Abstract
Background & Aims
ZBP-89 (also ZNF148 or Zfp148) is a butyrate-inducible zinc finger transcription factor that binds to GC-rich DNA elements. Deletion of the N-terminal domain is sufficient to increase mucosal susceptibility to chemical injury and inflammation. We investigated whether conditional deletion of ZBP-89 from the intestinal and colonic epithelium of mice increases their susceptibility to pathogens such as Salmonella typhimurium.
Methods
We generated mice with a conditional null allele of Zfp148 (ZBP-89FL/FL), using homologous recombination to flank Zfp148 with LoxP sites (ZBP-89FL/FL), and then breeding the resulting mice with those that express VillinCre. We used microarray analysis to compare gene expression patterns in colonic mucosa between ZBP-89FL/FL and C57BL/6 wild-type mice (controls). Mice were gavaged with 2 isogenic strains of S typhimurium after administration of streptomycin.
Results
Microarray analysis revealed that the colonic mucosa of ZBP-89FL/FL mice had reduced levels of tryptophan hydroxylase 1 (Tph1) mRNA, encoding the rate-limiting enzyme in enterochromaffin cell serotonin (5HT) biosynthesis. DNA affinity precipitation demonstrated direct binding of ZBP-89 to the mouse Tph1 promoter, which was required for its basal and butyrate-inducible expression. ZBP-89FL/FL mice did not increase mucosal levels of 5HT in response to S typhimurium infection and succumbed to the infection 2 days before control mice. The ΔhilA isogenic mutant of S typhimurium lacks this butyrate-regulated locus and stimulated, rather than suppressed, expression of Tph1 approximately 50-fold in control, but not ZBP-89FL/FL mice, correlating with fecal levels of butyrate.
Conclusions
ZBP-89 is required for butyrate-induced expression of the Tph1 gene and subsequent production of 5HT in response to bacterial infection in mice. Reductions in epithelial ZBP-89 increase susceptibility to colitis and sepsis following infection with S typhimurium, partly due to reduced induction of 5HT production in response to butyrate and decreased secretion of anti-microbial peptides.
Keywords: butyrate, 5HT, cryptdins, ΔhilA
Introduction
ZBP-89 (ZNF148, Zfp148) is a zinc finger transcription factor induced by the short chain fatty acid (SCFA) butyrate 1–3. Butyrate also induces the interaction of ZBP-89 with the tumor suppressor ataxia telangiectasia mutated and this protein-protein interaction is important in mucosal protection during dextran sulfate-induced colitis 4. Commensal bacterial species, e.g., Bifidobacteria and Lactobacillus, generate SCFAs (acetate, butyrate, propionate) from dietary fiber 5, 6. Although several beneficial effects are attributed to SCFAs in the colon, the most prominent effect ascribed to butyrate is its ability to inhibit histone deacetylases (HDACi) 7. Consistent with its HDACi activity, butyrate maintains the differentiated state of the colon and is a potent anti-inflammatory and anti-cancer agent 8, 9. Several studies have shown that butyrate stimulates the production of antimicrobial peptides and that this function contributes to the elimination of bacterial pathogens 10–12.
The food industry has effectively applied the medicinal effects of SCFAs by providing butyrate-laced feed to livestock to reduce Salmonella colonization and ostensibly food born illnesses 13, 14. In addition to stimulation of the host innate immune system 15–17, SCFAs inhibit genes within the Salmonella Pathogenicity Island 1 (SPI-1) that regulate bacterial invasion, e.g., hyperinvasion locus hilA 18–20. Moreover, there is evidence that specifically butyrate suppresses expression of the SPI-1 gene locus 19, 21.
Pluripotent stem cell progenitors differentiate into enterocytes, goblet cells, hormone-producing enteroendocrine cells (EECs) and Paneth cells, which synthesize antimicrobial peptides such as defensins 22. IECs express Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns (PAMPs), and participate in mucosal defense 23, 24. Like IECs, enteroendocrine cells of both the small and large intestine express TLRs 25,26. Specifically, the enteroendocrine cell line STC-1 expresses 5-hydroxytryptamine (5HT, serotonin) and the same spectrum of TLRs as primary enteroendocrine cells 25,27. Moreover, bacterial flagellin and CpG DNA motifs induce hormone secretion from STC-1 cells through TLR activation 27.
Salmonella enterica serovar Typhimurium (S. typhimurium) is the most common bacterial cause of food borne gastroenteritis 28. Symptoms of nausea, vomiting, diarrhea and crampy abdominal pain characteristic of pathogen-induced 5HT release appear within 8 hours of ingesting contaminated food or water 29, 30. 5HT is the primary bioamine secreted from gut enterochromaffin (EC) cells 31. When released from EC cells, platelets, mast cells and neurons, 5HT stimulates fluid secretion from enterocytes and activates submucosal ganglia to stimulate peristalsis and flush the GI tract of ingested pathogens. Residual 5HT secretion is a probable cause of post-infectious irritable bowel syndrome (IBS) following Salmonella gastroenteritis 32, 33.
We report here the generation of a mouse conditionally null for ZBP-89 in the intestine. A microarray analysis was used to identify genes modulated in the colon after ZBP-89 gene deletion and revealed suppression of tryptophan hydroxylase1 (Tph1) mRNA, which encodes the rate-limiting enzyme in 5HT biosynthesis. Therefore, we tested the hypothesis that ZBP-89 is essential for an effective innate mucosal defense against colonic pathogens such as S. typhimurium.
Methods and Materials
Targeting Vector
The mouse ZBP-89 locus (Zfp148) spans a genomic distance of ~130 kb preventing efficient deletion of the entire locus. Exons 8 and 9 encode greater than 60% of the protein coding domain and when deleted causes mRNA instability and subsequently an absence of ZBP-89 protein 34. High fidelity long range PCR was used to amplify the targeting arms from a mouse BAC clone #452J12 generated from a 129SvJ library (Incyte, Wilmington, DE) then sequenced and assembled into plasmid pBmZBP-89 (Genomatix, Cincinnati, OH). The left targeting arm consisted of the distal 3.1 kb of intron 7. The right targeting arm contained 5.6 kb of the 3′ untranslated region. Using the Vector pLM10-iKO-FinalTV, the neomycin resistance cassette driven by the phosphotransferase gene (PGK) promoter was in the reverse orientation and flanked by FRT sites. A single LoxP site was inserted into intron 7 upstream of the neomycin cassette and the second LoxP site was placed downstream of the poly A termination signal located within exon 9.
