Abstract
BACKGROUND
Infliximab is the most widely used biologic agent for Crohn’s disease (CD) and ulcerative colitis (UC), but requires outpatient infusion units due to its intravenous administration requirement.
OBJECTIVES
1) To determine the average non-drug costs associated with each outpatient use of infliximab for pediatric IBD. 2) To determine the proportion of non-drug costs associated with each outpatient infliximab use relative to the total cost of each encounter.
METHODS
Hospital administrative and pharmacy databases were queried for all short stay unit encounters at Lucile Packard Children’s Hospital at Stanford University linked to infliximab infusions for IBD between January 1, 2006 and December 31, 2011. Infliximab drug and non-drug costs associated with CD and UC were compared.
RESULTS
A total of 771 unique encounters were generated for 76 pediatric patients (53 CD, 23 UC). For direct costs related to infliximab infusions for either CD or UC patients, more than 77% of the total health care costs per encounter were related to personnel (e.g., nursing), facility operations, and laboratory costs. Only 23% of the total costs were related to the actual infliximab drug costs. Based on an 80/20 payor mix of managed care vs. government-subsidized insurance payors, 24.5% of the total reimbursements were applied to non-drug costs in CD; 20.9% in UC.
CONCLUSIONS
Non-drug costs represent a substantial proportion of the total cost of outpatient infliximab-related actual costs in IBD. Personnel costs represent the largest segment of the non-drug costs. The actual drug costs of infliximab represent a small proportion of the total costs.
Keywords: Remicade, biologics, costs, infusion units, Crohn’s disease, ulcerative colitis, pediatric
INTRODUCTION
Since the market availability of biologic agents, antibodies targeting tumor necrosis factor (TNF-α) are the latest therapeutic options for patients with Crohn’s disease (CD) and ulcerative colitis (UC). In the last decade, infliximab is demonstrated in numerous multi-center trials to provide beneficial outcomes in patients with CD1,2 and UC3. Although the data for children and adolescents are limited, current literature reported treatment with infliximab was associated with clinical improvement in pediatric patients with inflammatory bowel disease (IBD)4,5.
While the advent of infliximab improved the treatment of pediatric and adult IBD, the increased utilization of biologics focused the attention of payors and policy makers to manage the costs associated with infused biologic therapies. Majority of infliximab infusions are provided in a costly outpatient hospital-based setting due to its intravenous administration requirement. Based on a previously published report of outpatient infliximab infusions, privately insured health-plan paid an average $2793 per infusion and $583 per 100-mg vial of infliximab in 20066. Attempts to reduce costs related to administration resulted in alternative sites of care such as physician offices7 and home infusion pilot programs.8
Although biologics are expensive drugs, medications account for less than 3% of the average overall direct healthcare costs associated with CD.9 In 2008, the annual direct cost of outpatient medications used for the treatment of UC in the United States is estimated at $135310. Due to the increasing trends of biologics use at our center to treat IBD, we hypothesize additional costs due to non-drug expenditures are increasing and significant for infused therapies. To our knowledge, there is no study to date examining the drug and non-drug cost distribution of outpatient infliximab infusions for IBD. Therefore, the primary aims of this study are: 1) to determine the average non-drug costs associated with each outpatient administration of infliximab for pediatric IBD, and 2) to determine the proportion of non-drug costs associated with each outpatient infliximab use relative to the total cost of each encounter.
METHODS
Data Source & Study Population
Using pharmacy records, a data query of all infliximab infusions administered to patients at Lucile Packard Children’s Hospital (LPCH) at Stanford between January 1, 2006 and December 31, 2011 was requested to the Information Services Department. STRIDE Database (Stanford Translational Research Integrated Database) was used in identifying these patient encounters with CD or UC using ICD-9 codes between the range of 555.0 and 556.9. Only infliximab infusions administered to patients with IBD in the outpatient hospital-based setting were included in the final database. Hospital administrative account linked to each patient encounter of interest was accessed for financial data accuracy through the financial billing office. Each patient encounter in the final database was validated for a transaction for infliximab infusion during the study period. A separate financial administrative database generated by the hospital finance office was used to confirm our original data from the billing office. Stanford University Institutional Review Board approved the protocol for this study.
Cost and Database Analysis
In our report, costs are implied to be actual direct costs to LPCH. Examples of actual costs are pharmacy acquisition costs and facility operations cost; this is in contrast to costs related to charges and reimbursements (see Note on Reimbursements). Patient baseline characteristics and infusion related costs were collected. Patient demographic variables included age, gender, race, and insurance type. IBD diagnosis was stratified between CD and UC. Patient records containing both CD and UC ICD-9 codes were individually assessed via chart review to determine one IBD diagnosis, depending on clinical impression documented in patient records or predominance of one particular ICD-9 code for each patient.
