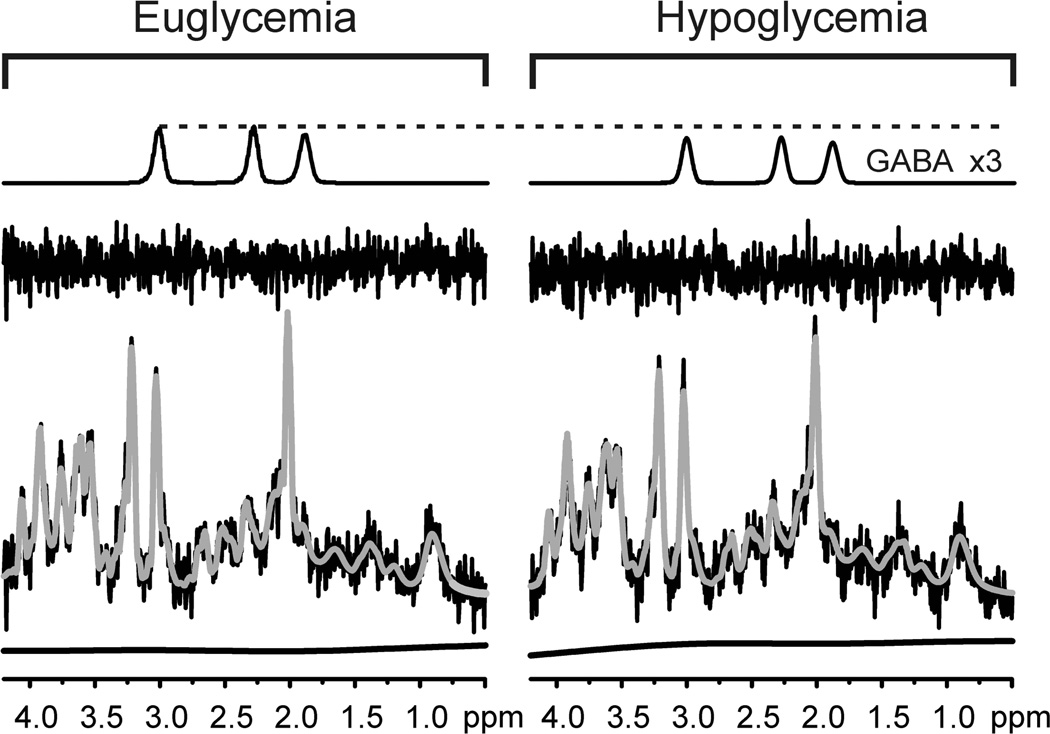
Figure 4.
LCModel fitting of spectra acquired during euglycemia and hypoglycemia in one volunteer. Note the reproducibility in the fitting, spline baselines and residuals under the two conditions. Also shown are the GABA contributions LCModel attributed to the two spectra (shown with a scale 3 times that of the in vivo spectrum).
Table 2.
Peak plasma counterregulatory hormone levels during euglycemia and hypoglycemia in subjects undergoing hypothalamus and/or occipital cortex studies (data are from 11 independent experiments, 6 from hypothalamus and 5 from occipital cortex, on 9 subjects).
| Euglycemia | Hypoglycemia | P | |
|---|---|---|---|
| Epinephrine (pg/mL) | 21±10 | 489±196 | <0.01 |
| Norepinephrine (pg/mL) | 326±154 | 466±199 | <0.01 |
| Glucagon (pg/mL) | 67±11 | 128±35 | <0.01 |
| Cortisol (µg/dL) | 25±10 | 31±10 | 0.01 |
| Growth hormone (ng/mL) | 6±6 | 16±12 | 0.01 |
Discussion and Conclusions
To our knowledge this is the first report of the measurement of hypothalamic GABA concentrations in healthy humans. We detected high GABA concentrations in the hypothalamus relative to cortical areas and were able to measure these with excellent within-session test-retest reproducibility. In addition, we investigated the feasibility of monitoring hypothalamic GABA levels during hyperinsulinemic euglycemic and hypoglycemic clamps in the scanner. These exploratory studies underlined the sensitivity of the hypothalamic measurements to subject motion during hypoglycemia, but also revealed a trend for a decrease in GABA in response to hypoglycemia.
