Abstract
B lymphocyte stimulator (BLyS) is a cytokine involved in differentiation and survival of follicular B cells along with humoral response potentiation. Lymphopenia is known to precipitate dramatic elevation in serum BLyS; however, the use of this effect to enhance humoral responses following vaccination has not been evaluated. We evaluated BLyS serum levels and antigen-specific antibody titers in 8 patients undergoing therapeutic temozolomide (TMZ)-induced lymphopenia, with concomitant vaccine against a tumor-specific mutation in the epidermal growth factor receptor (EGFRvIII). Our studies demonstrate that TMZ-induced lymphopenia corresponded with spikes in serum BLyS that directly preceded the induction of anti-EGFRvIII antigen-specific antibody titers, in some cases as high as 1:2,000,000. Our data are the first clinical observation of BLyS serum elevation and greatly enhanced humoral immune responses as a consequence of chemotherapy-induced lymphopenia. These observations should be considered for the development of future vaccination strategies in the setting of malignancy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1405-y) contains supplementary material, which is available to authorized users.
Keywords: Temozolomide, BLyS, BAFF, Lymphopenia, Immunotherapy, Vaccines
Introduction
Intentional lymphodepletion, commonly used in patients undergoing T-cell-based immunotherapy, increases serum levels of homeostatic T-cell cytokines (i.e., IL-7 and IL-15), thereby potentiating cellular immune responses [1, 2]. However, intentional lymphodepletion-inducing B-cell homeostatic cytokines to augment humoral responses have not been evaluated. Recent preclinical studies, however, have demonstrated that the provision of B lymphocyte stimulator (BLyS), a homeostatic B-cell cytokine [3–5], during vaccination leads to enhanced antibody titers [6, 7] and antibodies with higher affinity [8].
BLyS, also known as B-cell-activating factor (BAFF), is a cytokine in the tumor necrosis factor superfamily essential for the differentiation and survival of follicular B cells [9, 10]. Regulation of BLyS expression by myeloid cells through homeostatic and inflammatory cytokines (i.e., G-CSF and IFNγ) [11, 12] together with BLyS consumption by mature follicular B cells results in constant BLyS serum levels [13–17]. However, these levels can be augmented upon the induction of lymphopenia through decreased B-cell consumption of BLyS thereby increasing its expression [15, 18–21]. Consequently, BLyS blockade results in the absence of B-cell rebound, highlighting BLyS’ paramount role in B-cell recovery from lymphodepletion [18].
We have previously demonstrated that patients with glioblastoma (GBM) undergoing vaccination against the tumor-specific epidermal growth factor mutation, EGFRvIII, in the context of temozolomide (TMZ)-induced lymphopenia develop high serum levels of anti-EGFRvIII antibodies [22]. Paradoxically, patients who experience more profound lymphopenia were found to have higher peak titers. This prompted us to analyze the impact of homeostatic increases in BLyS on humoral responses. In patients with GBM undergoing TMZ-induced lymphopenia, we hypothesized that BLyS serum levels would be concomitantly elevated with TMZ-induced lymphodepletion and correlate with antibody titers against EGFRvIII. Thus, we evaluated BLyS serum levels from 8 patients with GBM and correlated these results with peak antibody titers. In this report, we demonstrate that a surge in BLyS serum level precedes the induction of EGFRvIII-specific antibody titers and peak BLyS levels directly correlate with peak antibody titers. These data are the first clinical observation of BLyS’ pivotal role inducing humoral immunity after lymphopenia in patients.
Methods
Patient selection and clinical protocol
Adults with newly diagnosed GBM who had gross total resection of their tumor and a Karnofsky Performance Scale (KPS) score of ≥80 were eligible for vaccination if tumor cells expressed EGFRvIII by immunohistochemistry and they had no radiographic evidence of progression after radiation therapy. The trial design and informed consent were approved by the FDA (under BB-IND-9944) and the local institutional review boards [22–24].