Generation of ZBP-89ΔInt mice
The University of Michigan Animal Care and Use Committee, which maintains an American Association of Assessment and Accreditation of Laboratory Animal Care (AAALAC) facility, approved all procedures used in this study. The University of Michigan Transgenic Animal Model Core electroporated Bruce4 (C57BL/6 derived) embryonic stem (ES) cells with the pLM10-iKO-FinalTV targeting vector (Intrexon Corporation, Blacksburg, VA). G418 was used to select ES cells expressing the targeting vector. Homologous recombination occurred in 18 of 480 ES clones (3.75% success rate). All animals were housed in microisolater polycarbonate cages under SPF conditions. The neomycin cassette was removed by breeding the F1 mice to the Flpe deleter mouse strain in which the Flpe recombinase was expressed from the human beta actin promoter (Jackson Laboratory, Bar Harbor, ME) 35, 36. Mice expressing the targeted ZBP-89 locus with flanking LoxP sites (ZBP-89FL/FL) were maintained on the C57BL/6 genetic background. The mouse colons analyzed from the ZBP-89FL/FL x VillinCre (from D. Gumucio 37) cross were designated ZBP-89ΔInt for deleted in the small and large intestinal mucosa.
Microarray Analysis
Mucosa was scraped from the colons of WT and ZBP-89ΔInt mice. Total RNA was prepared using TRizol followed by RNA clean-up using the RNEasy Microkit (Qiagen, Valencia, CA), and the quality was assessed on an Agilent nucleic acid analyzer using the Mouse Genome 430 2.0 Perfect Match Peg Array (Affymetrix). The Microarray Core Facility at the University of Michigan performed the gene chip analysis.
Statistics
Data were analyzed by unpaired 2-tailed Student’s t test, non-parametric Mann-Whitney U test (quantitative cultures), or ANOVA for multiple comparisons. For survival cures, the log-rank/Mantel-Cox test was used to determine significance. Results were presented as means ± SEM using GraphPad Prism version 5 (San Diego, CA). P-values of < 0.05 were considered significant.
Results
Characterization of ZBP-89 null allele in the colon
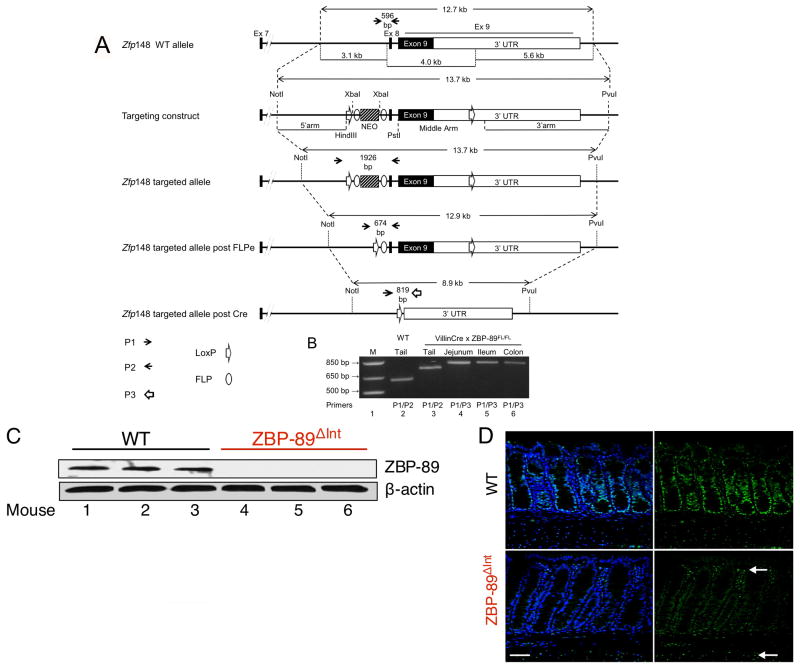
ZBP-89 is a ubiquitous transcription factor regulated by butyrate that inhibits cell growth when ectopically expressed in cells lines 1, 3, 38. However, the in vivo function of ZBP-89 has been difficult to study since homozygous disruption of the locus in mice was previously shown to be non-viable 34. Therefore, we generated a floxed ZBP-89 allele with the goal of generating a conditional knockout of the gene in intestinal and colonic mucosa when bred to the VillinCre-expressing mouse line (Figure 1A,B). We focused on the colon due to our prior study implicating ZBP-89 in colonic mucosal protection 4. A Western blot confirmed reduced levels of ZBP-89 protein in the homozygous conditionally null colons (ZBP-89ΔInt, Figure 1C). ZBP-89 protein expression in WT colons was ubiquitous and located in the nuclei of epithelial, lamina propria and smooth muscle cells (Figure 1D). Since VillinCre expression is exclusively epithelial, there was residual staining of the lamina propria and smooth muscle cells in the conditional null colons (ZBP-89ΔInt) (Figure 1D), which is consistent with the known expression of ZBP-89 in T lymphocytes, myeloid and smooth muscle cells 39.
Figure 1. Conditional deletion of locus encoding transcription factor Zfp148.
(A) Schematic representation of mouse ZBP-89 locus (Zfp148) and strategy for gene targeting. Intestine specific deletion was achieved by breeding to the VillinCre line (ZBP-89ΔInt). (B) PCR Genotyping. Lane 1= DNA markers, lane 2 = wild-type (+/+) tail DNA, lane 3 = targeted locus, lanes 4–6, intestinal mucosa where Cre is active. ZBP-89 expression in the colon of WT and ZBP-89ΔInt mice detected by (C) Western blot of ZBP-89 normalized to β-actin from after colonic epithelium separated by chelation. (D) Immunofluorescence. FITC label = ZBP-89, DAPI = nuclei. Arrows indicate residual ZBP-89 nuclear staining in the lamina propria/smooth muscle layer.
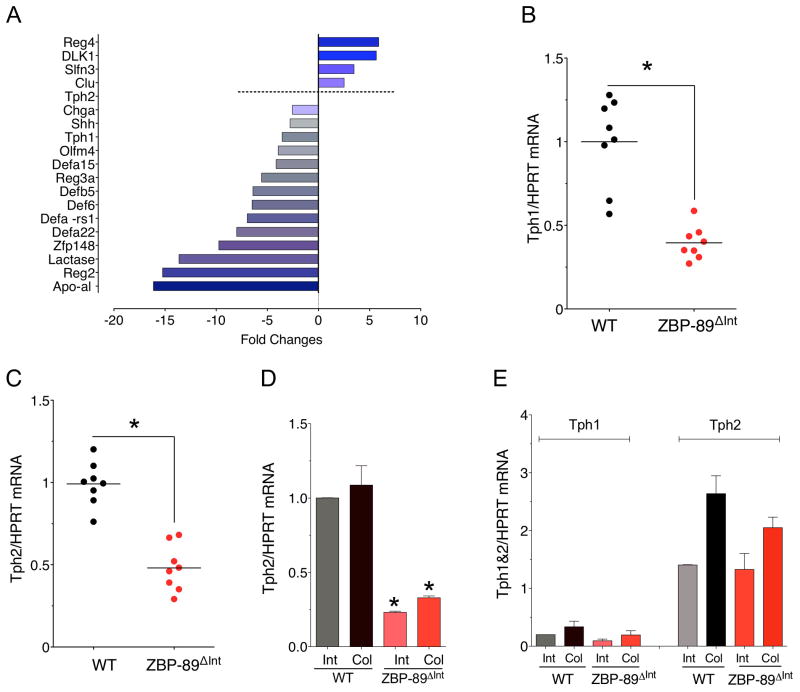
Despite significant reduction of ZBP-89 in the epithelium, there was no spontaneous phenotype in either the small intestine or colon. Therefore we performed a genome-wide analysis of the colons from these mice (Figure 2A). Of interest was the decrease in Tph1, defensin 5 (Defcr5), defensin 22 (Defcr22) and chromogranin A (ChgA) on the microarray that was confirmed by RT-qPCR (Figure 2B). Although we did not observe a change in Tph2 on the microarray, we also measured the mRNA levels of this Tph isoform in the total colon extracts (Figure 2C) and extracts in which the mucosa was separated from the mesenchyme (Figure 2D,E). AlthoughTph2 mRNA is generally expressed in neural tissues 50, apparently some Tph2 is expressed in the epithelium and was reduced in the ZBP-89ΔInt mice (Figure 2D, E).
