Abstract
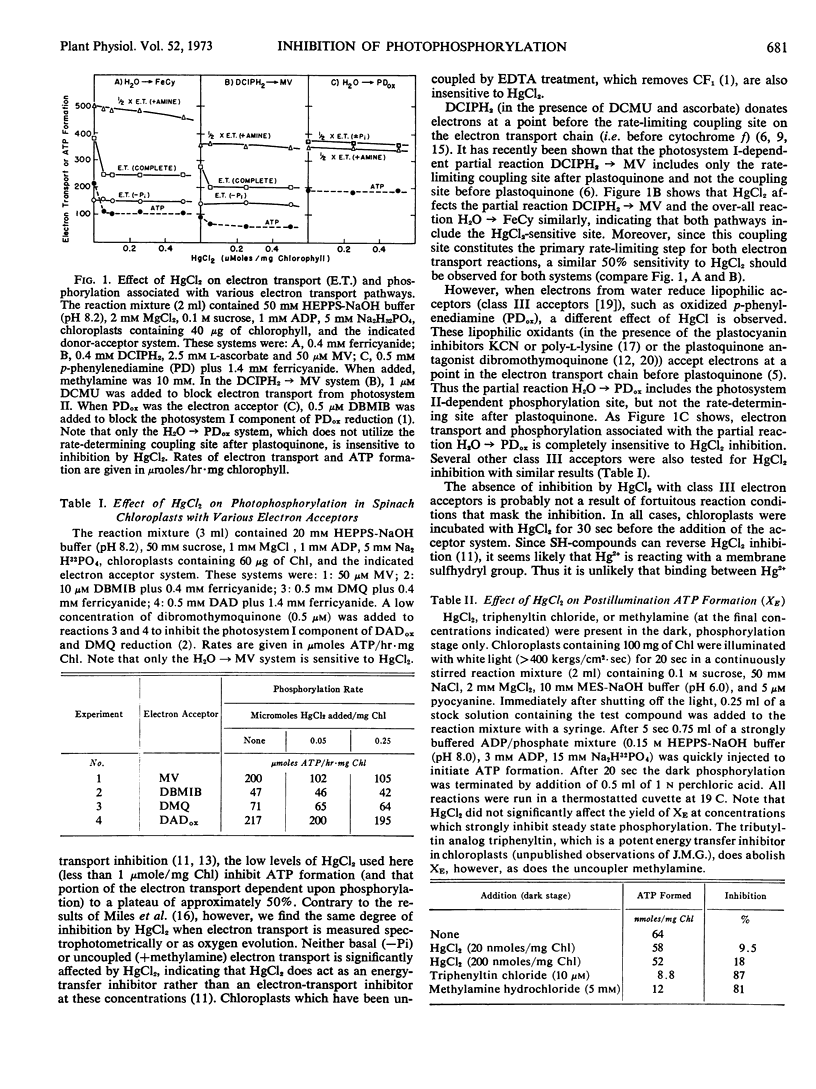
Photophosphorylation associated with noncyclic electron transport in isolated spinach (Spinacia oleracea) chloroplasts is inhibited to approximately 50% by low concentrations of HgCl2 (less than 1 μmole Hg2+/mg chlorophyll) when the electron transport pathway includes both sites of energy coupling. Reactions involving only a part of the electron transport system can give a functional isolation of at least two sites coupled to phosphorylation. Only one of these sites, located between the oxidation of plastoquinone and the reduction of cytochrome f, is sensitive to mercuric chloride. The energy conservation site located before plastoquinone and close to photosystem II is unaffected by HgCl2 concentrations up to 10-fold those required to inhibit phosphorylation by the coupling site after plastoquinone. This site-specific inhibition may reflect a mechanistic difference in the mode of energy coupling at the two coupling sites or a variable accessibility of HgCl2 to these sites.