After tumor resection and conformal external beam radiotherapy (XRT) with concurrent TMZ at a targeted dose of 75 mg/m2, informed consent was obtained. The initial 3 vaccinations of a 13-mer peptide conjugated to KLH were given biweekly starting within 6 weeks of completing radiation [22, 23]. Subsequent vaccines were given until clinical or radiographic evidence of tumor progression or death. Patients were assigned to receive TMZ at a targeted dose of 100 mg/m2 for the first 21 days of a 28-day cycle (n = 8). Patient’s characteristics including BLyS and peak titer information are in Table S1.
Lymphocyte counts
Absolute lymphocyte counts were quantified by flow cytometry using a direct immunofluorescence, single platform, and FDA-approved method in the clinical laboratory at the primary study center (Duke University) and evaluated at vaccine 1 (pre—prior to any vaccination or TMZ treatment at 100 mg/m2) and at vaccine 6 (post—which occurred after TMZ cycle 3).
Serum collection and processing
Patient sera were collected before and after each vaccine consisting of PEPvIII conjugated to KLH. Blood was drawn into serum blood collection tubes (Vacutainer BD, Franklin Lakes, NJ) and allowed to clot after which time tubes were centrifuged at 2,000g for 10 min. Serum was harvested from tubes, separated into 1 ml aliquots, and stored at −135 °C. Samples were collected before every vaccine cycle until tumor recurred.
BLyS ELISA
ELISA for human BLyS was performed using Quantikine ELISA for Human BAFF/BLyS/TNFSF13B (Cat #: DBLYS0, R&D Systems Minneapolis, MN). Patient serum was thawed, diluted 1:4 in Calibrator Diluent RD6Q, and submitted to procedure as recommended by manufacturer.
Anti-PEPvIII antibodies ELISA
Serum was analyzed using ELISA. A result was considered positive if it was more than twice background. Humoral responses were compared based on maximum titer obtained.
Statistical analysis
A paired t test was used to compare assessments obtained pre- and post-treatment relative to lymphocyte counts/µl of blood and BLyS serum levels. Linear regression was used to assess the association between these variables assuming pre- and post-measurements were independent. Statistical significance was determined at the level of p < 0.05. All analyses were conducted using GraphPad Prism, version 5.01.
Results and discussion
Chemotherapy-induced lymphopenia results in elevated serum BLyS levels
B lymphocyte stimulator (BLyS) serum levels are tightly regulated and can be altered by extrinsic events such as lymphopenia, and although this has been shown in preclinical murine studies, it has not been evaluated in the clinical setting. The standard of care for GBM involves high-dose TMZ chemotherapy, which has lymphopenia as its major side effect [25]. Therefore, in a cohort of 8 GBM patients undergoing TMZ chemotherapy with concomitant vaccination against the EGFRvIII tumor-specific mutation [22–24], we studied whether TMZ-induced lymphopenia increased BLyS serum levels.
As expected, compared with lymphocyte levels prior to treatment, our studies demonstrate significantly decreased circulating lymphocytes in six patients post-therapy (Fig. 1a, p = 0.0296), five of which developed Grade III lymphopenia. Serum analysis pre- and post-treatment revealed elevated BLyS serum levels (with an average twofold increase; Fig. 1b, p = 0.0075) that were inversely related to lymphocyte counts (Fig. 1c, r = 0.6311; p = 0.0116). Therefore, TMZ chemotherapy alone results in decreased lymphocyte counts precipitating BLyS serum level spikes. This is the first clinical evaluation of BLyS levels in patients undergoing lymphodepletive chemotherapy that emphasizes this relationship.
Fig. 1.