Figure 2. Microarray analysis of ZBP-89ΔInt mice.
(A) Mean relative expression (N=2 mice) of several genes in the ZBP-89ΔInt versus WT mice. (B) RT-qPCR for Tph1 mRNA and (C) for Tph2 mRNA. (D) Tph2 mRNA in isolated epithelium. (E) Tph1 versus Tph2 in isolated mesenchymal/smooth muscle layer. Shown is the mean ± SEM for N = 5. *P < 0.05.
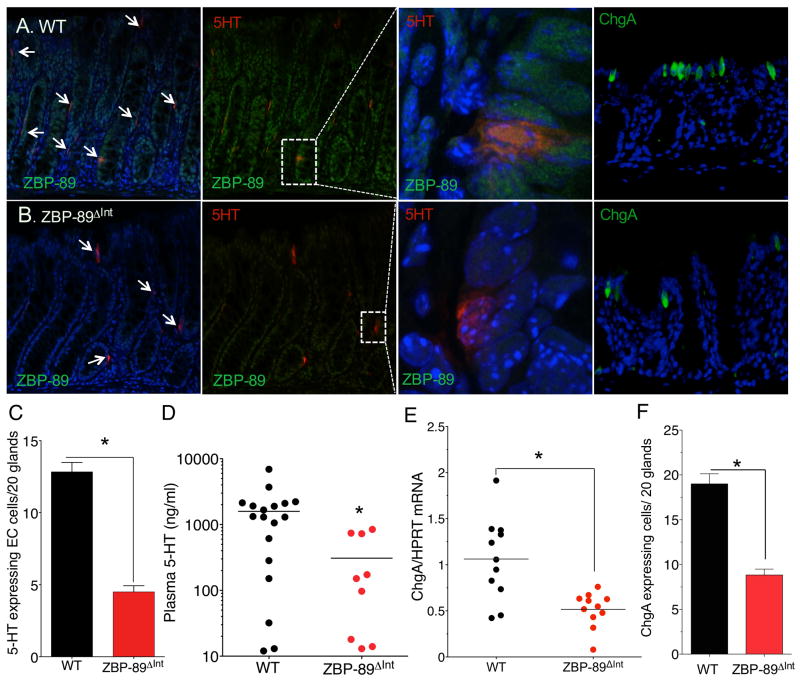
Tph1 is expressed in a subset of enteroendocrine cells that also express ChgA 40. Therefore, we used confocal microscopy analysis to confirm that ZBP-89 protein co-localizes to colonic 5HT-expressing EC cells (Figure 3A,B). Enumerating the number of EC cells in the colons of WT and ZBP-89ΔInt mice revealed a greater than 50% reduction in the number of 5HT-staining cells (Figure 3C). Moreover, plasma 5HT levels were significantly reduced in these mice (Figure 3D). ChgA protein is a neuroendocrine marker whose expression was also reduced in the ZBP-89ΔInt mice (Figure 3E,F). Thus we concluded that ZBP-89 expression modulates Tph1 and Tph2 gene expression consistent with reduced 5HT in the circulation. However since ChgA levels were also reduced, the effect was likely due to an overall decrease in the enteroendocrine cell lineage, suggesting that ZBP-89 contributes to EC cell development.
Figure 3. Reduced 5HT+ cells in ZBP-89ΔInt mice.
(A) Immunofluorescent staining for 5HT (red), and ZBP-89 (FITC, confocal) or ChgA (FITC) with DAPI (blue) in WT and (B) ZBP-89ΔInt mice. Low power merged views on left, (Scale bar, 100 μm). High power view of ZBP-89 with 5HT (600x) (confocal image) or ChgA with DAPI (400x). (C) Morphometric analysis of 5HT-expressing cells, N= 12 mice; (D) Plasma 5HT (ng/ml); (E) ChgA mRNA and (F) Morphometric analysis of ChgA-expressing cells in WT and ZBP-89ΔInt mouse colons, N = 8 mice. *P < 0.05.
Tph1 is a direct target of transcription factor ZBP-89
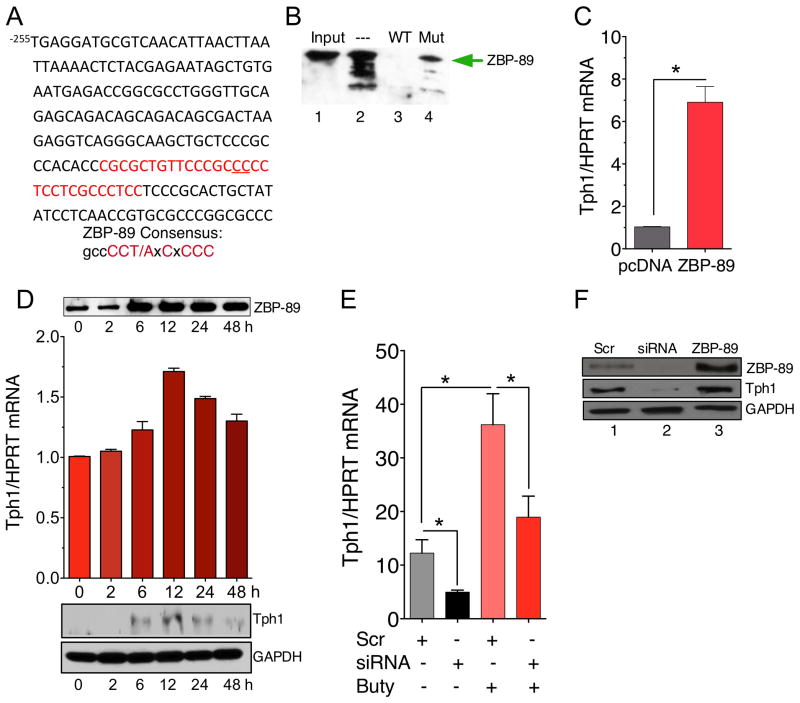
We identified several potential ZBP-89 consensus sites within the proximal promoter (Figure 4A). To demonstrate that ZBP-89 bound to a mTph1 DNA element, DNA affinity precipitation (DAPA) was performed with nuclear extracts from the 5HT-producing mouse STC-1 cell line using a putative ZBP-89 binding site at −123 bp upstream from the cap site. The DAPA showed that ZBP-89 is indeed present at the mTph1 promoter and that a 2 bp mutation within the GC-rich site did not compete effectively for ZBP-89 protein binding (Figure 4B). Ectopic expression of ZBP-89 induced endogenous mTph1 mRNA (Figure 4C). ZBP-89 is a transcription factor induced by butyrate, one of the major fatty acids produced by bacterial fermentation of dietary fiber 41. Butyrate induced ZBP-89 protein then Tph1 mRNA and protein in the mouse 5HT-producing STC-1 cell line (Figure 4D,E). Moreover, a knockdown of ZBP-89 with siRNA oligos (Figure 4E) reduced basal expression of Tph1 mRNA as well as Tph1 mRNA induced by butyrate (Figure 4E). A western blot confirmed that transfection of siRNA oligonucleotides decreased while ectopic expression increased ZBP-89 protein levels consistent with parallel changes in Tph1 protein levels (Figure 4F). Therefore we concluded that ZBP-89 binds the mTph1 promoter and is required for basal promoter as well as butyrate-inducible mTph1 gene expression.
