Abstract
Background
Prevalence data for heartburn in the urban Black American community is lacking. In order to estimate prevalence for this community we analyzed data from an ongoing cohort study in progress at our hospital. Comprehensive interviews allowed for exploration of factors associated with heartburn.
Methods
Complex, stratified sampling design. Survey invitations are hand delivered to random blocks in a single zip code tabulation area. One member per eligible household is invited to complete a computer-based survey. Heartburn was defined as ≥ 3 days/week of symptoms as defined by the Montreal Definition and Classification of GERD. Scaling and weighting factors were utilized to estimate population-level prevalence. Multivariate logistic regression was used to identify independent predictor variables for heartburn.
Results
Enrolled 379 participants corresponding to a weighted sample size of 22,409 (20,888–23,930) citizens. Demographic characteristics of the sample closely matched those of the entire targeted population. Overall, the weighted prevalence of heartburn ≥ 3 times per week was 17.6% (16.4%–18.8%). Variables independently associated with heartburn were BMI, daily caloric and fat intake, diabetes mellitus (OR=2.95; 2.59–3.36), cigarette smoking, and alcohol consumption (OR=2.55; 2.25–2.89). Factors inversely associated included illicit drug use and increased physical activity. Waist: hip ratio showed no relationship.
Conclusions
The prevalence of heartburn ≥ 3 times per week is high in the Black American community. Adverse lifestyle behaviors showed particularly important associations. Our study needs to be replicated in other communities with similar demographics.
Introduction
Heartburn is one of the most prevalent symptoms referable to the gastrointestinal tract. (1–3) In the United States, according to a nationwide population-based study conducted by the Gallup Organization, 44% of respondents suffered from heartburn at least monthly while daily heartburn was reported by 7–10% of respondents. (4) A survey of 2,200 Olmstead county residents found the prevalence of monthly heartburn to be 42%, while weekly heartburn was 20%. (5) The prevalence of heartburn amongst different racial groups using convenience sampling has also been investigated. El-Serag et al conducted a cross-sectional survey at the Houston Veterans Affairs hospital finding weekly heartburn symptoms in 27% of Black Americans, 23% of White Americans and 24% in other races. (6) In contrast, a review of endoscopy reports of 2,477 patients demonstrated complicated GERD in 12% of White Americans, 3% of Black Americans, and 2% of Asians. (7)
The prevalence of GERD, and its principle symptom heartburn, has been increasing in North America over the past 30 years. (8) Prevalence changes have been attributed to several factors including changes in diet, physical exercise, central adiposity, smoking, and alcohol consumption. (9–12) A lowered prevalence of H pylori may also be contributing.(13) Around the world, particularly in Asia, parallel increases in GERD prevalence appear to be due to similar etiologic factors. (14,15)
Community-based screening for GERD has primarily relied on the frequency and severity of heartburn, along with other variables such as the presence of regurgitation, dysphagia, and chest pain. Commonly used questionnaires have generally shown high reliability but variable validity when compared to objective testing. (16) In 2006, an international consensus group in Montreal devised a global definition and classification of GERD that could be used by primary care physicians and practitioners from all over the world. The Montreal Definition and Classification of GERD (MDCG) was constructed using the modified Delphi process. (17) Heartburn was defined as a burning sensation in the retrosternal area. Symptoms related to GERD are classified as `troublesome' if they adversely affect an individual's well-being. (17) Field testing of the MDCG was recently performed in 57 morbidly obese individuals.(18) Using upper endoscopy and pH testing as the gold standard for the diagnosis of GERD, the overall accuracy, sensitivity, and specificity of heartburn subjectively defined as “troublesome” was 78.7%, 72%, and 100%. (18) In a study of patients referred for pH testing due to PPI failure at our institution, heartburn defined as “troublesome” correlated moderately well (r=−0.359;p=0.005) with quality of life scores using the validated Quality of Life in Reflux and Dyspepsia questionnaire (QOLRAD). (19) However, both the QOLRAD and MDCG performed poorly in discriminating those with and without acid reflux on objective testing. (19)
The aim of our study was to estimate the prevalence of heartburn in a heretofore understudied population – urban Black Americans. We analyzed data from our ongoing cohort study of the Black community surrounding our hospital. Our secondary purpose was to identify factors associated with heartburn. Using the MDCG, we aimed to determine the impact of heartburn on quality of life.
Methods
Sample Population
TRIAGE (Temple Registry for the Investigation of African American Gastrointestinal Disease Epidemiology) is an ongoing cohort study of Black Americans residing within the ZIP Code Tabulation Area (ZCTA) incorporating Temple University Hospital.
Survey Methodology
Our survey utilizes a complex, stratified sampling design. Our initial step was to visualize the ZCTA using satellite photographs. We excluded approximately 10% of the tract at the southeast border as the area is nearly completely occupied by Hispanic/Latino citizens. The remaining portion of the ZCTA (~ 90% Black American) was then divided into four adjacent territories (Zones 1–4) encompassing a roughly equal number of homes and apartment buildings (Figure 1). Zone stratification was necessary because there is a heterogeneous distribution of demographic characteristics of Black citizens in the census tract (e.g. employment, education level).
Figure 1.
Low resolution satellite image of Zip Code Tabulation Area with zones demarcated. Red area in southeast corner was not surveyed.
In order to obtain a simple random sample, we hand-delivered 250 invitations to participate in the survey to each zone. At apartment buildings 2–4 invitations were placed depending on the estimated number of occupied units (< 10 vs. ≥ 10). The invitation stated that to participate in the survey the subject had to be self-described as a Black American, older than age 18, and a resident of the dwelling for at least 3 years. Only one adult per household could participate.
