Metal complexes constitute an attractive class of compounds for applications in medicinal chemistry and chemical biology due to features such as unusual reactivities, tunable ligand exchange kinetics, stereochemical diversity, structural complexity, the availability of radioisotopes, and distinct physicochemical properties.[1] However, it is remarkable that although the discovery of the antiproliferative activity of cis-[PtCl2(NH3)2] dates back almost half a century,[2] cisplatin and its derivatives remain the most important metal-based anticancer drugs used in the clinic at the present day.[3] In the quest for a new generation of metal-containing anticancer agents we here wish to report a class of rhenium(I) complexes as highly potent visible-light-triggered anticancer organometallics.
We discovered that rhenium(I) indolato complexes 1-3 provoke a strong light-induced antiproliferative activity in cancer cells (Figure 1). This is unexpected since the related class of rhenium(I) tricarbonyl polypyridine complexes (e.g. 4) are well-established nontoxic luminescent probes routinely applied for biological imaging.[4] Thus, replacing just the 2,2′-bipyridine in 4 for 2-(2′-pyridyl)indolato and its derivatives (1-3) completely changes the physicochemical and biological properties of such rhenium(I) complexes by abolishing luminescence and instead causing light-induced anticancer activity.[5]
Figure 1.
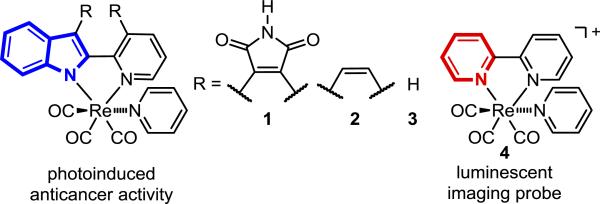
From luminescent to light-induced anticancer Re complexes. See Supporting Information for the synthesis of the new complexes 1-3.
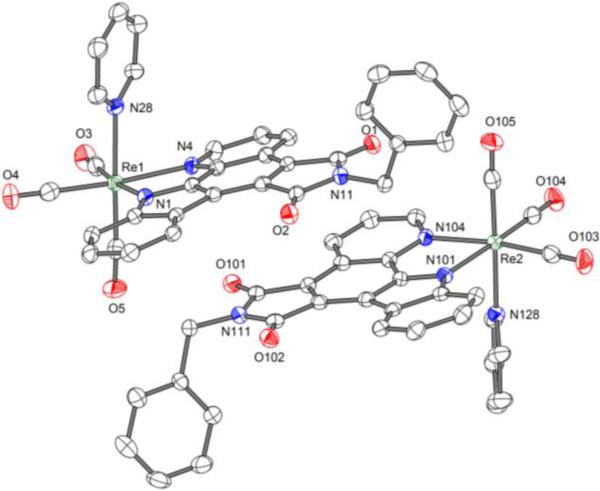
Figure 2 displays the structure of a benzylated derivative of rhenium(I) complex 1 in which rhenium is coordinated in a bidentate fashion to a pyrido[2,3-a]pyrrolo[3,4-c]carbazole-5,7(6H)-dione[6] ligand through a deprotonated indole, in addition to three CO and one pyridine ligand.
Figure 2.
Crystal structure of the N-benzylated derivative of Re complex 1. ORTEP drawing with 50 % probability thermal ellipsoids. Solvent is omitted for clarity.
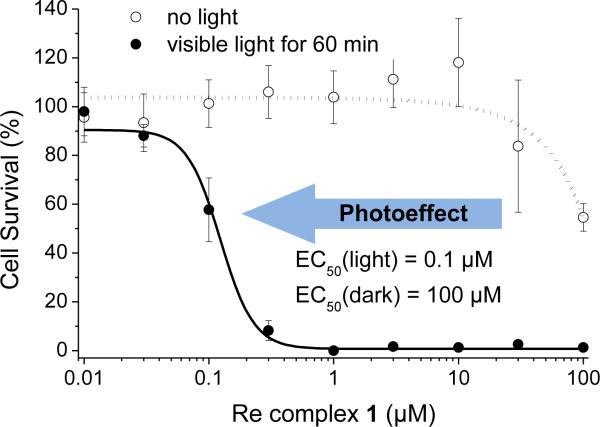
Astonishingly, whereas in the dark complex 1 does not exert any significant cytotoxicity in HeLa cancer cells with an EC50 (half maximum effective concentration) value of around 100 μm, when irradiated with visible light (λ ≥ 505 nm) the 50 % cellular survival dropped to 100 nm, representing a remarkable light effect by three orders of magnitude (Figure 3). This light-triggered antiproliferative activity is due to an induction of apoptosis as revealed by the activation of the executioner caspases 3 and 7 and an increasing number of cells in the sub-G1 phase upon green light exposure in the presence of 1 (see Supporting Information).