Figure 4. ZBP-89 binds and regulates mouse Tph1.
(A) mTph1 promoter from −255 to −55 bp. ZBP-89 DNA element highlighted (red); two bp mutation underlined. (B) DAPA using a 30 bp biotinylated DNA element at −123 bp. Lane 1: input, lane 2: probe alone with 50 μg of STC-1 nuclear extract, lane 3: 20x unlabeled probe competitor, lane 4: 20x 2 bp mutation competitor. (C) RT-qPCR analysis of mTph1 mRNA after ZBP-89 expression in STC-1 cells. (D) Butyrate (2.5 mM) induction of ZBP-89 protein, Tph1 mRNA and protein in STC-1 cells, N=5. Both ZBP-89 and Tph1 western blots normalized to same GAPDH. (E) ZBP-89 siRNA versus scrambled siRNA oligos transfected into STC-1 cells ± 2.5 mM butyrate for 6h. Tph1 mRNA levels determined by RT-qPCR. N = 3, *P < 0.05. (F) Western blot of ZBP-89 and mTph1 protein levels after transfection of scrambled (Scr) (lane 1) or siRNA oligos for mZBP-89 (lane 2) or a ZBP-89 expression vector (lane 3) into STC-1 cells.
ZBP-89ΔInt mice are more susceptible to S. typhimurium infection
Prior studies have suggested that gut EC cells contribute to the innate immune response during enteric infections 42, 43. However, there is little known regarding how EC cells sense the luminal content then subsequently increase production and release of 5HT to stimulate fluid secretion and peristalsis. Therefore to understand the significance of Tph1 gene regulation by ZBP-89 in vivo, we challenged the ZBP-89ΔInt mice with the enteric pathogen S. typhimurium SL1344.
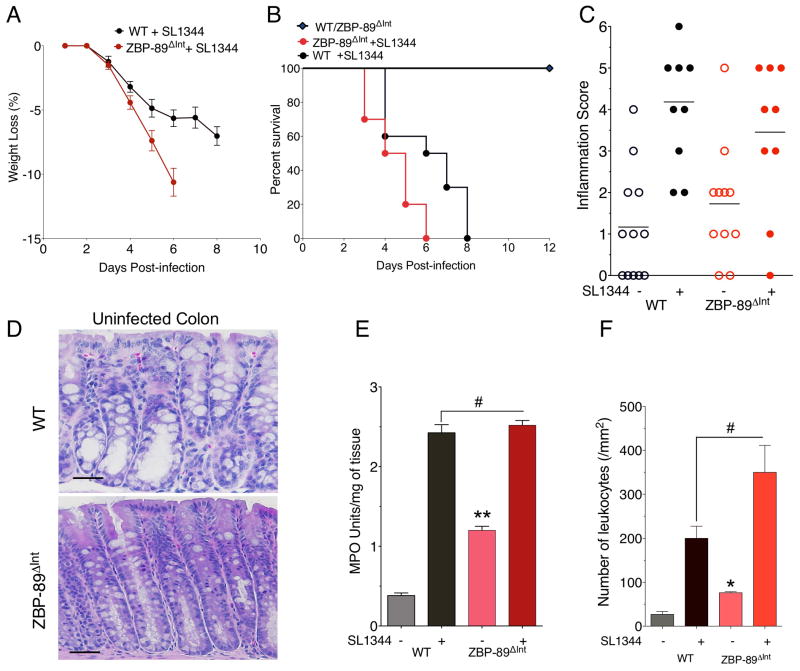
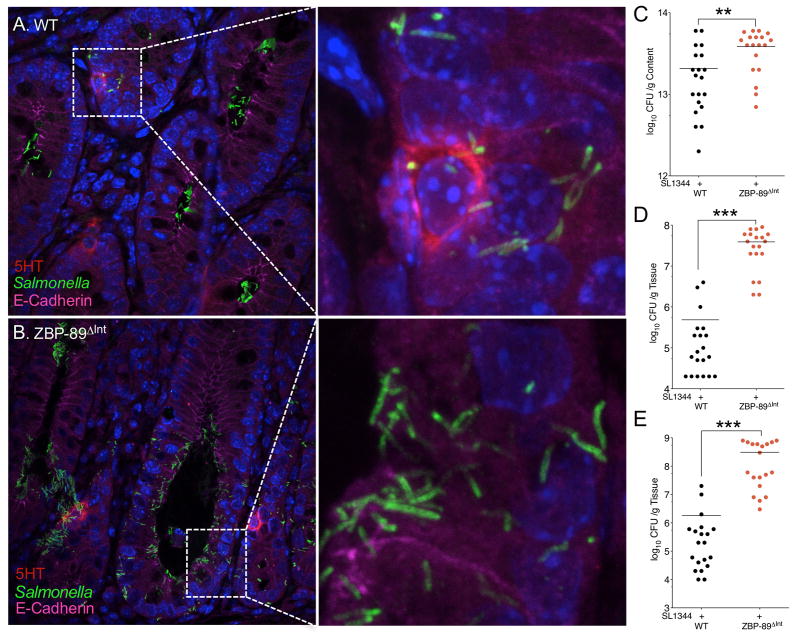
Both WT and ZBP-89ΔInt mice lost 7% and 10% of their body weight over time and eventually required euthanization by day 8 and 6 respectively (Figure 5A,B). Although ZBP-89ΔInt mice exhibited slightly more inflammation at baseline (Figure 5C), both groups reached about the same level of inflammation at the time of euthanization and as documented by the colitis score, myeloperoxidase activity (MPO) and number of leukocytes (Figure 5C–F, Suppl. Figure 1A). However, the ZBP-89 mice consistently succumbed to their infection 2 days before the WT mice. Since both groups are on a C57/BL6 genetic background, they are null for the natural resistance-associated macrophage protein 1 (Nramp1), which is required to control bacterial replication within the phagosome 44, 45. Confocal microscopy of 5HT+ EC cells after infection with GFP-labeled S. typhimurium SL1344 revealed fewer bacteria in the lumen of the wild type compared to the ZBP-89ΔInt mice (Figure 6A,B) and was consistent with the higher bacterial loads in the null mice (Figure 6C, Suppl. Figure 1B,C). ZBP-89ΔInt mice also exhibited significantly higher systemic levels of bacteria (Figure 6D–E), which correlated with reduced survival.