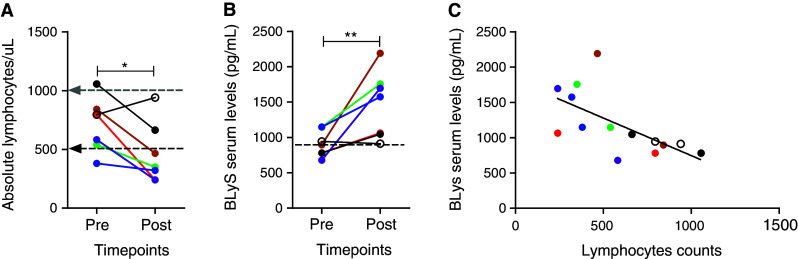
BLyS serum levels are elevated in GBM patients experiencing lymphopenia under temozolomide lymphodepletive chemotherapy. Following tumor resection and conformal XRT with concurrent TMZ at a targeted dose of 75 mg/m2, GBM patients initiated clinical protocol consisting of 3 PEPvIII-KLH vaccinations biweekly, followed by subsequent TMZ (100 mg/m2) 21 ± 2 during 28-day cycles of TMZ with vaccination at the end of the TMZ cycle. a Absolute lymphocytes were quantified by flow cytometry and evaluated at vaccine 1 (Pre prior to any vaccination or 100 mg/m2 TMZ treatment) and at vaccine 6 (Post which occurred after TMZ cycle 3) from peripheral blood collected from patients (n = 7). b BLyS analysis was performed from serum obtained from the periphery from patients and evaluated at vaccine 1 (Pre prior any vaccination or 100 mg/m2 TMZ treatment) and at vaccine 6 (Post which occurred after TMZ cycle 3; n = 8). Dotted line represents the average serum levels of BLyS in normal donors. c Linear regression was performed with the absolute lymphocyte counts and BLyS serum levels from GBM patients at pre- and post-treatment (r = 0.6311; p = 0.0116). Experiments were performed in duplicate at a minimum. Statistical analysis utilizing paired t test (a and b) and linear regression analysis (c) was performed. *p value ≤0.05, **p value ≤0.01 was statistically significant
BLyS serum surge precedes peak antibody responses to a vaccine and is positively associated with peak antibody titers
Preclinical data have annotated the dynamic relationship between lymphopenia and BLyS serum levels evolving over periods of weeks to months promoting increased antibody titers in circulation [13]. Accordingly, we evaluated BLyS serum levels from 8 GBM patients receiving an EGFRvIII-specific peptide vaccine at various time points during TMZ therapy and demonstrated that peak BLyS serum levels precede the induction of high titer antibodies (Fig. 2a) by 12 weeks (Fig. 2b, p = 0.0055). Following the peak, BLyS serum levels remained elevated from baseline. Meanwhile, after BLyS levels surge, anti-EGFRvIII antibody titers elevate to very high levels; titers up to 1:2,000,000 were observed.
Fig. 2.
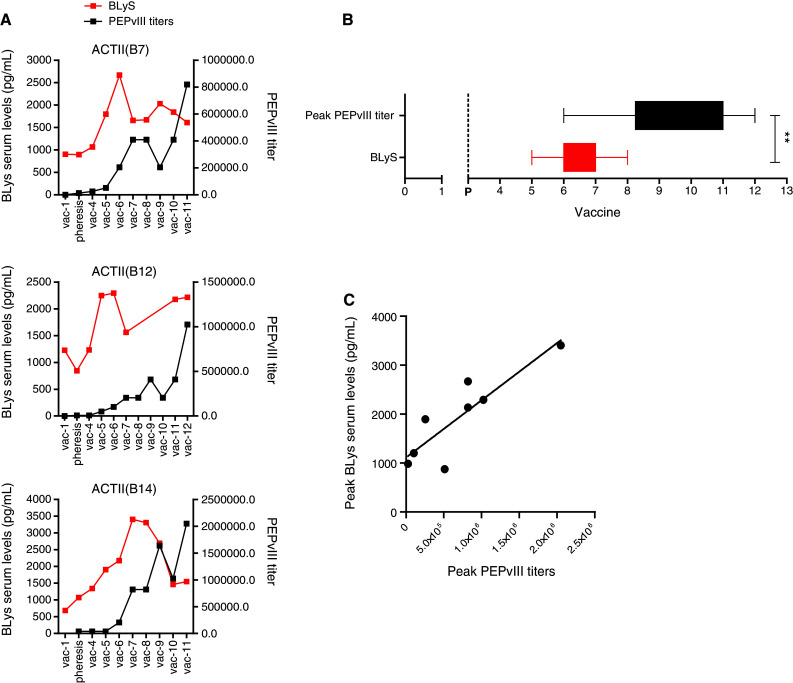