Survey Components
Dependent Variable
The TRIAGE survey asks 17 questions related to heartburn. The questions include the frequency of heartburn and regurgitation episodes per week. We use the descriptors put forth by the Montreal Committee.(17) To quantify how “troublesome” symptoms are we use a 1–5 Likert scale. For the purposes of classification we identified subjects complaining of ≥ 3 days/week of heartburn as “heartburn positive”, regardless of how “troublesome” the symptom. (20) Additional questions relate to nocturnal symptoms, heartburn-related medical visits, and medications used for treatment. We have previously field tested our questionnaire with 503 participants in the same community. (21)
Predictor Variables
Beyond demographic questions related to issues such as educational attainment and income, the survey incorporates several validated questionnaires to classify potential predictor variables. We require subjects to complete The Alcohol Use Disorders Identification Test (AUDIT). (22) For prescription and illegal drug abuse we use the Drug Abuse Screening Test (DAST). (23) Tobacco consumption is queried with the Fagerstrom Test for Nicotine Dependence (FTND).(24) Total calories is estimated using the Food Frequency Questionnaire developed by Kaiser Permanente. We adapted the Weight and Lifestyle Inventory (WALI) to estimate combined physical exertion for both work and home compared to the perceived level of activity for individuals of the same age and sex.(25)The survey also queries up to 10 medical problems and 10 prescription and non-prescription medications.
Survey Administration
Surveys are conducted at Temple University Hospital in our research department. A research coordinator records demographic information and measures the subject's height, weight, hip and waist circumference. Patients are then placed at a computer terminal. Our survey was developed using Microsoft Access 2007 (Microsoft Corporation, Redmond, WA). A coordinator assists participants with visual impairment and those unable to read at the 5th grade level. Subjects progress through the survey by selecting choices using a computer mouse. Subjects cannot progress through the survey without answering all questions. The survey was approved by our Institutional Review Board. The trial is registered on clinicaltrials.gov (NCT01262755).
Survey Analysis
Sample Weighting
Two levels of weighting were performed. An initial weight is applied to adjust for the unequal probability of selection within the household. For second-level weighting, we utilize data from the 2010 US Census which stratifies Black Americans living in the ZCTA by sex and age. An additional variable labeled “Scale” is used to estimate population-level prevalence data. The scale factor (v) represents the ratio of the entire Black population of the ZCTA divided by the number of subjects interviewed (v=NB/nB).
Sample Size and Statistical Analysis
We estimated that the prevalence of “heartburn positive” individuals would be between 17–20%. (21) To develop a well-calibrated regression model with 8–10 outcome events per predictor variable, our protocol calls for recruitment of 375 subjects. Exploratory data analysis identified that the distribution of waist: hip ratio (WHR), daily caloric intake, and daily fat intake were highly skewed and therefore these variables were re-coded into quartiles. We then designed a complex sample file plan using the Complex Sample Module of SPSS 19.0 (IBM SPSS, Armonk, New York). For design variables we chose “Zone” as the strata variable and “Final_Weight” as the sample weight. For variance estimates we performed sampling with replacement and applied the finite population correction.
Descriptive and inferential statistics were determined using the Complex Sample Module yielding point estimates with 95% confidence intervals. For categorical variables, an adjusted F statistic was calculated. General linear models were used to estimate the association of continuous predictor variables with categorical outcomes. P values were calculated based on the Wald statistic. A multivariable logistic regression was performed to identify variables independently associated with the prevalence of heartburn ≥ 3 days per week. Odds ratios along with their corresponding 95% confidence intervals were calculated for variables included in the model. All hypothesis testing was 2-tailed with α=0.05.
Results
Study Population Characteristics
We recruited 379 subjects corresponding to a weighted sample size of 22,409 (20,888–23,930) citizens. An additional 190 subjects responded however they were enrolled in the next phase of the TRIAGE study looking at irritable bowel syndrome (overall invitation response rate of 56.9%). Several lines of evidence support that our random sampling methodology was successful and results can be generalized to the entire ZCTA. For example, our prevalence estimate for the proportion of adult Black Americans in the ZTCA that are male is 39.5% (33.8–45.4%) while the actual proportion is 41.2%. The mean weighted age of our adult Black sample is 43.2 (40.8–45.6) years, similar to census results (ranging from 40.7 to 46.6 years using the upper and lower limits of each 5 year age strata multiplied by population percentage). The weighted proportional estimate for those who are apartment dwellers, 34.4% (29.0–40.3%), is reasonably close to the proportion of occupied apartments units in the census tract (41.0%). A recent survey found that only 9% of adults (not stratified by race) over the age of 25 had a college degree in the ZCTA; reasonably close to the weighted prevalence found in our survey of 7.7% (4.9–12.0%). Physical activity assessed on a 6-point Likert scale demonstrated higher than expected activity. The prevalence of drug, alcohol, and tobacco consumption was substantial.
As shown in table 1, the weighted prevalence of heartburn ≥ 3 times per week was 17.6% (95% CI, 16.4%–18.8%). Female gender, older age, increased BMI, higher levels of education and income, and harmful alcohol use were associated with an increased prevalence of heartburn ≥ 3 times per week. The complex relationship between gender and age is shown in Figure 2.