Figure 5. Increased susceptibility to S. typhimurium in ZBP-89ΔInt mice.
(A) Mouse body weight at euthanization. Percent survival of WT, N = 20 (black) versus ZBP-89ΔInt N = 20 SL1344 infected mice (red) from 4 independent experiments. Log-rank test demonstrated a statistically significant difference between the 2 groups (P = 0.0165) (C) Colitis scores (D) H&E stains of colon from WT and ZBP-89ΔInt mice prior to infection; (E) Myeloperoxidase (MPO) activity; (F) Leukocyte cell count from colons of WT versus ZBP-89ΔInt mice before and after SL1344 infection for 6 days. *P < 0.05.
Figure 6. Salmonella-infected colonic glands in WT and ZBP-89ΔInt mice.
(A) Confocal image of Salmonella with 5HT-expressing EC cells 4 day p.i. in wild type mice. (B) versus ZBP-89ΔInt. Quantitative cultures of Salmonella SL1344 in (C) colon, (D) liver, (E) spleen for in WT versus ZBP-89ΔInt mice at euthanization (CFU/g content, Log10) for two separate experiments. * P < 0.05. ** P < 0.01; *** P < 0.001.
To demonstrate that the 2-day difference in survival was due to lower levels of Tph1 enzyme and subsequent EC cell production of 5HT, we treated both WT and ZBP-89ΔInt mice with 5-hydroxytryptophan (5-HTP), the direct product of the Tph1 reaction to compensate for the ostensibly reduced EC cell 5HT levels. We observed a delay in weight loss that correlated with a 2-day improvement in the survival of both groups of mice (Suppl. Figure 2A,B). Histological examination of the colons and determination of the bacterial load demonstrated that the mice still exhibited colitis but had reduced bacterial loads in both the bowel lumen (data not shown) and systemically (Suppl. Figure 2C,D). At the time of euthanasia, both groups had lost about half of their blood volumes with hematocrits of ~20% (Suppl. Figure 2E), and positive fecal blood within two days of the gavage (data not shown). Thus as expected, the mice die from the combination of severe colitis resulting in anemia due to fecal blood loss and subsequent systemic infection. Since the mice are on a pure C57BL/6 background, which is NRAMP negative, mortality was ultimately observed in both groups. ELISAs confirmed the expected boost in tissue 5HT levels after 5HTP treatment (Suppl. Figure 2F). Therefore we concluded that the 2-day shift in survival was related to higher 5HT levels. Despite fewer 5HT positive EC cells, the basal and infection-induced tissue 5HT levels were similar in the colons of both the WT and ZBP-89ΔInt mice (Suppl. Figure 2F) and did not correlate with plasma levels (Figure 3). Thus other sources of 5HT such as platelets, which store 5HT and neurons which exclusively express Tph2, contributed to the observed tissue levels prior to and during infection 31, 46, 47.
5HT induces inflammatory cytokines
Ghia et al. demonstrated that 5HT enhances dextran sulfate-induced colitis by stimulating macrophage recruitment and secretion of IL-1β, TNFα and IL-6 48. Sustained colitis in the absence of a bona fide bacterial infection possibly contributes to inflammatory bowel disease or irritable bowel syndrome 46. Indeed, the infection induced several cytokine pathways (Th1, Th2 and Th17) to the same extent in both groups (Suppl. Table 1). Thus, differences in the 5HT levels of both the WT and ZBP-89ΔInt mice did not differentially affect the cytokine response to the bacterial infection.
5HT and 5HTP induce intestinal crypt killing activity
Since ZBP-89ΔInt mice also exhibited impaired defensin (Suppl. Figure 3A,B) and Tph1 gene expression and consequently increased susceptibility to Salmonella, we examined whether the ZBP-89 gene deletion decreased bacterial crypt killing activity. Crypts isolated from the colon of both WT and ZBP-89ΔInt mice were stimulated with LPS, 5HT, 5HTP, muramyl dipeptide (MDP) or inactive chiral MDP isomers (MDPDD) revealed reduced bacterial killing from colonic crypts isolated from ZBP-89ΔInt compared to wild type mice (Suppl. Figure 3D). MDP is a Nod2 activator 49, known to induce Paneth cell secretion of antimicrobial peptides, while the inactive isomer MDPDD does not (Suppl. Figure 3D). A pan-anti-defensin antibody blocked LPS induction of the crypt killing activity. Thus we concluded that 5HT-induced secretions contain antimicrobial peptides.