Figure 3.
Visible-light-induced antiproliferative activity of rhenium complex 1 in HeLa cancer cells. Experimental details: 1 h after the addition of 1, cells were irradiated for 60 min with λ ≥ 505 nm. The cytotoxicity was determined after additional 22 h by MTT assay. Standard deviations result from two independent experiments with overall 18 data points for each compound concentration.
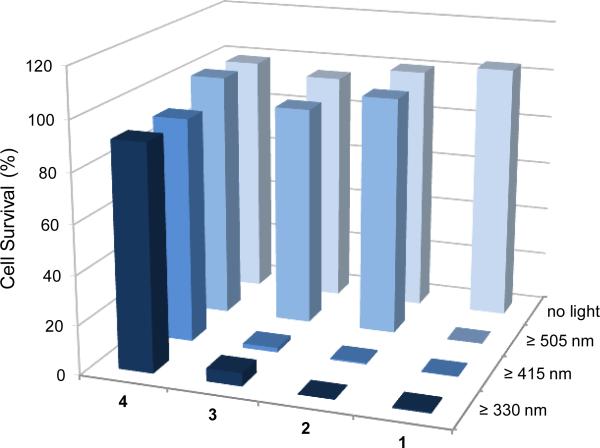
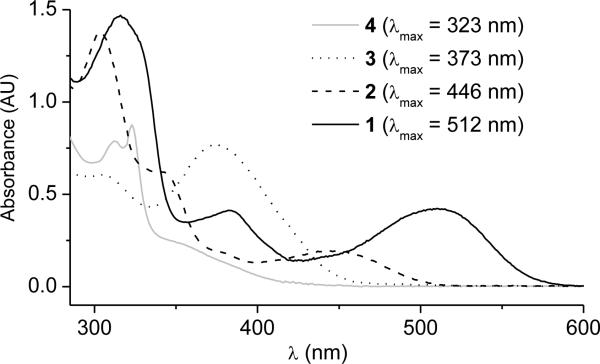
The wavelength-dependent bioactivity of complexes 1-4 is shown in Figure 4. Whereas the luminescent 2,2′-bipyridine complex 4 does not display any photoinduced cytotoxicity even under exposure to UV-light (λ ≥ 330 nm), complexes 2 and 3 induce cell death upon irradiation with UV and blue light, and complex 1 is even capable of activating cell death with green light (λ ≥ 505 nm). Thus, the stepwise expansion of the pyridocarbazole chromophore from complex 3 to 1 shifts the light-induced antiproliferative activity to longer wavelengths which is desired for phototherapy because it allows a deeper tumor penetration. This trend parallels the longest wavelength absorption band of the complexes 1-4 as shown in Figure 5.
Figure 4.
Wavelength-dependent photoactivity of Re complexes 1-4 in HeLa cells. Complexes (1 μm) were incubated for 1 h before irradiation (15 min for λ ≥ 330 nm, 60 min for λ ≥ 415 nm and λ ≥ 505 nm) and 24 h after compound administration cell survival was determined by the MTT assay. The presented data are based on the average of 18 measurements.
Figure 5.
UV/Vis-absorbance of Re complexes 1-4 measured in DMSO (60 μm).
To gain insight into the mechanism of action of these novel photoactivatable rhenium organometallics, we investigated changes in the cellular morphology upon exposure to complex 1 in the presence of visible light. Accordingly, HeLa cells were preincubated with 1 at 1 μm and thereafter irradiated with a standard LED light source (7 W) for 15 min. As documented by fluorescence and phase contrast microscopy in Figure 6, a very rapid response of the cells to phototherapy was observed. Already half an hour after exposure to visible light, a pronounced blebbing of the plasma membrane occurred followed by the formation of large blebs after an additional hour. Interestingly, a similar surface remodeling with an appearance of blebs on the outer membrane was reported by Moan et al. after the treatment of NHIK 3025 cancer cells with the well-established singlet oxygen photosensitizer haematoporphyrin.[7]
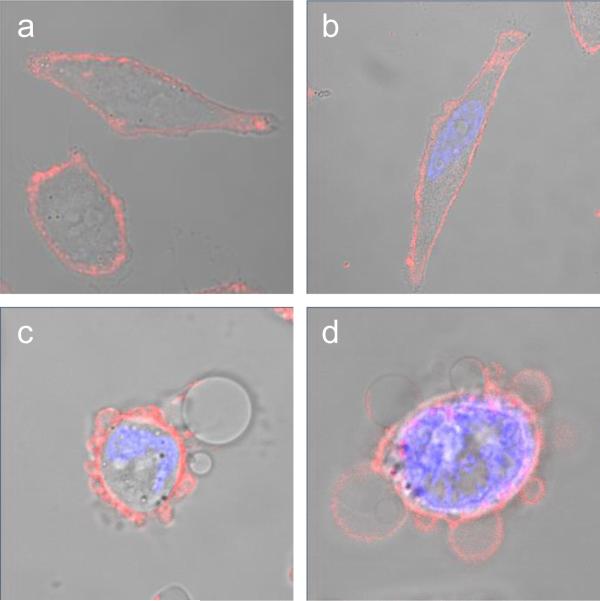
Figure 6.