Table 1.
| Total Weighted N = 22,409 | 95% CI 20,888−23,930 | Heartburn ≥3d/week + Weighted N = 3,934 | 95% CI 2,853−5,015 | Heartburn ≥3d/week − Weighted N = 18,475 | 95% CI 16,847−20,102 | |
|---|---|---|---|---|---|---|
| Male (%) | 8,852 (39.5) | 33.8−45.4 | 1,172 (29.8) | 17.3−46.1 | 7,667(41.5) | 35.2−48.1 |
|
| ||||||
| Mean Age, y | 43.2 | 40.8−45.6 | 46.4 | 41.5−51.3 | 42.5 | 39.9−45.2 |
|
| ||||||
| Body Mass Index (Kg/m2) | 29.03 | 28.12−29.94 | 31.00 | 28.80−33.20 | 28.61 | 27.61−29.61 |
|
| ||||||
| Male: Waist/Hip Ratio | 0.89 | 0.88−0.90 | 0.91 | 0.88−0.94 | 0.89 | 0.87−0.90 |
| Female: Waist/Hip Ratio | 0.87 | 0.86−0.88 | 0.87 | 0.84−0.90 | 0.87 | 0.86−0.88 |
|
| ||||||
| Socioeconomic | ||||||
|
| ||||||
| Educational Attainment (%) | ||||||
| < High School | 6,476 (28.9) | 23.9−34.4 | 1,180(30.0) | 18.4−44.8 | 5,284 (28.6) | 23.2−34.7 |
| High School Graduate | 14,207 (63.4) | 57.5−68.9 | 2,219 (56.4) | 40.9−70.7 | 11,990 (64.9) | 58.6−70.8 |
| College Graduate | 1,725 (7.7) | 4.9−12.0 | 535 (13.6) | 4.8−33.3 | 1,201 (6.5) | 4.0−10.2 |
|
| ||||||
| Marital Status (%) | ||||||
| Single | 16,941 (75.6) | 69.9−80.6 | 2,954 (75.1) | 58.9−86.3 | 14,004 (75.8) | 69.5−81.1 |
| Divorced | 2,711 (12.1) | 8.2−17.4 | 586 (14.9) | 5.7−33.6 | 2,125 (11.5) | 7.5−17.1 |
| Separated | 650 (2.9) | 1.7−5.0 | 122 (3.1) | 1.1−8.6 | 517 (2.8) | 1.5−5.3 |
| Married | 2,106 (9.4) | 6.6−13.3 | 271 (6.9) | 3.0−15.1 | 1,829 (9.9) | 6.7−14.5 |
|
| ||||||
| Household Income (USD) (%) | ||||||
| No earnings | 7,609 (34.0) | 28.6−39.8 | 936 (23.8) | 13.6−38.2 | 6,688 (36.2) | 30.1−42.7 |
| < 20K | 10,622 (47.4) | 41.3−53.5 | 1,920 (48.8) | 34.1−63.7 | 8,702 (47.1) | 40.4−53.9 |
| 20K−50K | 3,675 (16.4) | 12.3−21.6 | 1,054 (26.8) | 14.8−43.6 | 2,623 (14.2) | 10.2−19.5 |
| >50K | 493 (2.2) | 1.2−3.9 | 24 (0.6) | 0.1−4.7 | 462 (2.5) | 1.4−4.5 |
|
| ||||||
| Apartment Living (%) | 7,709 (34.4) | 29.0−40.3 | 1,043 (26.5) | 15.6−41.2 | 6,669 (36.1) | 30.0−42.6 |
| Health Insurance (%) | ||||||
| None | 4,594 (20.5) | 16.1−25.6 | 728(18.5) | 9.3−33.4 | 3,861 (20.9) | 16.2−26.6 |
| Mcare/Mcare HMO | 1,569 (7.0) | 1.8−11.5 | 586 (14.9) | 5.5−34.6 | 998 (5.4) | 3.1−9.1 |
| Mcaid/Mcaid HMO | 12,885 (57.5) | 49.8−67.8 | 2,294 (58.3) | 42.9−72.3 | 10,605 (57.4) | 48.6−69.1 |
| Commercial/Com HMO | 3,137 (14.0) | 9.4−20.7 | 327 (8.3) | 2.8−23.5 | 2,808 (15.2) | 9.9−23.0 |
| VA | 179 (0.8) | 0.3−2.1 | 0 (0.0) | 0.0−1.1 | 185 (1.0) | 0.4−2.6 |
|
| ||||||
| Lifestyle Behaviors | ||||||
|
| ||||||
| Physical Activity Level (%)* | ||||||
| Low | 2,598 (13.2) | 9.7−17.7 | 865 (22.0) | 12.3−36.1 | 2,088 (11.3) | 7.8−16.0 |
| Medium | 8,695 (38.8) | 33.0−44.9 | 1,377 (32.5) | 21.2−46.4 | 7,408(40.1) | 33.6−46.9 |
| High | 10,756 (48.0) | 42.0−54.1 | 1,786 (45.4) | 31.4−60.3 | 8,979 (48.6) | 42.0−55.3 |
|
| ||||||
| Tobacco Dependence (%) ** | ||||||
| Non-Smoker | 9,569 (42.7) | 36.7−48.9 | 1,475 (37.5) | 24.3−53.0 | 8,092 (43.8) | 37.2−50.6 |
| Low | 6,745 (30.1) | 25.0−35.8 | 1,314(33.4) | 21.5−47.8 | 5,432 (29.4) | 23.9−35.6 |
| Medium | 5,087 (22.7) | 18.1−28.0 | 960 (24.4) | 14.6−37.9 | 4,120 (22.3) | 17.3−28.2 |
| High | 1,188 (5.3) | 3.2−8.7 | 185 (4.7) | 1.2−16.9 | 1,016 (5.5) | 3.2−9.2 |
|
| ||||||
| Likelihood of Prescription or Illicit Drug Abuse† (%) | ||||||
| Low | 18,331 (81.8) | 77.1−85.7 | 3,552 (90.3) | 81.8−95.1 | 14,780 (80.0) | 74.5−84.5 |
| Moderate | 2,398 (10.7) | 7.9−14.4 | 244 (6.2) | 2.8−13.3 | 2,162(11.7) | 8.4−16.0 |
| High | 1,681 (7.5) | 5.0−11.3 | 138 (3.5) | 1.1−10.9 | 1,552 (8.4) | 5.4−12.8 |
|
| ||||||