RESULTS
Patient Demographics
Table 1 shows the baseline characteristics of the patients represented in the study between January 1, 2006 and December 31, 2011. Our study included 76 unique patients with IBD, representing 53 CD and 23 UC patients with an average age range of 14.4 to 14.9 at the time of patient encounter. Total patient encounters were 555 for CD and 216 for UC. Consistent with racial demographics reported in existing IBD literature, the majority of our patients were categorized as White 78.3 to 81.1%. However, there is significant proportion of Asian patients (11.3 to 13%) in the study, likely due to the larger percentage of Asians in the San Francisco Bay Area compared to other locales of the United States. More than three-fourths of our patients were privately insured at the time of patient encounter.
Table 1.
Patient Demographics
| No. (%) | ||
|---|---|---|
| Crohn’s Disease |
Ulcerative Colitis |
|
| # of encounters | 555 | 216 |
| # of unique patients | 53 | 23 |
| Gender | ||
| Female | 24 (45.2) | 11 (47.8) |
| Male | 29 (54.8) | 12 (52.2) |
| Race | ||
| White | 43 (81.1) | 18 (78.3) |
| Black | 2 (3.8) | 1 (4.3) |
| Asian | 6 (11.3) | 3 (13) |
| Other | 2 (3.8) | 1 (4.3) |
| Age, mean (SD), years | ||
| Initial encounter | 13.8 (3.5) | 13.7 (4.7) |
| Time of study | 14.9 (3.5) | 14.4 (3.7) |
| Insurance coverage | ||
| Private | 42 (79.2) | 18 (78.3) |
| Government subsidized | 11 (20.8) | 4 (17.3) |
| Unknown | 0 | 1 (4.4) |
Annual Outpatient Infliximab Drug Costs
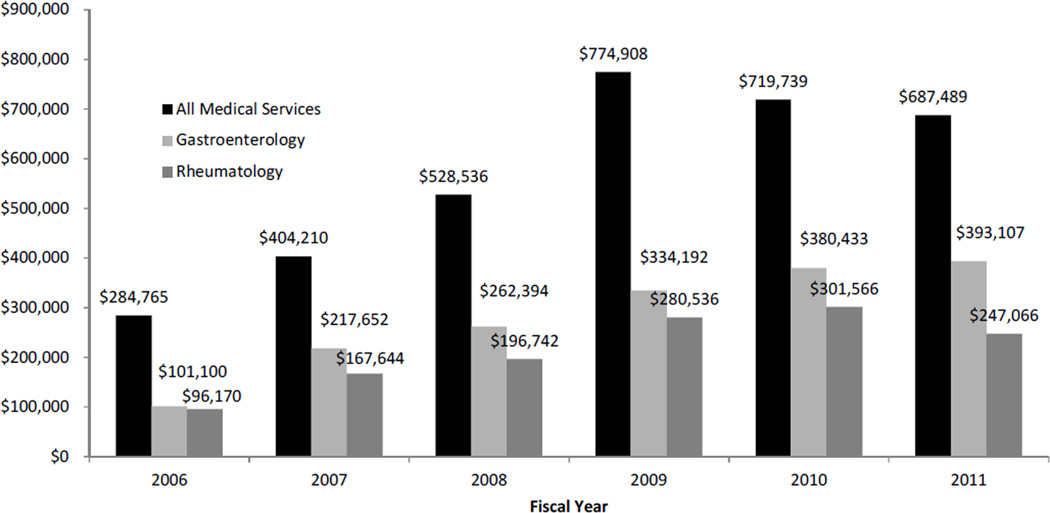
Figure 1 shows the actual outpatient hospital expenditures on infliximab since 2007 at LPCH, comparing outpatient infliximab use by pediatric gastroenterology service, pediatric rheumatology service, and total use by all medical services. Peak cumulative infliximab drug costs were reported in 2009 when direct annual infliximab cost approximately $775,000. Gradual decline was seen in 2010 ($720,000) and 2011 ($688,000). Meanwhile, outpatient gastroenterology infliximab utilization and subsequent drug cost have increased since 2007, with total annual drug cost of approximately $393,000 in 2011. Estimated by drug expenses only, pediatric gastroenterology service is using more than 57% of the total outpatient infliximab at LPCH.
Figure 1. Actual Costs of Infliximab at LPCH.
Annual infliximab expenditure at LPCH during 2006–2011. Estimated by drug expenditure, pediatric gastroenterology and rheumatology comprised majority of infliximab utilization. Total estimated infliximab use by all medical services at LPCH increased since 2007.