GABA concentrations have been measured by MRS in the frontal and occipital cortical areas previously (24–27). However, GABA measurements in the hypothalamus have not been possible before due to the small size and central location of the structure in the brain. In the current study, these limitations were overcome with recently developed MRS methodology at ultra-high field (12). This methodology takes advantage of the high sensitivity of 7T, utilizes B1+ shimming with a multi-channel transceiver array to optimize transmit efficiency and thereby enables acquisition of spectra with a low chemical shift displacement error (< 5% / ppm / direction) and, lastly, utilizes ultra-short TE to minimize J-evolution and signal loss to T2 relaxation. With our current approach, hypothalamic GABA was quantified with CRLB < 20%, which is generally accepted to demonstrate reliable quantification. We found that under euglycemic conditions, GABA levels were higher in the hypothalamus (~3.5 μmol/g) than the occipital cortex (~1.4 μmol/g), which is consistent with prior observations in rodents, both by postmortem analysis of brain extracts (28) and by in vivo 1H MRS at ultra-high field (29). In addition, the GABA levels obtained from the occipital cortex in this study were consistent with those reported by MRS from this region previously (24, 27). The CRLB obtained for GABA in the occipital cortex were lower than those in the hypothalamus despite the lower GABA concentrations in the occipital cortex due to the larger voxel size and the resulting substantially higher SNR (Fig. 2).
In this study, we chose to utilize non-edited spectroscopy to measure GABA levels despite challenges in quantification in the presence of overlapping resonances such as tCr. The use of the more conventional MEGA-PRESS editing method for GABA detection was not practical due to SNR limitations. First, the hypothalamic VOI was very small (1.6 mL). Second, perfect MEGA-PRESS editing for an AX2 spin system such as GABA reduces editing efficiency to a maximum of 50%. Finally, another 50% of the signal would be lost to T2 relaxation based on the longer echo time (TE = 69 ms) required to accommodate the editing pulses and the anticipated T2 (< 100 ms) for GABA (26). The GABA concentrations obtained from the hypothalamus using non-edited MRS were reliable not only based on CRLB, but also established cross-correlation criteria: Namely, GABA was quantified with correlation coefficients with tCr of ~ −0.4 (r > −0.5), indicating that GABA and tCr concentrations were not correlated to each other and thus the individual concentrations were reliable.
In this study we observed a trend for GABA drop in hypothalamus in response to hypoglycemia, while GABA levels remained constant in the occipital cortex, consistent with the potential involvement of hypothalamic GABAergic tone in the regulation of counterregulatory response by this structure. Since this study’s primary goal was to demonstrate the feasibility of GABA measurement in the hypothalamus, it was not powered to test whether there was a significant drop in hypothalamic GABA during hypoglycemia. On the other hand, the strong trend we observed for a GABA drop in the hypothalamus in response to hypoglycemia demonstrates that further studies of this response by ultra-high field MRS are warranted. If future studies demonstrate a GABA drop in hypothalamus in response to hypoglycemia, this would be consistent with prior fMRI results. Namely, the hypothalamus is activated during hypoglycemia (31), which implies a lower GABAergic tone in this region. Conversely, glucose intake lowers hypothalamic neuronal activity (32,33), which would presumably be associated with a higher GABAergic tone in this area. Note that other fMRI studies showed that oral intake of glucose decreases hypothalamic neuronal activity more effectively than IV glucose administration, suggesting other factors besides blood glucose, including insulin, may be involved in this response (34). In the current study we could generate a well-controlled hypoglycemic state only with IV insulin/glucose clamps; therefore insulin did not change during the transition from euglycemia to hypoglycemia. Future studies are needed to investigate the effects of insulin, as well as oral vs. IV glucose intake, in the hypothalamic GABA response to changes in blood glucose.
Another consideration in interpreting MRS-observed GABA changes is that 1H MRS measures the total concentration of GABA within a localized region without differentiating between separate functional pools of GABA. It primarily measures intracellular GABA since the extracellular concentrations are much lower than intracellular concentrations. The exploratory component of this study was based on the rationale that hypothalamic GABA release is decreased in response to hypoglycemia as assessed by microdialysis in the rodent brain (8). Such decreased release (lower extracellular GABA) could be either associated with increased intracellular GABA levels (due to higher uptake or lower utilization), or with decreased intracellular GABA levels (due to reduced synthesis). The rate of GABA synthesis has been shown to be reduced in rodents during insulin-induced hypoglycemia, possibly due to reduced substrate availability (35,36), providing the rationale for expecting lower GABA levels as measured by MRS in the current study.