| Harmful Drinking Behavior‡ (%) | 2,756 (12.3) | 9.2−16.2 | 692 (17.6) | 9.5−30.3 | 2,069 (11.2) | 8.0−15.4 |
|
| ||||||
| Medical Care | ||||||
|
| ||||||
| Primary Care Physician (%) | 17,636 (78.7) | 73.5−83.2 | 3,269 (83.1) | 69.0−91.6 | 14,374 (77.8) | 72.0−82.7 |
| Diabetes Mellitus (%) | 1,860 (8.3) | 5.6−12.1 | 555 (14.1) | 6.5−28.0 | 1,312(7.1) | 4.5−11.0 |
|
| ||||||
| Medications (%) | ||||||
| Calcium Channel Blocker | 1,367 (6.1) | 3.4−10.7 | 386 (9.8) | 4.6−19.7 | 998 (5.4) | 2.5−11.0 |
| Beta-Agonist | 2,510 (11.2) | 7.8−16.0 | 810 (20.6) | 11.6−34.0 | 1,700 (9.2) | 5.7−14.5 |
| Anti-Cholinergic | 2,062 (9.2) | 6.5−12.9 | 327 (8.3) | 3.7−17.6 | 1,737 (9.4) | 6.4−13.7 |
| NSAID | 2,689 (12.0) | 8.8 16.0 | 661 (16.8) | 9.1−28.8 | 2,032 (11.0) | 7.7−15.3 |
Adapted from Paffenberger
Fagerstrom Test for Nicotine Dependence
The Drug Abuse Screening Test (DAST) is a 28-item self-report scale that consists of items that parallel those of the Michigan Alcoholism Screening Test (MAST). The DAST has “exhibited valid psychometric properties” and has been found to be “a sensitive screening instrument for the abuse of drugs other than alcohol.
Alcohol Use Inventory (AUDIT) – score >=8.
USD – United States Dollars
Figure 2.
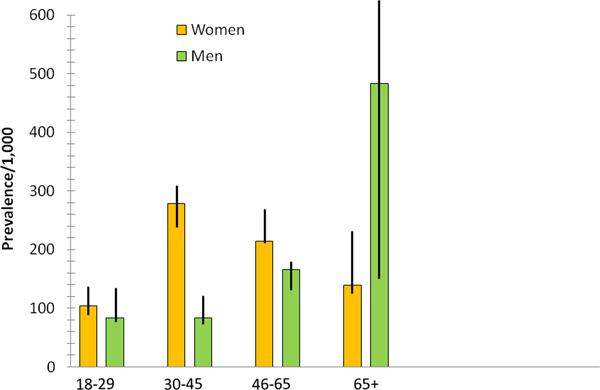
Relationship between age, gender and the prevalence of heartburn ≥ 3 days per week in the surveyed population.
Characteristics of Heartburn Subjects - Table 2
Table 2.
Univariate characterization of subpopulation complaining of heartburn ≥ 3 times per week.
| Point Estimate (%) | 95% Confidence Interval | |
|---|---|---|
| Heartburn Days/week | ||
| 3−4 | 67.4 | 52.3−79.6 |
| 5−7 | 32.6 | 20.4−47.7 |
|
| ||
| How Troublesome is heartburn? | ||
| “Not at all” | 0 | 0.0−7.1 |
| “Mildly-Moderate” | 62.6 | 38.4−93.3 |
| “Severe” | 37.4 | 22.0−62.0 |
|
| ||
| Do you have episodes of regurgitation?* | ||
| Yes | 61.0 | 46.0−74.2 |
| If yes, days/week | ||
| 1−2 | 43.9 | 18.1−89.8 |
| 3−4 | 33.6 | 15.3−66.2 |
| 5−7 | 22.4 | 7.1−63.3 |
|
| ||
| How Troublesome is regurgitation? | ||
| “Not at all” | 8.3 | 2.0−29.0 |
| “Mild-Moderate” | 53.9 | 25.8−96.0 |
| “Severe” | 37.8 | 20.5−70.0 |
|
| ||
| Do you get a sharp chest pain that is different from heartburn? | ||
| Yes | 69.3 | 52.5−82.2 |
|
| ||
| Does heartburn disturb your sleep? | ||
| Yes | 65.7 | 49.3−79.0 |
| If yes, how many nights/week? | ||
| 1−2 | 27.1 | 11.9−61.8 |
| 3−4 | 54.3 | 28.8−90.3 |
| 5−7 | 18.7 | 7.4−43.6 |
|
| ||
| Do you discuss treatment for your heartburn with your doctor? | ||
| Yes | 42.3 | 28.0−57.9 |
| If yes, how many times a year? | ||
| 1−2 | 38.7 | 15.4−86.7 |
| >2 | 61.4 | 26.2−100.0 |
|
| ||
| Do you take medicine for heartburn? | ||
| Yes | 57.2 | 42.0−71.1 |
| If yes, how many days per week | ||
| 1−2 | 20.7 | 6.3−48.0 |
| 3−4 | 22.5 | 9.3−53.1 |
| 5−7 | 58.8 | 34.3−98.6 |
| Do the medicines work? | ||
| No | 2.6 | 0.6−10.9 |
| Sometimes | 42.9 | 25.5−62.2 |
| Most of the Time | 25.6 | 10.4−50.6 |
| All of the Time | 28.9 | 14.9−48.6 |
|
| ||
| Treatment | ||
| Proton Pump Inhibitor | 57.7 | 33.3−100.0 |
| H2 Blocker | 42.3 | 16.9−92.9 |
defined as a bitter or sour-tasting fluid coming into the throat or mouth.
For the subset of subjects with heartburn ≥ 3 days per week, the majority characterized the episodes as mild-moderate. More than half also had associated regurgitation, chest pain, and disturbing nocturnal symptoms. Those with regurgitation had frequent episodes that were quite troublesome. Slightly more than half utilized anti-secretory therapy and only approximately half of this group found them beneficial.