Distribution of Costs per Outpatient Infliximab Encounter
Table 2 describes the composition of direct costs related to an average of the 771 infliximab infusions. Cost categories are assigned to 3 groups: 1) pharmacy-related costs, 2) personnel costs, and 3) other overhead costs. Pharmacy-related costs are subdivided as the direct pharmacy acquisition cost of the drug infliximab. Other pharmacy costs include pharmacy costs of materials and processing costs generated during the encounter. Other incidental medications and supplies required during the short stay unit visit include, but not limited to, premedications, epinephrine, topical anesthetics, intravenous starter kits, and saline solutions. Personnel costs comprise of nursing and short stay unit staff costs (e.g., unit scheduler). Other overhead costs include facility costs, maintenance costs, and laboratory processing costs from concomitant serological tests (e.g., complete blood counts, C-reactive protein, liver function tests, etc).
Table 2.
Direct Costs of Outpatient Infliximab Infusions in Pediatric IBD
| IBD Type | Distribution of Costs |
|---|---|
| Crohn’s disease (n=53) | |
| Pharmacy-related | |
| Infliximab drug cost | 22.6% |
| Other pharmacy costs | 23.0% |
| Personnel (i.e., nursing, short stay unit staff) | 31.8% |
| Other overhead (e.g., facilities, maintenance) | 22.6% |
| Non-Drug Cost Per Infusion in CD | 77.4% |
| Ulcerative colitis (n=23) | |
| Pharmacy-related | |
| Infliximab cost | 22.7% |
| Other pharmacy costs | 23.1% |
| Personnel (i.e., nursing, short stay unit staff) | 31.5% |
| Other overhead (e.g., facilities, maintenance) | 22.7% |
| Non-Drug Cost Per Infusion in UC | 77.3% |
Non-drug costs of infliximab
Table 2 shows 77.4% of the total health care costs per CD encounter were related to non-drug costs. Similarly, 77.3% of total costs were attributed to non-drug costs in UC. 22.6% and 22.7% of the total costs in CD and UC, respectively, were assigned to the actual infliximab drug cost. The proportions of drug and non-drug costs were based on an aggregate average of 771 (555 CD and 216 UC) actual costs per infliximab infusion over the 6 years of observation.
Note on Reimbursements
Based on an 80/20 payor-mix of managed care vs. government-subsidized insurance payors, LPCH reported in 2011 that 24.5% of the total reimbursements were applied to non-drug costs in CD; 20.9% in UC. In contrast, when limited to government-subsidized programs and payors, 90.4% of the total reimbursements were applied towards non-drug costs related to patient care.
DISCUSSION
To our knowledge, our investigation is the first report to estimate the non-drug costs associated with outpatient infliximab use in IBD. Our analysis suggests that actual non-drug costs (77.4% of total actual costs per infusion) comprise a majority of the total costs for each outpatient infliximab encounter. Nursing and personnel costs appear to be a substantial proportion of the non-drug costs. Although recent adult data suggest that patients may tolerate a shortened infusion time,11 most patients requiring infliximab still undergo a typical 2 to 3 hour infusion period in the outpatient hospital setting. Our study results may not be fully generalizable if institutional payor-mix is substantially different from LPCH or if there is high variability in nursing and facility costs between institutions. However, hourly compensation for registered nurses – although highest in California – remain relatively uniform between states and hourly rate differences remain small.12 Therefore, based on our findings, we conclude that non-drug costs of infliximab administration for IBD are likely to be greater than the actual drug costs in most medical practices and institutions.
Maximizing cost-efficiency through expanding outpatient services is a commonly adopted goal of health care systems.13,14,15 The growing popularity of short stay units, infusion units, in-home infusion services, and the like represent the general trend to avoid hospitalization for routine maintenance therapies. In gastroenterology, infliximab represents an important drug with implications for a greater role in future standard of care, as the “top-down” approach to biologics use becomes evidence-based practice.16 Our report highlights the need to address how providers should to consider the total cost per encounter – especially in limited resource settings – rather than single drug costs.
Comparative studies between infliximab versus self-injectable biologics and precise qualitative evaluations assessing patient preferences are needed to understand whether the added non-drug costs of intravenous biologics are acceptable to optimize patients’ health and quality-of-life while maintaining cost-efficiency. Furthermore, especially in the pediatric patient population, the indirect costs can be substantial when 3 hour infusions require potential disturbances to daily parents’ work and patients’ school schedules. Subcutaneous adalimumab may represent one therapeutic alternative to minimize non-drug and indirect costs to patients and families, especially considering the latest safety and efficacy evidence of adalimumab in the pediatric CD population.17 However, self injections may introduce additional medication adherence issues compared to outpatient infusion visits, particularly in the pediatric population.