Morphological changes of HeLa cells upon exposure to visible light in the presence of organorhenium complex 1. HeLa cells were incubated with 1 (1 μm) for 1 h before irradiation with a 7 W LED light source (15 min). a) 1 h after administration of 1; b) 60 min after irradiation without 1; c) 30 min and d) 120 min after irradiation in the presence of 1. To highlight alterations of the cell membrane (red fluorescence) and the nuclei (blue fluorescence), cells were stained with the fluorescent dyes Alexa Fluor 594 wheat germ agglutinin and 4′,6-diamidino-2-phenylindole. Show are overlays of fluorescence and phase contrast images taken with a 63× lens setting.
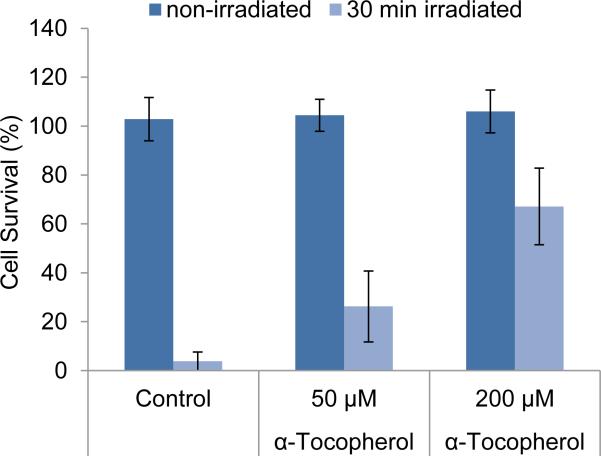
Since the microscopy study suggested that the organorhenium complex 1 might induce apoptosis through the production of singlet oxygen, we determined the light-induced formation of singlet oxygen with complexes 1-4 and, revealingly, found a correlation between the irradiation-dependent production of singlet oxygen of the rhenium complexes and their photocytotoxicities (Figure 7). We next reasoned that if indeed singlet oxygen is responsible for the induction of apoptosis, an antioxidant should suppress the light-induced cell death. This is in fact the case as shown in Figure 8. Preincubation of HeLa cells with the membrane-located natural antioxidant α-tocopherol (vitamin E)[8] ahead of the administration of complex 1 (1 μm) and light exposure, resulted in a dose-dependent increase of cell survival with reaching 26 ± 15 % at 50 μm and 67 ± 16 % at 200 μm α-tocopherol compared to a cell survival of just 4 ± 4 % in the absence of the antioxidant (Figure 8).
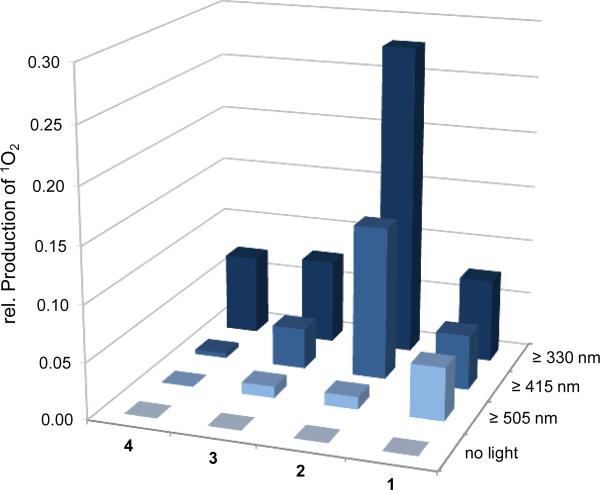
Figure 7.