S. typhimurium inhibits Tph1 gene expression
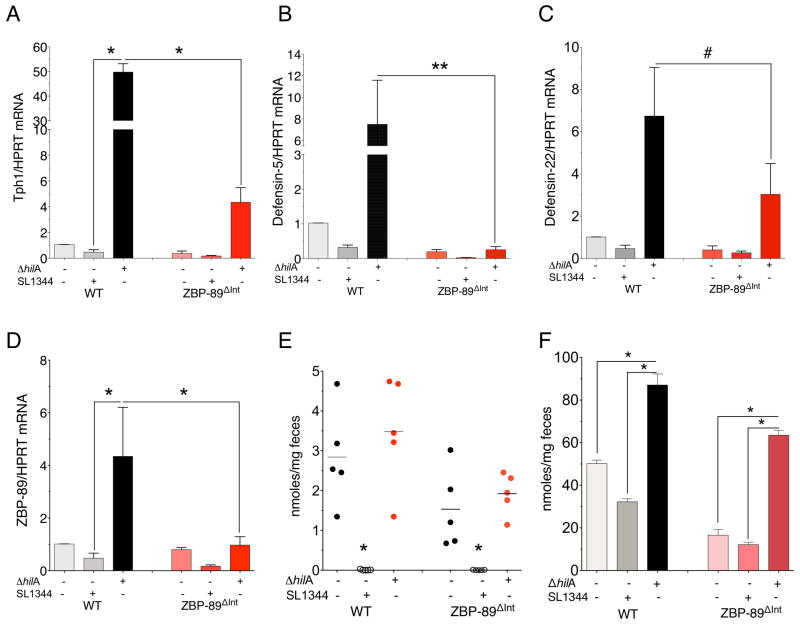
Due to low levels of 5HT generated in the infected WT mice, we examined Salmonella’s effect on Tph1 gene expression in WT mice and were surprised to find a decrease in Tph1 and defensin 5/22 mRNA expression within 3 days (Suppl. Figures 4 and 3A,B). The significantly lower Tph1 mRNA levels were nearly equivalent to the levels in ZBP-89ΔInt mice (Figure 7A). Moreover, S. typhimurium suppressed other putative ZBP-89 targets, e.g., mRNA for defensin 5 and defensin 22 (Figure 7B,C). Although pathogens typically induce 5HT production via TLRs 50, Tph1 suppression by S. typhimurium suggested an additional mechanism. Therefore we examined whether bacterial invasion genes encoded by the SPI-1 pathogenicity island were responsible. Indeed, the suppression was due to bacterial effectors encoded by the SPI-1 locus, since infecting WT mice with the isogenic hilA SPI-1 mutant S. typhimurium strain (STΔhilA) induced rather than suppressed both Tph1 and defensin 5/22 mRNAs (Figure 7A–C). The induction required ZBP-89 since Tph1 and defensin 5 were not significantly induced in the ZBP-89ΔInt mice infected with STΔ hilA. Moreover, ZBP-89 mRNA levels were induced by the STΔ hilA isogenic mutant strain (Figure 7D). Therefore bacterial effectors encoded by the SP1-1 Salmonella locus modulated ZBP-89 gene expression, which is ultimately required for butyrate induction of Tph1 gene expression and subsequently secretion of 5HT and antimicrobial peptides. In particular, we found that butyrate and total SCFA levels in the colonic lumen were nearly absent in mice infected with the WTSL1344 but not the STΔhilA mutant strain (Figure 7E, F).
Figure 7. S. typhimurium ΔhilA induces Tph1 and defensin mRNA levels.
mRNA levels 4 days after gavage with ΔhilA mutant for (A)Tph1; (B) defensin 5; (C) defensin 22; (D) ZBP-89. N = 6 mice per WT versus ZBP-89ΔInt for two independent experiments. Mean ± SEM. #P = 0.42; *P < 0.05; **P < 0.01. (E) Fecal butyrate and (F) Fecal SCFA levels in the colon. *P < 0.05.
Both bacterial strains induced Tph1 and βcatenin gene expression ex vivo when co-cultured with STC-1 cells (Suppl. Figure 5A). Moreover, the mutant STΔhilA strain induced significant levels of ZBP-89 mRNA and subsequently downstream targets Tph1 and defensin 5 mRNA compared to the wild type strain (Figure 7A,B,D). Considering the known presence of TLRs on EECs and that TLR signaling is required for Salmonella virulence 50, we concluded that the mutant strain elicits the expected cell response by inducing Tph1. Salmonella activation of TLRs increases βcatenin 51. Indeed cotransfection of WT βcatenin with ZBP-89 cooperated to induce Tph1 protein and Tph1 mRNA in STC-1 cells (Suppl. Figure 5B,C). However, reduced luminal butyrate levels correlated with reduced ZBP-89 gene expression (Figure 7E) and presumably its ability to cooperate with TLR induction of mucosal defense gene targets, e.g., βcatenin. TLRs have been shown to increase transcriptional activation of βcatenin and its nuclear accumulation in response to bacteria 52. In particular, Salmonella induce host epithelial defense mechanisms through the activation of the Wnt/βcatenin pathway 53, which includes epithelial cell proliferation, maintenance of tight junctions and antimicrobial peptide expression 26. Thus gut homeostasis is a balance between cell damage due to the collateral effects of bacteria killing and epithelial repair 54.
Discussion
The goal of this study was to identify the transcriptional targets of ZBP-89 that mediate mucosal protection. A microarray analysis of colonic mucosa from WT versus ZBP-89ΔInt mice revealed several genes encoding antimicrobial peptides and Tph1 as potential ZBP-89 gene targets. We show here that ZBP-89 was essential for the production of 5HT in colonic EC cells through its ability to directly regulate Tph1 gene expression. Moreover, we demonstrated that butyrate strongly induces Tph1 gene expression in vitro and that the induction requires ZBP-89. Targeted ZBP-89 deficiency revealed that an important consequence is decreased survival to infection from the clinically-relevant pathogen S. typhimurium. Unexpectedly, S. typhimurium infection suppressed colonic butyrate levels, which also reduced ZBP-89 and Tph1 gene expression acutely in WT mice. However removing the butyrate-regulated hilA locus abolished this suppressive effect resulting in robust induction of Tph1 and defensin expression. Salmonella induction of epithelial proliferation through βcatenin stabilization has been previously reported 51, 55, 56. Indeed, we also found that Salmonella induces βcatenin and synergizes with ZBP-89 to induce Tph1. Therefore, we concluded that ZBP-89 regulates several essential mucosal defense genes activated during bacterial pathogenesis (Suppl. Fig. 6, Model). Moreover consistent with its in vitro regulation by butyrate, ZBP-89 gene expression in vivo correlated with fecal SCFA levels, which were also modulated by the Salmonella infection.
The overall levels of 5HT at both baseline and during colonic injury vary widely due to multiple mechanisms impacting the tissue levels such as its reuptake mediated by SERT, storage in platelets, in addition to production by myeloid cells and enteric neurons 31. 5HT has been implicated in the inflammation generated after chemical injury to the colon, and as such has been an important therapeutic target considered in the treatment of both inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) 57. However, the results here underscore the importance of 5HT in the colon’s initial defense against an invading pathogen. 5HT targets multiple processes in mucosal defense, e.g., as a regulator of immune cells, the enteric nervous system and fluid secretion by colonocytes. Indeed, we show here that reduced serotonin levels compromised mucosal defenses since increasing tissue serotonin levels with 5HTP administration dramatically reduced the bacterial load and subsequently improved survival. An underappreciated result observed with the burst of 5HT included the secretion of antimicrobial peptides, e.g., defensins. Regulated production of antimicrobial peptides has been examined in studies focused on suppressing pathogen colonization of livestock 10–12, but has not been extensively studied as a mechanism by which susceptible mammalian hosts mount a defense against food borne pathogens. Moreover, there are multiple examples implicating IBD and IBS as multigenic disorders developing surreptitiously after a distant episode of bacterial gastroenteritis 32, 58, 59.
Studying the acute mucosal defense response to S. typhimurium in the ZBP-89 mutant colons uncovered the potential role of SCFAs. SCFAs especially butyrate are required to maintain homeostasis of the normal colon. The millimolar butyrate concentration generated from commensal colonic bacteria is purported to also exhibit anti-inflammatory properties 5. Despite the intense focus regarding how butyrate induces apoptosis, cell cycle arrest and epigenetic modifications in mammalian cells, there is little mechanistic understanding regarding its actual antimicrobial effects in vivo 60. Moreover, any antimicrobial activity mediated by butyrate has only been studied in livestock 61.