Given the historical trend of previously successful and profitable drugs, patent extensions to maintain “market exclusivity” are common and may be in the foreseeable future for infliximab.18,19 To the patient-advocating clinician, superb IBD care should continue to drive decision-making, but the gravity of the present health care challenges require further thought about the economics of individual medical choices creating downstream societal impact. Based on our experience, we find that reimbursement rates by payors will be of increasing importance. In reference to our above note on reimbursements, our center encounters a negative profit margin each time a patient with government-subsidized insurance receives infliximab – one example among many which will drive future sustainability of certain clinical practices. Our report highlights the need for greater investigation into clinical decisions around pharmaco-economics and transparency in identifying substantial non-drug costs related standard clinical practice in gastroenterology.
Acknowledgments
Grant support:
Authors acknowledge the generous support of Stanford Society of Physician Scholars (SSPS) and funds available from the SSPS Collaborative Research Grant for data collection. KTP is supported by NIH K08 DK094868. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential competing interests:
None
Disclosures
Authors report no conflicts of interest as described by Inflammatory Bowel Diseases
Author contributions:
May Wu, PharmD – planning and conducting the study, collecting data, writing the first draft of the manuscript
Aaron Sin, BS – obtaining funding source, planning and conducting the study, collecting and interpreting data, editing the manuscript
Fred Nishioka, PharmD – planning the study, interpreting data, editing the manuscript
KT Park, MD, MS – obtaining funding source, planning and conducting the study, collecting and interpreting data, and drafting/editing the manuscript
All authors approve the final draft submitted.
References
- 1.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002 May 4;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 2.Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004 Feb 26;350(9):876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005 Dec 8;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 4.Hyams JS, Markowitz J, Wyllie R. Use of infliximab in the treatment of Crohn's disease in children and adolescents. J Pediatr. 2000 Aug;137(2):192–196. doi: 10.1067/mpd.2000.107161. [DOI] [PubMed] [Google Scholar]
- 5.Serrano MS, Schmidt-Sommerfeld E, Kilbaugh TJ, Brown RF, Udall JN, Jr, Mannick EE. Use of infliximab in pediatric patients with inflammatory bowel disease. Ann Pharmacother. 2001 Jul-Aug;35(7–8):823–828. doi: 10.1345/aph.10395. [DOI] [PubMed] [Google Scholar]
- 6.Ollendorf DA, Lidsky L. Infliximab drug and infusion costs among patients with Crohn's disease in a commercially-insured setting. Am J Ther. 2006 Nov-Dec;13(6):502–506. doi: 10.1097/01.mjt.0000245223.43783.45. [DOI] [PubMed] [Google Scholar]
- 7.Johnson R, Freeman EN. Addressing costs and continuity of care through innovative solutions for infused therapies: A collaborative experience with infliximab. American health & drug benefits. 2011;4(1):39. [PMC free article] [PubMed] [Google Scholar]
- 8.Condino AA, Fidanza S, Hoffenberg EJ. A home infliximab infusion program. J Pediatr Gastroenterol Nutr. 2005 Jan;40(1):67–69. doi: 10.1097/00005176-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Vreeland MG, Larson LR, Bala MV. Annual cost of care for Crohn’s Disease: A payor perspective. Am J Gastroenterol. 2000;95:1955–1960. doi: 10.1111/j.1572-0241.2000.02261.x. [DOI] [PubMed] [Google Scholar]
- 10.Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA, Finkelstein JA. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology. 2008 Dec;135(6):1907–1913. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breynaert C, Ferrante M, Fidder H, Van Steen K, Noman M, Ballet V, Vermeire S, Rutgeerts P, Van Assche G. Tolerability of shortened infliximab infusion times in patients with inflammatory bowel diseases: a single-center cohort study. Am J Gastroenterol. 2011 Apr;106(4):778–785. doi: 10.1038/ajg.2011.61. Epub 2011 Mar 15. [DOI] [PubMed] [Google Scholar]
- 12. http://www.cbsalary.com/salaries/RN/
- 13.Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010 Jun 2;303(21):2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak LJ, Owings MF, Hall MJ. National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2005 Mar;(158):1–199. [PubMed] [Google Scholar]
- 15.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010 Aug;126(2):204–213. doi: 10.1542/peds.2009-3109. Epub 2010 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010 Apr 15;362(15):1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 17.Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA, Jr, Colletti RB, Dubinsky M, Kierkus J, Rosh J, Wang Y, Huang B, Bittle B, Marshall M, Lazar A. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012 Aug;143(2):365.e2–374.e2. doi: 10.1053/j.gastro.2012.04.046. Epub 2012 May 2. [DOI] [PubMed] [Google Scholar]
- 18.Psaty BM, Redberg RF. Evidence of Pharmaceutical Innovation and Therapeutic Enthusiasm: Strategies for Patent Extension. Arch Intern Med. 2012 Apr 9; doi: 10.1001/archinternmed.2012.382. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Carrier MA. Unsettling drug patent settlements: a framework for presumptive illegality. Mich Law Rev. 2009 Oct;108(1):37–80. [PubMed] [Google Scholar]