Glucose + taurine and myo-inositol levels also decreased in the hypothalamus during hypoglycemia (Table 1). A decrease in glucose in the hypothalamus is expected during hypoglycemia; however the etiology for change in myo-inositol is not clear and could be related to changes in osmolarity.
The hypoglycemia studies further revealed the sensitivity of the hypothalamic measurement to minor subject motions. Namely, even minimal head movements on the order of 1–2 mm were enough to substantially displace the voxel from the region of interest since voxel dimensions were 10–13 mm. Such head movement is almost unavoidable during hypoglycemia because of the strong adrenergic response that is elicited by the fall in blood glucose. Study participants typically experience tremor, diaphoresis, and anxiety during the hypoglycemic phase of the experiment, which often leads to head movement. We expect that this limitation will be overcome in the near future by utilizing prospective motion correction, e.g. by optical tracking (37).
In summary, good quality 1H spectra can be obtained from the human hypothalamus despite the small size and central location of this region. GABA concentrations can be reliably quantified from these spectra. These methods can be used to further investigate the role of GABA signaling in regulation of the counterregulatory response to hypoglycemia in humans, given that issues with subject motion can be minimized using motion tracking methodology.
Acknowledgments
Sources of support: This work was supported by NIH grants R01 NS035192 (E.R.S.,G.Ö., L.E.E) and 2 T32 DK7203 (A.M.), in addition to a grant from the American Diabetes Association (E.R.S., L.E.E.). This project was also supported by the NIH National Center for Research Resources (P41 RR008079, M01 RR0400, and UL1 RR033183) and the National Institute of Biomedical Imaging and Bioengineering (P41 EB015894). Additional CMRR funding is from: Minnesota Medical Foundation, NIH P30 NS057091, NIH P30 NS5076408, NIH S10 RR026783 and WM KECK Foundation.
References
- 1.Chang L, Cloak C, Ernst T. Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J Clin Psychiatry. 2003;64:7–14. [PubMed] [Google Scholar]
- 2.Sanacora G, Mason G, Rothman D, Hyder F, Ciarcia J, Ostroff R, Berman R, Krystal J. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 3.Chan O, Zhu W, Ding Y, McCrimmon R, Sherwin R. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes. 2006;55:1080–1087. doi: 10.2337/diabetes.55.04.06.db05-0958. [DOI] [PubMed] [Google Scholar]
- 4.Routh VH. Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF) Diabetes Metab Res Rev. 2003;19:348–356. doi: 10.1002/dmrr.404. [DOI] [PubMed] [Google Scholar]
- 5.Thorens B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab. 2011;13:82–88. doi: 10.1111/j.1463-1326.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- 6.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 7.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu W, Czyzyk D, Paranjape S, Zhou L, Horblitt A, Szab G, Seashore M, Sherwin R, Chan O. Glucose prevents the fall in ventromedial hypothalamic GABA that is required for full activation of glucose counterregulatory responses during hypoglycemia. Am J Physiol Endocrinol Metab. 2010;298:E971–E977. doi: 10.1152/ajpendo.00749.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory reponses after antecedent hypoglycemia. Diabetes. 2008;57:1363–1370. doi: 10.2337/db07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs GE, van der Grond J, Teeuwisse WM, Langeveld TJC, van Pelt J, Verhagen JCM, de Kam ML, Cohen AF, Zitman FG, van Gerven JMA. Hypothalamic glutamate levels following serotonergic stimulation: A pilot study using 7-tesla magnetic resonance spectroscopy in healthy volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:486–491. doi: 10.1016/j.pnpbp.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Van den Bogaard SJA, Dumas EM, Teeuwisse WM, Kan HE, Webb A, Roos RAC, van der Grond J. Exploratory 7-tesla magnetic resonance spectroscopy in huntington's disease provides in vivo evidence for impaired energy metabolism. J Neurol. 2011;258:2230–2239. doi: 10.1007/s00415-011-6099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emir UE, Auerbach EJ, Van De Moortele P, Marjanska M, Ugurbil K, Terpstra M, Tkáč I, Öz G. Regional neurochemical profiles in the human brain measured by 1H MRS at 7 T using local B1 shimming. NMR Biomed. 2012;25:152–160. doi: 10.1002/nbm.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seaquist ER, Damberg GS, Tkáč I, Gruetter R. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes. 2001;50:2203–2209. doi: 10.2337/diabetes.50.10.2203. [DOI] [PubMed] [Google Scholar]