Peak antigen-specific antibody titer is positively associated with a preceding surge in BLyS serum levels in GBM patients receiving a peptide vaccine. Peripheral blood from primary GBM patients, enrolled in clinical protocol receiving PEPvIII-KLH vaccinations with concomitant TMZ treatment (100 mg/m2), was collected at the indicated time points. Serum was obtained by centrifugation and was submitted to PEPvIII and BLyS ELISA. a Serum BLyS levels kinetics from three representative patients are depicted in red lines, while the kinetics of PEPvIII titer are in black lines. b Graph illustrates the peak of BLyS serum surge and PEPvIII antibody titers with regard to time of vaccination. BLyS levels precedes peak PEPvIII titers (p = 0.0055, Mann–Whitney test). p in graph stands for pheresis. c Linear regression was performed with peak BLyS serum levels and peak anti-PEPvIII antibody titers from GBM (r = 0.8687; p = 0.0051). Experiments were performed at least duplicate. *p value ≤0.05, **p value ≤0.01 was statistically significant
Based on preclinical murine studies [6], the magnitude of BLyS levels in circulation could dictate the magnitude of humoral responses. Our data demonstrate that peak BLyS serum levels were directly proportional to peak anti-EGFRvIII antibody titers (Fig. 2c, r = 0.8687; p = 0.0051) in GBM patients undergoing vaccination with lymphodepletive TMZ chemotherapy, suggesting that higher BLyS serum levels correlate with higher vaccine-induced antibody titers.
BLyS overexpression stimulates abnormally increased levels of mature follicular B cells, a harbinger for antibody-mediated pathology, while under-expression markedly reduces these levels [5, 14]. Our data are the first clinical observation of BLyS’ pivotal role in the induction of vaccine-specific humoral immunity upon intentional lymphodepletion. Further evaluation, however, is necessary to discern whether BLyS elevation and lymphopenia act alone or in conjunction with one another to generate supraphysiological vaccine-specific antibodies. Nonetheless, BLyS incorporation into immunotherapeutic regimens may augment humoral immunity requisite of vaccination strategies aimed at inducing high titers of neutralizing antibodies in malignancies in need of superior targeted therapeutic modalities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank Tecca Wright and Tracy A. Chewning for their assistance in the preparation of grant applications supporting these studies. In addition, special thanks go to Shicheng Yang, MD, PhD, Carter Suryadevara, and Tony Shan for their contribution on data discussion.
Conflict of interest
All other authors do not have any conflict of interest.