Considering both the biochemical and in vivo studies reported here, we conclude that a plausible mechanism by which Tph1 enzyme levels and therefore 5HT production are modulated by commensal effectors and their byproducts (e.g., butyrate) is through the transcriptional regulatory protein ZBP-89. Our studies are consistent with reports showing that butyrate suppresses the S. typhimurium regulatory locus hilA 18, 19. In addition, we found that deletion of this bacterial locus permits induction of ZBP-89 gene expression and downstream targets such as Tph1 and defensin 5. Since infection by S. typhimurium apparently must occur in a butyrate-poor environment 62, 63, it appears that the pathogen might be capable of suppressing bacteria generating SCFAs. In that way, Salmonella could partially inhibit butyrate-responsive genes like ZBP-89 and its downstream targets such as Tph1 and the antimicrobial peptides secreted during colonic mucosal defense.
In summary, a conditional null ZBP-89 allele reduced innate mucosal defenses against a bacterial pathogen in the colon. The mechanism is in part due to the requirement of this transcription factor to induce butyrate-dependent target genes, e.g., Tph1 and subsequently to generate optimal amounts of 5HT and antimicrobial peptides.
Supplementary Material
Acknowledgments
The studies were supported by NIH grant R01 DK55732 (to JLM). The authors thank Lisa Travnikar for technical assistance and Laura Johnson for the SL1344 Salmonella strain. The authors acknowledge the expertise of the University of Michigan Transgenic Animal Model Core, especially Thom Saunders, Linda Samuelson and Elizabeth Hughes supported by NIH Cancer Center grant P30 CA46592 and Gut Peptide Center grant P30 DK34933 as well as the University of Michigan Metabolic Core Service supported by NIH grant P30 DK089503. The authors also thank the Intrexon Corporation (Blacksburg, VA) for assembly of the pLM10-iKO-FinalTV construct and the Unit for Laboratory Animal Medicine at the University of Michigan for assistance with animal husbandry. We thank Deborah Gumucio (University of Michigan) for the VillinCre mouse line and Beth McCormick (University of Massachusetts, Worcester) for the hilA mutant Salmonella strain.
Biographies
Bryan Essien: experimental design; acquisition of data; drafting of the manuscript; statistical analysis; technical and material support
Helmut Grasberger: experimental design; acquisition of data; analysis and interpretation of data
Rachael D. Romain: experimental design; acquisition of data; analysis and interpretation of data; technical and material support; drafting of the manuscript
David J. Law: experimental design; acquisition of data; analysis and interpretation of data; technical and material support; drafting of the manuscript
Natalia A. Veniaminova: acquisition of data; technical, or material support
Milena Saqui-Salces: acquisition of data; technical and material support
Mohamad El-Zaatari: acquisition of data; technical and material support
Arthur Tessier: experimental design; acquisition of data; technical and material support
Michael M. Hayes: experimental design; acquisition of data; technical and material support
Alexander C. Yang: technical and material support
Juanita L. Merchant: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
Footnotes
The authors have all declared that no conflict of interest exists.
Website for Microarray data: file:///Microarray%20ZBP-89%2010.09%20results/VillinCre%20xZBP-89%20%20v%20WT%20colon.html
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bai L, Merchant JL. Transcription factor ZBP-89 cooperates with histone acetyltransferase p300 during butyrate activation of p21waf1 transcription in human cells. J Biol Chem. 2000;275:30725–33. doi: 10.1074/jbc.M004249200. [DOI] [PubMed] [Google Scholar]
- 2.Merchant JL, Bai L, Okada M. ZBP-89 mediates butyrate regulation of gene expression. J Nutr. 2003;133:2456S–2460S. doi: 10.1093/jn/133.7.2456S. [DOI] [PubMed] [Google Scholar]
- 3.Merchant JL, Iyer GR, Taylor BR, et al. ZBP-89, a Krüppel-type zinc finger protein, inhibits EGF induction of the gastrin promoter. Mol Cell Biol. 1996;16:6644–6653. doi: 10.1128/mcb.16.12.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai L, Kao JY, Law DJ, et al. Recruitment of Ataxia-Telangiectasia Mutated to the p21(waf1) Promoter by ZBP-89 Plays a Role in Mucosal Protection. Gastroenterology. 2006;131:841–52. doi: 10.1053/j.gastro.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 6.Delcenserie V, Martel D, Lamoureux M, et al. Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol. 2008;10:37–54. [PubMed] [Google Scholar]
- 7.Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–21. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- 8.Lawrance IC. Topical agents for idiopathic distal colitis and proctitis. J Gastroenterol Hepatol. 2011;26:36–43. doi: 10.1111/j.1440-1746.2010.06497.x. [DOI] [PubMed] [Google Scholar]
- 9.Greer JB, O’Keefe SJ. Microbial induction of immunity, inflammation, and cancer. Front Physiol. 2011;1:168. doi: 10.3389/fphys.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raqib R, Sarker P, Bergman P, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A. 2006;103:9178–83. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schauber J, Iffland K, Frisch S, et al. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol. 2004;41:847–54. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Schauber J, Svanholm C, Termen S, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–41. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Immerseel F, Russell JB, Flythe MD, et al. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 2006;35:182–8. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- 14.Hill TM, Vandehaar MJ, Sordillo LM, et al. Fatty acid intake alters growth and immunity in milk-fed calves. J Dairy Sci. 2011;94:3936–48. doi: 10.3168/jds.2010-3935. [DOI] [PubMed] [Google Scholar]
- 15.Schwab M, Reynders V, Loitsch S, et al. The dietary histone deacetylase inhibitor sulforaphane induces human beta-defensin-2 in intestinal epithelial cells. Immunology. 2008;125:241–51. doi: 10.1111/j.1365-2567.2008.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichenametla S, South E, Exon J. Interaction of conjugated linoleic acid, sphingomyelin, and butyrate on formation of colonic aberrant crypt foci and immune functions in rats. J Toxicol Environ Health A. 2004;67:469–81. doi: 10.1080/15287390490276494. [DOI] [PubMed] [Google Scholar]
- 17.Zasloff M. Inducing endogenous antimicrobial peptides to battle infections. Proc Natl Acad Sci U S A. 2006;103:8913–4. doi: 10.1073/pnas.0603508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durant JA, Corrier DE, Ricke SC. Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella typhimurium. J Food Prot. 2000;63:573–8. doi: 10.4315/0362-028x-63.5.573. [DOI] [PubMed] [Google Scholar]