Singlet oxygen production of rhenium complexes 1-4 (50 μm) in PBS/DMSO 1:1. Singlet oxygen was determined according to a method by Kraljić and El Mohsni (ref. 9) using the redox-sensitive dye p-nitrosodimethylaniline (50 μm) in the presence of imidazole in the dark and after 30 min irradiation with cut-on filters of different wavelengths. The bars indicate the average values of six data points.
Figure 8.
Influence of α-tocopherol on the photoinduced cytotoxic effect of complex 1. HeLa cells were cultured in the presence of α-tocopherol for 24 h and afterwards treated with complex 1 (1 μm) for 1 h followed by irradiation with λ ≥ 505 nm for 30 min. Cell survival was determined the next day by the MTT method. Error bars indicate standard deviations of 18 data points determined in two independent experiments.
Taken together, these experiments not only explain the photocytotoxicity of rhenium complex 1 but also indicate a location inside the membrane. In this case, lipid peroxidation and/or other types of oxidative cell membrane damage constitute a probable explanation for the observed cellular effects.[10] This assumption is supported by the absent photocytotoxicity of complex 4 despite its ability to produce singlet oxygen upon exposure to UV-light because its positive charge will prevent a localization at the plasma membrane (Figures 4 and 7). Finally this emphasizes the importance of the neutral character of complexes 1-3 for the photocytotoxic effect. In fact, membrane localization is a common feature of the most successful photosensitizers used in photodynamic therapy (PDT).[11] The advantage of membrane localization is a very rapid and direct activation of apoptosis without the need for intermediate signal transduction pathways that might be inactivated in certain neoplastic cells.
Since one promising field of application in photodynamic therapy is the treatment of skin cancer, we finally examined the light induced antiproliferative effect of complex 1 in melanoma spheroids.[12] Accordingly, 1 (5 μm) was incubated with the spheroids for 1 h before irradiation. To increase the effect, irradiation was repeated on the next day and images were taken 24 h after the second stimulation. The results shown in Figure 9 exhibit that the photocytotoxicity already demonstrated in 2D culture experiments is maintained in 3-dimensional organized cells, as indicated by an increase in dead cells (red fluorescence) and loss of spheroid integrity on the edges.
Figure 9.
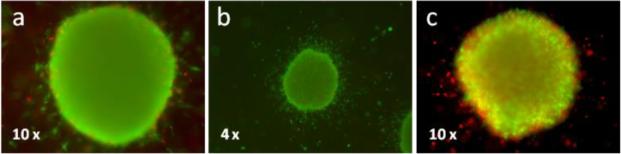
Photodynamic treatment of 1205Lu melanoma spheroids embedded in collagen with 5 μm of complex 1. Complex 1 was added to the spheroids 1 h before irradiation with a 7 W LED light source for a duration of 60 min. After one day, irradiation was repeated and another 24 h later spheroids were stained for live (green fluorescence) and dead (red fluorescence) cells, documented by fluorescence microscopy (4× or 10× lens setting). a) Irradiated DMSO control; b) 5 μm 1, no irradiation; c) 5 μm 1, irradiated.
Obvious advantages of photodynamic therapy, such as a spatially controlled cytotoxicity and a low risk of resistance development, have made it an attractive alternative to conventional chemotherapy during the last two decades.[13] With this study we introduced a novel class of photosensitizers quite different to the well established scaffolds based on porphyrins, porphycenes or phthalocyanines but still fulfilling the main criteria suitable for photodynamic therapy, which include the light-dependant generation of singlet oxygen, no cytotoxicity in the dark, stability under physiological conditions, and an excitation wavelength in the upper range of visible light. According to our knowledge, these are the first examples of rhenium complexes with a proven in vitro light-induced anticancer activity.[14,15] Finally, it is worth noting that the here disclosed visible-light-induced anticancer rhenium(I) complex 1 is based on a metallo-pyridocarbazole scaffold previously used for the design of metal-containing protein kinase inhibitors.[16] Work is ongoing in our laboratory to combine photodynamic therapy activity with the inhibition of protein kinases involved in cancer, thus creating complementary, dual anticancer activity in a single compound.[17]
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (CA114046).
Footnotes
Supporting information for this article is available on the WWW under http://www.chemmedchem.org or from the author.