Independent Predictors of Heartburn ≥ 3 Days per week – Table 3
Table 3.
Independent predictors of heartburn from regression analysis.
| Heartburn >=3d/week Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Weighted N | Weighted % | Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | |
|
| ||||||
| BMI, Kg/m2 | ||||||
| >=25 | 7277 | 35.8 | ref | ref | ||
|
|
||||||
| 25.1−30 | 5574 | 27.4 | 1.34 | 1.21−1.47 | 1.36 | 1.22−1.52 |
|
|
||||||
| 30.1−35 | 3381 | 16.6 | 2.59 | 2.34−2.87 | 2.27 | 2.00−2.58 |
|
|
||||||
| ≥35.1 | 4095 | 20.1 | 2.54 | 2.31−2.80 | 3.27 | 2.87−3.72 |
|
| ||||||
| Waist/Hip Ratio, Quartiles | ||||||
| 1 | 5548 | 27.3 | ref | ref | ||
|
|
||||||
| 2 | 4955 | 24.4 | 1.90 | 1.72−2.10 | 1.88 | 1.67−2.10 |
|
|
||||||
| 3 | 4920 | 24.2 | 1.40 | 1.26−1.55 | 0.98 | 0.86−1.11 |
|
|
||||||
| 4 | 4903 | 24.1 | 1.48 | 1.33−1.64 | 0.97 | 0.85−1.10 |
|
| ||||||
| Daily Kcal, Quartiles | ||||||
| 1 | 5527 | 27.2 | ref | ref | ||
|
|
||||||
| 2 | 5257 | 25.9 | 1.49 | 1.34−1.65 | 2.07 | 1.79−2.40 |
|
|
||||||
| 3 | 4648 | 22.9 | 1.56 | 1.40−1.73 | 4.77 | 3.97−5.73 |
| 4 | 4894 | 24.1 | 2.38 | 2.15−2.63 | 4.96 | 4.00−6.14 |
|
| ||||||
| Daily Fat Intake (g), Quartiles | ||||||
| 1 | 5478 | 26.9 | ref | ref | ||
|
|
||||||
| 2 | 5174 | 25.5 | 1.34 | 1.21−1.48 | 1.14 | 0.99−1.31 |
|
|
||||||
| 3 | 5117 | 25.2 | 1.27 | 1.15−1.41 | 0.74 | 0.62−0.88 |
|
|
||||||
| 4 | 4557 | 22.4 | 2.24 | 2.03−2.47 | 1.31 | 1.07−1.60 |
|
| ||||||
| Physical Activity Level | ||||||
| Low | 2833 | 13.9 | ref | ref | ||
|
| ||||||
| Moderate | 7827 | 38.5 | 0.42 | 0.38−0.46 | 0.32 | 0.28−0.36 |
|
| ||||||
| High | 9667 | 47.6 | 0.48 | 0.44−0.53 | 0.40 | 0.35−0.45 |
|
| ||||||
| Diabetes Mellitus | ||||||
| No | 18632 | 91.7 | ref | ref | ||
| Yes | 1695 | 8.3 | 2.16 | 1.94−2.40 | 2.95 | 2.59−3.36 |
|
| ||||||
| Harmful Drinking | ||||||
| No | 17759 | 87.4 | ref | ref | ||
|
|
||||||
| Yes | 2568 | 12.6 | 1.71 | 1.55−1.88 | 2.55 | 2.25−2.89 |
|
| ||||||
| Tobacco Use | ||||||
| None | 8571 | 42.2 | ref | ref | ||
|
| ||||||
| Low | 6045 | 29.7 | 1.30 | 1.19−1.41 | 1.42 | 1.29−1.57 |
|
| ||||||
| Medium | 4732 | 23.3 | 1.25 | 1.15−1.37 | 1.42 | 1.27−1.59 |
|
| ||||||
| High | 979 | 4.8 | 0.98 | 0.83−1.15 | 1.20 | 0.94−1.53 |
|
| ||||||
| Likelihood Drug Use | ||||||
| Low | 16391 | 80.6 | ref | ref | ||
|
|
||||||
| Moderate | 2251 | 11.1 | 0.47 | 0.41−0.54 | 0.33 | 0.28−0.39 |
|
|
||||||
| High | 1685 | 8.3 | 0.37 | 0.31−0.45 | 0.18 | 0.15−0.23 |
Adjusted for age, gender, and all other variables in the model.
Increasing BMI levels were associated with heartburn, however this relationship was not seen for waist: hip ratio. In fact, individuals in the second lowest quartile of waist: hip ratio had an increased risk in the regression model (1.88;1.67–2.10). Progressive levels of caloric intake were associated with heartburn. For total fat intake, only the highest quartile was independently associated with heartburn. Level of physical activity was inversely associated with the heartburn prevalence. Diabetes status was a strongly associated independent risk. The presence of potentially harmful alcohol consumption behavior was strongly associated with heartburn (2.55;2.25–2.89). Low-medium inhaled tobacco consumption was associated with heartburn, but the relationship was not seen for the highest level. Drug use was inversely associated with heartburn. Those with the highest likelihood of drug use had roughly one-fifth the risk for heartburn.
Discussion
Our study represents the first truly population-based survey of urban, adult Black Americans in the United States reporting on the prevalence and risk factors associated with heartburn. We found that the prevalence of heartburn ≥ 3 times per week was 17.6% (95% CI, 16.4%–18.8%). Table 4 compares this prevalence rate with studies from other centers. Heartburn was rated as “severely troublesome” by 37.4% (22.0–62.0%) of symptomatic patients. Overall, 65.7% (49.3–79.0%) of heartburn sufferers complained of nocturnal heartburn severe enough to disturb their sleep with roughly 3 in 4 experiencing this symptom at least 3 nights per week. Roughly half of the subjects (57.2%; 42.0–71.1%) were on therapy for heartburn with a near even split between daily and on-demand treatment.