- 14.Adriany G, De Moortele PV, Ritter J, Moeller S, Auerbach EJ, Akguen C, Snyder CJ, Vaughan T, Ugurbill K. A geometrically adjustable 16-channel transmit/receive transmission line array for improved RF efficiency and parallel imaging performance at 7 tesla. Magn Reson Med. 2008;59:590–597. doi: 10.1002/mrm.21488. [DOI] [PubMed] [Google Scholar]
- 15.Tkáč I, Öz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: Metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echoplanar techniques. Magn Reson Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Tkáč I, Starcuk Z, Choi I, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Natt O, Bezkorovaynyy V, Michaelis T, Frahm J. Use of phased array coils for a determination of absolute metabolite concentrations. Magn Reson Med. 2005;53:3–8. doi: 10.1002/mrm.20337. [DOI] [PubMed] [Google Scholar]
- 19.Provencher S. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 20.Govindaraju V, Young K, Maudsley A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Tkáč I. Refinement of simulated basis set for LCModel analysis. In Proceedings of the 16th Annual Meeting of ISMRM; Toronto, Canada. 2008. p. 1624. [Google Scholar]
- 22.Behar K, Rothman D, Spencer D, Petroff O. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 23.Provencher S. LCModel & LCMgui user's manual. 2001 [Google Scholar]
- 24.Rothman D, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of γ-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumner P, Edden RAE, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: A neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 26.Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 tesla. Magn Reson Med. 2002;47:1009–1012. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- 27.Hetherington H, Newcomer BR, Pan JW. Measurements of human cerebral GABA at 4.1 T using numerically optimized editing pulses. Magn Reson Med. 1998;39:6–10. doi: 10.1002/mrm.1910390103. [DOI] [PubMed] [Google Scholar]
- 28.Tappaz M, Brownstein M, Kopin I. Glutamate decarboxylase (GAD) and gammaaminobutyric acid (GABA) in discrete nuclei of hypothalamus and substantia nigra. Brain Res. 1977;125:109–121. doi: 10.1016/0006-8993(77)90363-8. [DOI] [PubMed] [Google Scholar]
- 29.Lei H, Poitry-Yamate C, Preitner F, Thorens B, Gruetter R. Neurochemical profile of the mouse hypothalamus using in vivo 1H MRS at 14.1T. NMR Biomed. 2010;23:578–583. doi: 10.1002/nbm.1498. [DOI] [PubMed] [Google Scholar]
- 30.Edden RAE, Intrapiromkul J, Zhu H, Cheng Y, Barker PB. Measuring T2 in vivo with J-difference editing: Application to GABA at 3 tesla. JMRI. 2012;35:229–234. doi: 10.1002/jmri.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musen G, Simonson DC, Bolo NR, Driscoll A, Weinger K, Raji A, Theberge J, Renshaw PF, Jacobson AM. Regional brain activation during hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab. 2008;93:1450–1457. doi: 10.1210/jc.2007-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smeets PAM, de Graaf C, Stafleu A, van Osch MJP, van der Grond J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage. 2005;24:363–368. doi: 10.1016/j.neuroimage.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda M, Liu YJ, Mahankali S, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999;48:1801–1806. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]
- 34.Smeets PAM, Vidarsdottir S, De Graaf C, Stafleu A, Van Osch MJP, Viergever MA, Pijl H, Van der Grond J. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am J Physiol Endocrinol Metab. 2007;293:E754–E758. doi: 10.1152/ajpendo.00231.2007. [DOI] [PubMed] [Google Scholar]
- 35.Martin DL, Rimvall K. Regulation of γ-Aminobutyric acid synthesis in the brain. J Neurochem. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- 36.Paulsen RE, Fonnum F. Regulation of transmitter γ-Aminobutyric acid (GABA) synthesis and metabolism illustrated by the effect of γ-vinyl GABA and hypoglycemia. J Neurochem. 1988;50:1151–1157. doi: 10.1111/j.1471-4159.1988.tb10586.x. [DOI] [PubMed] [Google Scholar]
- 37.Andrews-Shigaki BC, Armstrong BSR, Zaitsev M, Ernst T. Prospective motion correction for magnetic resonance spectroscopy using single camera retro-grate reflector optical tracking. JMRI. 2011;33:498–504. doi: 10.1002/jmri.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]