References
- 1.Metal complexes for medicine and the life sciences: Schatzschneider U, Metzler-Nolte N. Angew. Chem. 2006;118:1534–1537. doi: 10.1002/anie.200504604.Angew. Chem. Int. Ed. 2006;45:1504–1507. doi: 10.1002/anie.200504604.Mukherjee A, Sadler PJ. Wiley Encycl. Chem. Biol. 2009:1–47.Haas KL, Franz KJ. Chem. Rev. 2009;109:4921–4960. doi: 10.1021/cr900134a.Meggers E. Chem. Commun. 2009:1001–1010. doi: 10.1039/b813568a.Che C-M, Siu F-M. Curr. Opin. Chem. Biol. 2010;14:255–261. doi: 10.1016/j.cbpa.2009.11.015.Hillard EA, Jaouen G. Organometallics. 2011;30:20–27.Gasser G, Ott I, Metzler-Nolte N. J. Med. Chem. 2011;54:3–25. doi: 10.1021/jm100020w.Lo KK-W, Choi AW-T, Law WH-T. Dalton Trans. 2012;41:6021–6047. doi: 10.1039/c2dt11892k.Ma D-L, Ma VP-Y, Chan DS-H, Leung K-H, He H-Z, Leung C-H. Coord. Chem. Rev. 2012;256:3087–3113.Sasmal PK, Streu CN, Meggers E. Chem. Commun. 2013;49:1581–1587. doi: 10.1039/c2cc37832a.
- 2.Rosenberg B, van Camp L, Krigas T. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 3.Kelland L. Nature Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 4.a Lo KK-W, Louie M-W, Zhang KY. Coord. Chem. Rev. 2010;254:2603–2622. [Google Scholar]; b Lo KK-W, Zhang KY, Li SP-Y. Eur. J. Inorg. Chem. 2011:3551–3568. [Google Scholar]
- 5.Reviews on metal complexes with photoactivated biological activities: Farrer NJ, Salassa L, Sadler PJ. Dalton Trans. 2009:10690–10701. doi: 10.1039/b917753a.Schatzschneider U. Eur. J. Inorg. Chem. 2010:1451–1467.Crespy D, Landfester K, Schubert US, Schiller A. Chem. Commun. 2010;46:6651–6662. doi: 10.1039/c0cc01887b.Schatzschneider U. Inorg. Chim. Acta. 2011;374:19–23.
- 6.a Bregman H, Williams DS, Atilla GE, Carroll PJ, Meggers E. J. Am. Chem. Soc. 2004;126:13594–13595. doi: 10.1021/ja046049c. [DOI] [PubMed] [Google Scholar]; b Bregman H, Williams DS, Meggers E. Synthesis. 2005:1521–1527. [Google Scholar]
- 7.Moan J, Pettersen EO, Christensen T. Br. J. Cancer. 1979;39:398–407. doi: 10.1038/bjc.1979.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudière J, Ferrari-Iliou R. Food Chem. Toxicol. 1999;37:949–962. doi: 10.1016/s0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 9.Singlet oxygen determination: Kraljić I, El Mohsni S. Photochem. Photobiol. 1978;28:577–581.
- 10.No visible-light-dependent disintegration of complex 1 occurs as monitored by 1H-NMR (see Supporting Information).
- 11.Photodynamic therapy: Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. J. Natl. Cancer. Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889.
- 12.Smalley KSM, Lioni M, Noma K, Haass NK, Herlyn M. Exp. Opin. Drug Discov. 2008;3:1–10. doi: 10.1517/17460441.3.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Photodynamic therapy for cancer: Dolmans DEJGJ, Fukumura D, Jain RK. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071.
- 14.For a report on a rhenium(I) complex with DNA photocleavage properties, see: Yam VW-W, Lo KK-W, Cheung K-K, Kong RY-C. J. Chem. Soc., Dalton Trans. 1997:2067–2072.
- 15.For a report on a biocompatible photoactivated CO-releasing rhenium(I) complex, see: Pierri AE, Pallaoro A, Wu G, Ford PC. J. Am. Chem. Soc. 2012;134:18197–18200. doi: 10.1021/ja3084434.
- 16.For recent examples, see: Blanck S, Maksimoska J, Baumeister J, Harms K, Marmorstein R, Meggers E. Angew. Chem. 2012;124:5335–5338. doi: 10.1002/anie.201108865.Angew. Chem. Int. Ed. 2012;51:5244–5246. doi: 10.1002/anie.201108865.Feng L, Geisselbrecht Y, Blanck S, Wilbuer A, Atilla-Gokcumen GE, Filippakopoulos P, Kräling K, Celik MA, Harms K, Maksimoska J, Marmorstein R, Frenking G, Knapp S, Essen L-O, Meggers E. J. Am. Chem. Soc. 2011;133:5976–5986. doi: 10.1021/ja1112996.
- 17.Kastl A, Wilbuer A, Merkel AL, Feng L, Di Fazio P, Ocker M, Meggers E. Chem. Commun. 2012;48:1863–1865. doi: 10.1039/c1cc15378a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.