Table 4.
Prevalence of heartburn in selected other studies.
| Author (ref) | Country | Year | Prevalence of Heartburn (%) | Frequency Assessed |
|---|---|---|---|---|
|
| ||||
| Locke GR et al.5 | USA | 1997 | 19.8 | Weekly |
|
| ||||
| Ho KY et al. 42 | Singapore | 1998 | 1.6 | Monthly |
|
| ||||
| Spechler SJ et al.7 | USA | 2002 | 28.7 | Monthly |
|
| ||||
| Wong WM et al. 43 | China | 2003 | 29.8 | Annually |
| 8.9 | Monthly | |||
| 2.5 | Weekly | |||
|
| ||||
| El-Serag HB et al. 6 | USA | 2004 | Blacks | Weekly |
| Whites 23.0 | ||||
| Other races 24.0 | ||||
|
| ||||
| Nilsson M et al. 9 | Sweden | 2004 | (only those with servere symptoms included) | |
|
| ||||
| 72.0 | Monthly | |||
| 23.0 | Weekly | |||
| 5.0 | Daily | |||
|
| ||||
| Wong WM et al. 44 | China | 2004 | 34.1 | Annually |
| 10.1 | Monthly | |||
| 2.7 | Weekly | |||
|
| ||||
| Ho KY et al. 45 | Singapore | 2005 | 10.5 | Monthly |
|
| ||||
| El-Serag HB et al. 46 | USA | 2005 | 26.0 | Weekly |
|
| ||||
| Mohammad et al.10 | England | 2005 | 21.0 | Weekly |
|
| ||||
| Nocon M etal. 11 | Germany | 2006 | 18.0 | Not defined |
|
| ||||
| Jacobson BC et al. 47 | USA | 2006 | 22.0 | Weekly |
|
| ||||
| Corley DA etal.32 | USA | 2007 | 11.0 | Not defined |
|
| ||||
| Yuen E et al.31 | USA | 2010 | 34.6 | Monthly |
| 26.2 | Weekly | |||
| 8.2 | Daily | |||
|
| ||||
| Sharma PK et al.34 | India | 2011 | 17.7 | Monthly |
| 5.9 | Weekly | |||
| 3.6 | Daily | |||
Our survey provides insight into additional symptoms patients with heartburn experience. For example, 61.0 % (46.0–74.2%) of heartburn sufferers complained of regurgitation, and over half had this symptom at least 3 days per week. Chest pain was experienced by 69.3% (52.5–82.2%) episodically.
It appears that our random sampling methodology was successful and the results of this study can be generalized. As a result, our study sheds light on heartburn in impoverished Black Americans compared to most prior US studies which have focused on other populations of interest. (26) Our questionnaire was field tested previously and modified extensively based on participant feedback. A research coordinator provided assistance to all participants as necessary in completing the survey to accommodate differences in age, education, and literacy levels. A major strength was the use of descriptors put forth by the Montreal Committee on the Global Assessment of Reflux. (17) By requiring patients to be residents of the targeted ZTCA for at least 3 years we minimized the potential for ecological confounding.
This study aimed to overcome shortcomings of our previous study which utilized convenience sampling and therefore likely biased estimates for heartburn prevalence. (21) Other studies have used convenience samples such as hospital employees.(6) Another example is a recent study which interviewed 1,212 subjects at health fairs in Philadelphia. Hispanics had the highest prevalence of monthly (50.0%), weekly (38.0%) and daily (13.6%) heartburn.(31) Thirty percent of Whites reported weekly heartburn compared to 22.1% of Blacks. A notable shortcoming of convenience samples is the high likelihood of oversampling individuals who differ from the general population with respect to many potential factors. Other studies have suffered the limitations of administrative databases in which selection bias and missing information are important limitations. For example, in the study by Corley et al, only patients seen for a health check-up were included, not members of the general population, potentially introducing selection bias. (32)
Many factors previously shown to be associated with heartburn were also found in our study group. For example, increasing levels of obesity, and increasing intake of calories and fat were associated with heartburn ≥ 3 times per week. In contrast to previous studies, we found that central adiposity (waist: hip ratio) was not linearly associated with heartburn prevalence. We also found that moderate-high physical activity was inversely associated with heartburn. We found that individuals with diabetes mellitus, independent of BMI or waist circumference, had a nearly 3-fold increased risk for heartburn (OR=2.95; 2.59–3.36). This independent finding corroborates results from a community survey in Sweden. (27) Robust data regarding the pathophysiologic basis for this association is lacking, however it appears that poor glycemic control and autonomic neuropathy are at the root cause. (28–30)
A second important goal of our study was to determine the role of adverse lifestyle choices, particularly tobacco, alcohol, and drug consumption on the prevalence of heartburn. We found that patients with low (OR=1.42; 1.29–1.57) and medium (OR=1.42; 1.27–1.59) levels of tobacco consumption had an increased risk of heartburn relative to non-smokers. However, the highest consumers were not at risk (OR=1.20; 0.94–1.53). Our findings are congruent with a study which found that smokers had a significantly decreased lower esophageal sphincter (LES) pressure compared to non-smokers. (33) Population-based survey data from Norway found an increased risk for recurrent heartburn in those who smoke. (9) In HUNT 1, a dose-dependent association between increasing duration of daily tobacco smoking and heartburn symptoms was found. A cross-sectional study from New Delhi of 4,039 hospital employees also demonstrated an association between current smoking and heartburn. (34) Current smoking of cigarettes (OR=1.48) and previous smoking (OR=1.36) were found to be risks for frequent heartburn.