References
- 1.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8 + T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancro MP. The BLyS family of ligands and receptors: an archetype for niche-specific homeostatic regulation. Immunol Rev. 2004;202:237–249. doi: 10.1111/j.0105-2896.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- 4.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198(6):937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodland RT, Schmidt MR, Thompson CB. BLyS and B cell homeostasis. Semin Immunol. 2006;18(5):318–326. doi: 10.1016/j.smim.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Bosello S, Youinou P, Daridon C, Tolusso B, Bendaoud B, Pietrapertosa D, Morelli A, Ferraccioli G. Concentrations of BAFF correlate with autoantibody levels, clinical disease activity, and response to treatment in early rheumatoid arthritis. J Rheumatol. 2008;35(7):1256–1264. [PubMed] [Google Scholar]
- 7.Gor DO, Ding X, Li Q, Sultana D, Mambula SS, Bram RJ, Greenspan NS. Enhanced immunogenicity of pneumococcal surface adhesin A (PsaA) in mice via fusion to recombinant human B lymphocyte stimulator (BLyS) Biol Direct. 2011;6:9. doi: 10.1186/1745-6150-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dosenovic P, Soldemo M, Scholz JL, O’Dell S, Grasset EK, Pelletier N, Karlsson MC, Mascola JR, Wyatt RT, Cancro MP, Karlsson Hedestam GB. BLyS-mediated modulation of naive B cell subsets impacts HIV Env-induced antibody responses. J Immunol. 2012;188(12):6018–6026. doi: 10.4049/jimmunol.1200466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9(7):491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 10.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2(7):465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 11.Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F, Tamassia N, Pieropan S, Biasi D, Sbarbati A, Sozzani S, Bambara L, Cassatella MA. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. 2005;105(2):830–837. doi: 10.1182/blood-2004-02-0564. [DOI] [PubMed] [Google Scholar]
- 12.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, Cassatella MA. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197(3):297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, Plebani A, Lougaris V, Quinti I, Thon V, Litzman J, Schlesier M, Warnatz K, Thiel J, Rolink AG, Eibel H. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188(1):497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 14.Schneider P, Tschopp J. BAFF and the regulation of B cell survival. Immunol Lett. 2003;88(1):57–62. doi: 10.1016/S0165-2478(03)00050-6. [DOI] [PubMed] [Google Scholar]
- 15.Crowley JE, Treml LS, Stadanlick JE, Carpenter E, Cancro MP. Homeostatic niche specification among naive and activated B cells: a growing role for the BLyS family of receptors and ligands. Semin Immunol. 2005;17(3):193–199. doi: 10.1016/j.smim.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Waldschmidt TJ, Noelle RJ. Immunology. Long live the mature B cell–a baffling mystery resolved. Science. 2001;293(5537):2012–2013. doi: 10.1126/science.1065591. [DOI] [PubMed] [Google Scholar]
- 17.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 18.Cassani B, Poliani PL, Marrella V, Schena F, Sauer AV, Ravanini M, Strina D, Busse CE, Regenass S, Wardemann H, Martini A, Facchetti F, van der Burg M, Rolink AG, Vezzoni P, Grassi F, Traggiai E, Villa A. Homeostatic expansion of autoreactive immunoglobulin-secreting cells in the Rag2 mouse model of Omenn syndrome. J Exp Med. 2010;207(7):1525–1540. doi: 10.1084/jem.20091928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter JE, Rucci F, Patrizi L, Recher M, Regenass S, Paganini T, Keszei M, Pessach I, Lang PA, Poliani PL, Giliani S, Al-Herz W, Cowan MJ, Puck JM, Bleesing J, Niehues T, Schuetz C, Malech H, DeRavin SS, Facchetti F, Gennery AR, Andersson E, Kamani NR, Sekiguchi J, Alenezi HM, Chinen J, Dbaibo G, ElGhazali G, Fontana A, Pasic S, Detre C, Terhorst C, Alt FW, Notarangelo LD. Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. J Exp Med. 2010;207(7):1541–1554. doi: 10.1084/jem.20091927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley JE, Scholz JL, Quinn WJ, III, Stadanlick JE, Treml JF, Treml LS, Hao Y, Goenka R, O’Neill PJ, Matthews AH, Parsons RF, Cancro MP. Homeostatic control of B lymphocyte subsets. Immunol Res. 2008;42(1–3):75–83. doi: 10.1007/s12026-008-8036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treml LS, Crowley JE, Cancro MP. BLyS receptor signatures resolve homeostatically independent compartments among naive and antigen-experienced B cells. Semin Immunol. 2006;18(5):297–304. doi: 10.1016/j.smim.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling R, Shi W, Vredenburgh JJ, Bigner DD, Heimberger AB. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, II, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, II, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, Friedman HS, Bigner DD. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8(10):2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neyns B, Tosoni A, Hwu WJ, Reardon DA. Dose-dense temozolomide regimens: antitumor activity, toxicity, and immunomodulatory effects. Cancer. 2010;116(12):2868–2877. doi: 10.1002/cncr.25035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