- 19.Gantois I, Ducatelle R, Pasmans F, et al. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol. 2006;72:946–9. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CA, Jones BD, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci U S A. 1992;89:1847–51. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawhon SD, Maurer R, Suyemoto M, et al. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol. 2002;46:1451–64. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- 22.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–12. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 23.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov R. TLR-mediated innate immune recognition. Semin Immunol. 2007;19:1–2. doi: 10.1016/j.smim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogunovic M, Dave SH, Tilstra JS, et al. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1770–83. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 27.Palazzo M, Balsari A, Rossini A, et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol. 2007;178:4296–303. doi: 10.4049/jimmunol.178.7.4296. [DOI] [PubMed] [Google Scholar]
- 28.Surveillance for foodborne disease outbreaks--United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60:1197–202. [PubMed] [Google Scholar]
- 29.Mintz ED, Cartter ML, Hadler JL, et al. Dose-response effects in an outbreak of Salmonella enteritidis. Epidemiol Infect. 1994;112:13–23. doi: 10.1017/s095026880005737x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbara G, Cremon C, Pallotti F, et al. Postinfectious irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48 (Suppl 2):S95–7. doi: 10.1097/MPG.0b013e3181a15e2e. [DOI] [PubMed] [Google Scholar]
- 31.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Spiller R. Serotonin, inflammation, and IBS: fitting the jigsaw together? J Pediatr Gastroenterol Nutr. 2007;45 (Suppl 2):S115–9. doi: 10.1097/MPG.0b013e31812e66da. [DOI] [PubMed] [Google Scholar]
- 33.Smith JL, Bayles D. Postinfectious irritable bowel syndrome: a long-term consequence of bacterial gastroenteritis. J Food Prot. 2007;70:1762–9. doi: 10.4315/0362-028x-70.7.1762. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi A, Mishina Y, Miyaishi O, et al. Heterozygosity with respect to Zfp148 causes complete loss of fetal germ cells during mouse embryogenesis. Nat Genet. 2003;33:172–176. doi: 10.1038/ng1072. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez CI, Buchholz F, Galloway J, et al. High-efficiency deleter mice show that FLPe is an alternative to cre- loxP. Nat Genet. 2000;25:139–40. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 36.Schaft J, Ashery-Padan R, van der Hoeven F, et al. Efficient FLP recombination in mouse ES cells and oocytes. Genesis. 2001;31:6–10. doi: 10.1002/gene.1076. [DOI] [PubMed] [Google Scholar]
- 37.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 38.Bai L, Merchant JL. ZBP-89 promotes growth arrest through stabilization of p53. Mol Cell Biol. 2001;21:4670–83. doi: 10.1128/MCB.21.14.4670-4683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Law GL, Itoh H, Law DJ, et al. Transcription factor ZBP-89 regulates the activity of the ornithine decarboxylase promoter. J Biol Chem. 1998;273:19955–64. doi: 10.1074/jbc.273.32.19955. [DOI] [PubMed] [Google Scholar]
- 40.Yu PL, Fujimura M, Okumiya K, et al. Immunohistochemical localization of tryptophan hydroxylase in the human and rat gastrointestinal tracts. J Comp Neurol. 1999;411:654–65. doi: 10.1002/(sici)1096-9861(19990906)411:4<654::aid-cne9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 41.Clausen MR, Bonnen H, Mortensen PB. Colonic fermentation of dietary fibre to short chain fatty acids in patients with adenomatous polyps and colonic cancer. Gut. 1991;32:923–8. doi: 10.1136/gut.32.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grondahl ML, Jensen GM, Nielsen CG, et al. Secretory pathways in Salmonella Typhimurium-induced fluid accumulation in the porcine small intestine. J Med Microbiol. 1998;47:151–7. doi: 10.1099/00222615-47-2-151. [DOI] [PubMed] [Google Scholar]
- 44.Valdez Y, Grassl GA, Guttman JA, et al. Nramp1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell Microbiol. 2009;11:351–62. doi: 10.1111/j.1462-5822.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- 45.Valdez Y, Ferreira RB, Finlay BB. Molecular mechanisms of Salmonella virulence and host resistance. Curr Top Microbiol Immunol. 2009;337:93–127. doi: 10.1007/978-3-642-01846-6_4. [DOI] [PubMed] [Google Scholar]
- 46.Margolis KG, Pothoulakis C. Serotonin has a critical role in the pathogenesis of experimental colitis. Gastroenterology. 2009;137:1562–6. doi: 10.1053/j.gastro.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghia JE, Li N, Wang H, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–60. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 49.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 50.Arpaia N, Godec J, Lau L, et al. TLR signaling is required for Salmonella typhimurium virulence. Cell. 2011;144:675–88. doi: 10.1016/j.cell.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tahoun A, Mahajan S, Paxton E, et al. Salmonella transforms follicle-associated epithelial cells into m cells to promote intestinal invasion. Cell Host Microbe. 2012;12:645–56. doi: 10.1016/j.chom.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Monick MM, Carter AB, Robeff PK, et al. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J Immunol. 2001;166:4713–20. doi: 10.4049/jimmunol.166.7.4713. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Lu R, Wu S, et al. Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Lett. 2010;584:911–6. doi: 10.1016/j.febslet.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchon N, Broderick NA, Poidevin M, et al. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–11. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Martinez Rodriguez NR, Eloi MD, Huynh A, et al. Expansion of Paneth cell population in response to enteric Salmonella enterica serovar Typhimurium infection. Infect Immun. 2012;80:266–75. doi: 10.1128/IAI.05638-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu R, Liu X, Wu S, et al. Consistent activation of the beta-catenin pathway by Salmonella type-three secretion effector protein AvrA in chronically infected intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1113–25. doi: 10.1152/ajpgi.00453.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee KJ, Tack J. Altered intestinal microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:493–8. doi: 10.1111/j.1365-2982.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- 58.Man SM. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol. 2011;8:669–85. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 59.Jess T, Simonsen J, Nielsen NM, et al. Enteric Salmonella or Campylobacter infections and the risk of inflammatory bowel disease. Gut. 2011;60:318–24. doi: 10.1136/gut.2010.223396. [DOI] [PubMed] [Google Scholar]
- 60.Fauser JK, Prisciandaro LD, Cummins AG, et al. Fatty acids as potential adjunctive colorectal chemotherapeutic agents. Cancer Biol Ther. 2011;11:724–31. doi: 10.4161/cbt.11.8.15281. [DOI] [PubMed] [Google Scholar]
- 61.Namkung H, Yu H, Gong J, et al. Antimicrobial activity of butyrate glycerides toward Salmonella Typhimurium and Clostridium perfringens. Poult Sci. 2011;90:2217–22. doi: 10.3382/ps.2011-01498. [DOI] [PubMed] [Google Scholar]
- 62.Garner CD, Antonopoulos DA, Wagner B, et al. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infect Immun. 2009;77:2691–702. doi: 10.1128/IAI.01570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis K, Lutgendorff F, Phan V, et al. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. 2010;16:1138–48. doi: 10.1002/ibd.21177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.