We found harmful alcohol consumption, as defined by AUDIT, to be an important independent risk for heartburn (OR=2.55; 2.25–2.89). The relationship is biologically plausible as intake of certain alcoholic beverages such as beer, wine, and vodka has been demonstrated to lead to acid reflux. (35–37) For example, Kaufman et al investigated reflux episodes after combining 180 ml of 100 proof vodka with a standard meal in crossover fashion in twelve normal subjects. (36) In 11 subjects, reflux episodes and total reflux scores were higher with vodka than without.
Clinical studies investigating the association of alcohol consumption and heartburn symptoms and acid reflux complications have yielded contradictory results. A cross-sectional study from Ireland found that alcohol consumption in early adulthood was associated with the development of reflux esophagitis while more recent consumption did not appear to increase the risk. (38) A Japanese study examined 463 men who underwent upper endoscopy as part of health screening.(39) Subjects were divided into never drinkers, light drinkers (< 25 g of alcohol per day), moderate drinkers (25–50 g of alcohol per day), and heavy drinkers (> 50 g of alcohol per day). Compared to never drinkers, moderate drinkers (1.88;1.02–3.48) and heavy drinkers (1.99;1.12–5.54) had an increased risk for erosive esophagitis. Only heavy drinking was associated with an increased risk of Barrett's epithelium (1.91; 1.19–3.09). (39)
In our study individuals with a moderate (0.33; 0.28–0.39) or high (0.18; 0.15–0.23) likelihood of drug use demonstrated a substantially reduced risk for heartburn. This finding is consistent with both pharmacologic and clinical data. For example, Penagini et al evaluated the effect of morphine on the rate of TLESRs and motor function of the proximal stomach in 19 healthy subjects. (40) In those given morphine the rate of TLESRs was greatly decreased during pressure controlled distention of the fundus with a barostat. Gastric distention also decreased after morphine during both pressure and volume controlled distention.
One of the main weaknesses of our study is that we cannot provide additional information on non-respondents. There are many reasons subjects may not have responded; no interest, unable to take off from work, and so on. These are always issues surrounding community surveys and unless every individual from the community participates, some form of bias is likely to occur. However, our methodology, we believe, is far superior to mailed surveys and the use of convenience samples.
In conclusion, our study represents the only population-based survey of an urban, Black American community in the US reporting on the prevalence of heartburn and factors associated with this symptom. As such, our study results can only be generalized to other urban Black American communities. Similar to other studies in other populations we found that obesity, low physical activity, and high consumption of fat and calories were risks. Unique to our study is the focus on adverse lifestyle behaviors in which we found that both tobacco and alcohol use were risks, while drug abuse was inversely associated with heartburn. Also, unique to our study was the identification of diabetes, independent of obesity parameters, as a risk for heartburn. We encourage researchers from other Black American communities to survey their citizens in order to determine whether these epidemiologic associations are reproducible.
Acknowledgments
Source of Funding: Award K24DK083268 from the DHHS to FKF.
Abbreviations
- AUDIT
The Alcohol Use Disorders Identification Test
- DAST
Drug Abuse Screening Test
- FTND
Fagerstrom Test for Nicotine Dependence
- GERD
gastroesophageal reflux disease
- MDCG
Montreal Definition and Classification of GERD
- NSAID
non-steroidal anti-inflammatory drug
- QOLRAD
Quality of Life in Reflux and Dyspepsia questionnaire
- TRIAGE
Temple Registry for the Investigation of African American Gastrointestinal Disease Epidemiology
- TRLES
transient relaxation of the lower esophageal sphincter
- WALI
Weight and Lifestyle Inventory
- ZCTA
ZIP Code Tabulation Area
References
- 1.Kahrilas PJ, Shaheen NJ, Vaezi MF, American Gastroenterological Association Institute. Clinical Practice and Quality Management Committee American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392, 1413, 1413.e1–5. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 2.Katelaris P, Holloway R, Talley N, et al. Gastro-oesophageal reflux disease in adults: Guidelines for clinicians. J Gastroenterol Hepatol. 2002;17:825–33. doi: 10.1046/j.1440-1746.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 3.Berstad A, Hatlebakk JG. The predictive value of symptoms in gastro-oesophageal reflux disease. Scand J Gastroenterol Suppl. 1995;211:1–4. doi: 10.3109/00365529509090284. [DOI] [PubMed] [Google Scholar]
- 4.Shaker R, Castell DO, Schoenfeld PS, Spechler SJ. Nighttime heartburn is an under-appreciated clinical problem that impacts sleep and daytime function: the results of a Gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol. 2003;98:1487–93. doi: 10.1111/j.1572-0241.2003.07531.x. [DOI] [PubMed] [Google Scholar]
- 5.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126:1692–9. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 7.Spechler SJ, Jain SK, Tendler DA, Parker RA. Racial differences in the frequency of symptoms and complications of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16:1795–800. doi: 10.1046/j.1365-2036.2002.01351.x. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5:17–26. doi: 10.1016/j.cgh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut. 2004;53:1730–5. doi: 10.1136/gut.2004.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammed I, Nightingale P, Trudgill NJ. Risk factors for gastro-oesophageal reflux disease symptoms: a community study. Aliment Pharmacol Ther. 2005;21:821–7. doi: 10.1111/j.1365-2036.2005.02426.x. [DOI] [PubMed] [Google Scholar]