Concentrations of HgCl2, which inhibit steady state phosphorylation, do not inhibit dark phosphorylation after illumination (XE), suggesting that HgCl2 affects a step in the coupling mechanism prior to the terminal step of ATP formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Böhme H., Cramer W. A. Localization of a site of energy coupling between plastoquinone and cytochrome f in the electron-transport chain of spinach chloroplasts. Biochemistry. 1972 Mar 28;11(7):1155–1160. doi: 10.1021/bi00757a007. [DOI] [PubMed] [Google Scholar]
- Etienne A. L., Lavergne J. Action du m-dinitrobenzène sur la phase thermique d'induction de fluorescence en photosynthèse. Biochim Biophys Acta. 1972 Nov 17;283(2):268–278. doi: 10.1016/0005-2728(72)90243-5. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Izawa S. Photosystem-II electron transport and phosphorylation with dibromothymoquinone as the electron acceptor. Eur J Biochem. 1973 Aug 1;37(1):185–192. doi: 10.1111/j.1432-1033.1973.tb02974.x. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Izawa S. Studies on the energy coupling sites of photophosphorylation. I. Separation of site I and site II by partial reactions of the chloroplast electron transport chain. Biochim Biophys Acta. 1973 Aug 31;314(2):211–223. doi: 10.1016/0005-2728(73)90136-9. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Ort D. R. Studies on the energy coupling sites of photophosphorylation. 3. The different effects of methylamine and ADP plus phosphate on electron transport through coupling sites I and II in isolated chloroplasts. Biochim Biophys Acta. 1973 Oct 19;325(1):157–166. doi: 10.1016/0005-2728(73)90161-8. [DOI] [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Connolly T. N., Winget G. D., Good N. E. Inhibition and uncoupling of photophosphorylation in chloroplasts. Brookhaven Symp Biol. 1966;19:169–187. [PubMed] [Google Scholar]
- Izawa S., Gould J. M., Ort D. R., Felker P., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. 3. A dibromothymoquinone-insensitive phosphorylation reaction associated with photosystem II. Biochim Biophys Acta. 1973 Apr 27;305(1):119–128. doi: 10.1016/0005-2728(73)90237-5. [DOI] [PubMed] [Google Scholar]
- Kraayenhof R., Izawa S., Chance B. Use of uncoupling acridine dyes as stoichiometric energy probes in chloroplasts. Plant Physiol. 1972 Dec;50(6):713–718. doi: 10.1104/pp.50.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum A. W., Bonner W. D. The effect of artificial electron donor and acceptor systems on light-induced absorbance responses of cytochromes f and other pigments in intact chloroplasts. Biochim Biophys Acta. 1972 Apr 20;267(1):149–159. doi: 10.1016/0005-2728(72)90146-6. [DOI] [PubMed] [Google Scholar]
- Miles D., Bolen P., Faraq S., Goodin R., Lutz J., Moustafa A., Rodriquez B., Weil C. Hg ++ - a DCMU independent electron acceptor of photosystem II. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1113–1119. doi: 10.1016/0006-291x(73)91521-0. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Izawa S., Good N. E., Krogmann D. W. Effects of the plastocyanin antagonists KCN and poly-L-lysine on partial reactions in isolated chloroplasts. FEBS Lett. 1973 Apr 1;31(1):119–122. doi: 10.1016/0014-5793(73)80087-0. [DOI] [PubMed] [Google Scholar]
- Ouitrakul R., Izawa S. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. II. Acceptor-specific inhibition by KCN. Biochim Biophys Acta. 1973 Apr 27;305(1):105–118. doi: 10.1016/0005-2728(73)90236-3. [DOI] [PubMed] [Google Scholar]
- Saha S., Ouitrakul R., Izawa S., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971 May 25;246(10):3204–3209. [PubMed] [Google Scholar]
- Trebst A., Reimer S. Properties of photoreductions by photosystem II in isolated chloroplasts. An energy-conserving step in the photoreduction of benzoquinones by photosystem II in the presence of dibromothymoquinone. Biochim Biophys Acta. 1973 Apr 27;305(1):129–139. doi: 10.1016/0005-2728(73)90238-7. [DOI] [PubMed] [Google Scholar]
- Winget G. D., Izawa S., Good N. E. The inhibition of photophosphorylation by phlorizin and closely related compounds. Biochemistry. 1969 May;8(5):2067–2074. doi: 10.1021/bi00833a043. [DOI] [PubMed] [Google Scholar]



