Abstract
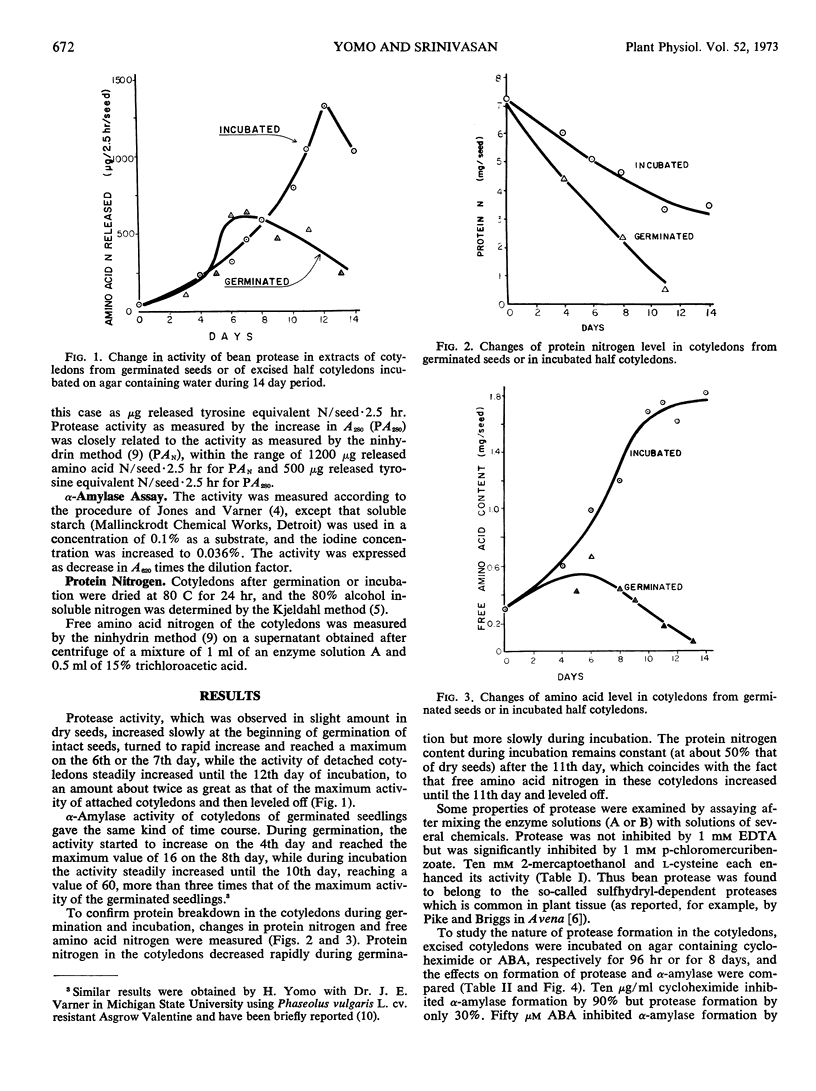
In contrast to earlier reported results of similar experiments in peas, in which almost no increase in protease activity occurred in incubated detached cotyledons, we report here an increase in protease activity in both attached and detached bean cotyledons. Detached bean cotyledons showed continually increasing protease activity up to the 12th day, while that in attached cotyledons declined after 6 days. The free amino acid level in detached cotyledons reached a maximum at the 11th day; protease formation leveled off after 50% of the original seed protein was digested. These data suggest that high free amino acid levels may inhibit protease formation.
The activity of partially purified protease in aqueous extracts was enhanced by 10 mm 2-mercaptoethanol or cysteine, indicating a sulfhydryl requirement for activation. Protease formation in detached cotyledons was inhibited 30% by 10 μg/ml cycloheximide and 50% by 100 μm abscisic acid. In contrast, α-amylase formation was inhibited 90% by 10 μg/ml cycloheximide and 95% by 20 μm abscisic acid. The cycloheximide data suggest that only a part of the protease, but all of the α-amylase, is synthesized de novo; the similar pattern of inhibition by abscisic acid emphasizes the concept that protease may exist in two forms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Pike C. S., Briggs W. R. Partial Purification and Characterization of a Phytochrome-degrading Neutral Protease from Etiolated Oat Shoots. Plant Physiol. 1972 Apr;49(4):521–530. doi: 10.1104/pp.49.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]