- 11.Nocon M, Labenz J, Willich SN. Lifestyle factors and symptoms of gastro-oesophageal reflux -- a population-based study. Aliment Pharmacol Ther. 2006;23:169–74. doi: 10.1111/j.1365-2036.2006.02727.x. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenberg A. Effects of environment and lifestyle on gastroesophageal reflux disease. Dig Dis. 2011;29:229–34. doi: 10.1159/000323927. [DOI] [PubMed] [Google Scholar]
- 13.Richter JE, Falk GW, Vaezi MF. Helicobacter pylori and gastroesophageal reflux disease: the bug may not be all bad. Am J Gastroenterol. 1998;93:1800–2. doi: 10.1111/j.1572-0241.1998.00523.x. [DOI] [PubMed] [Google Scholar]
- 14.Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil. 2011;17:14–27. doi: 10.5056/jnm.2011.17.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang MS, Park DI, Oh SY, et al. Abdominal obesity is an independent risk factor for erosive esophagitis in a Korean population. J Gastroenterol Hepatol. 2007;22:1656–61. doi: 10.1111/j.1440-1746.2006.04518.x. [DOI] [PubMed] [Google Scholar]
- 16.Fass R. Symptom assessment tools for gastroesophageal reflux disease (GERD) treatment. J Clin Gastroenterol. 2007;41:437–44. doi: 10.1097/MCG.0b013e31802e849f. [DOI] [PubMed] [Google Scholar]
- 17.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus Group The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900, 20. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 1943. [DOI] [PubMed] [Google Scholar]
- 18.Madalosso CA, Fornari F, Callegari-Jacques SM, Madalosso CA, Gurski RR. Performance of the Montreal Consensus in the diagnosis of gastroesophageal reflux disease in morbidly obese patients. Obes Surg. 2008;18:668–74. doi: 10.1007/s11695-008-9462-6. [DOI] [PubMed] [Google Scholar]
- 19.Sawaya RA, Macgill A, Parkman HP, Friedenberg FK. Use of the Montreal global definition as an assessment of quality of life in reflux disease. Dis Esophagus. 2011 doi: 10.1111/j.1442-2050.2011.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwizer W, Thumshirn M, Dent J, et al. Helicobacter pylori and symptomatic relapse of gastro-oesophageal reflux disease: a randomised controlled trial. Lancet. 2001;357:1738–42. doi: 10.1016/S0140-6736(00)04894-7. [DOI] [PubMed] [Google Scholar]
- 21.Friedenberg FK, Rai J, Vanar V, et al. Prevalence and risk factors for gastroesophageal reflux disease in an impoverished minority population. Obes Res Clin Pract. 2010;4:e261–9. doi: 10.1016/j.orcp.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 23.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 24.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 25.Wadden TA, Foster GD. Weight and Lifestyle Inventory (WALI) Surg Obes Relat Dis. 2006;2:180–99. doi: 10.1016/j.soard.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P, Wani S, Romero Y, Johnson D, Hamilton F. Racial and geographic issues in gastroesophageal reflux disease. Am J Gastroenterol. 2008;103:2669–80. doi: 10.1111/j.1572-0241.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- 27.Spangeus A, El-Salhy M, Suhr O, Eriksson J, Lithner F. Prevalence of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroenterol. 1999;34:1196–202. doi: 10.1080/003655299750024706. [DOI] [PubMed] [Google Scholar]
- 28.Lauffer A, Forcelini CM, Ruas LO, Madalosso CA, Fornari F. Gastroesophageal reflux disease is inversely related with glycemic control in morbidly obese patients. Obes Surg. 2011;21:864–70. doi: 10.1007/s11695-011-0372-7. [DOI] [PubMed] [Google Scholar]
- 29.Lluch I, Ascaso JF, Mora F, et al. Gastroesophageal reflux in diabetes mellitus. Am J Gastroenterol. 1999;94:919–24. doi: 10.1111/j.1572-0241.1999.987_j.x. [DOI] [PubMed] [Google Scholar]
- 30.Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–11. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 31.Yuen E, Romney M, Toner RW, et al. Prevalence, knowledge and care patterns for gastrooesophageal reflux disease in United States minority populations. Aliment Pharmacol Ther. 2010;32:645–54. doi: 10.1111/j.1365-2036.2010.04396.x. [DOI] [PubMed] [Google Scholar]
- 32.Corley DA, Kubo A, Zhao W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. Gut. 2007;56:756–62. doi: 10.1136/gut.2006.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut. 1990;31:4–10. doi: 10.1136/gut.31.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma PK, Ahuja V, Madan K, Gupta S, Raizada A, Sharma MP. Prevalence, severity, and risk factors of symptomatic gastroesophageal reflux disease among employees of a large hospital in northern India. Indian J Gastroenterol. 2011;30:128–34. doi: 10.1007/s12664-010-0065-5. [DOI] [PubMed] [Google Scholar]
- 35.Chari S, Teyssen S, Singer MV. Alcohol and gastric acid secretion in humans. Gut. 1993;34:843–7. doi: 10.1136/gut.34.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman SE, Kaye MD. Induction of gastro-oesophageal reflux by alcohol. Gut. 1978;19:336–8. doi: 10.1136/gut.19.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bor S, Capanoglu D. The additive effect of ethanol and extract of cigarette smoke on rabbit esophagus epithelium. J Gastroenterol Hepatol. 2009;24:316–21. doi: 10.1111/j.1440-1746.2008.05634.x. [DOI] [PubMed] [Google Scholar]
- 38.Anderson LA, Cantwell MM, Watson RG, et al. The association between alcohol and reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma. Gastroenterology. 2009;136:799–805. doi: 10.1053/j.gastro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama T, Inamori M, Iida H, et al. Alcohol consumption is associated with an increased risk of erosive esophagitis and Barrett's epithelium in Japanese men. BMC Gastroenterol. 2008;8:58. doi: 10.1186/1471-230X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penagini R, Allocca M, Cantu P, et al. Relationship between motor function of the proximal stomach and transient lower oesophageal sphincter relaxation after morphine. Gut. 2004;53:1227–31. doi: 10.1136/gut.2003.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]


