Abstract
Introduction
Alpha particles possess an exquisite degree of cytotoxicity when employed for targeted α–particle therapy (TAT) or radioimmunotherapy (RIT). 212Pb, which acts as an in vivo generator of the α-emitting nuclide 212Bi has shown great promise in pre-clinical studies when used to label the HER2 binding antibody, trastuzumab. Currently, the first RIT clinical trial employing 212Pb radiolabeled trastuzumab is in progress. This report provides detailed current protocol operations and steps that were generated for use in the clinical trial as well as the relevant pre-clinical experimentation, and describes in detail the labeling of proteins or peptides with 212Pb as provided via a 224Ra based generator system.
Methods
212Pb was eluted from the 224Ra/212Pb generator using hydrochloric acid (2 M). The generator eluate was evaporated and digested with nitric acid (8M) followed by extraction of the 212Pb with dilute nitric acid (0.1 M). The dilute nitric acid solution of 212Pb was used to label the immunoconjugate Trastuzumab-TCMC (2-(4-isothiocyanatobenzyl-1,4,7,10-tetraaza-1,4,7,10,tetra-(2-carbamonylmethyl)-cyclododecane) at pH 5.5.
Results
Elution of 212Pb from the generator was efficient yielding > 90% of available 212Pb. Trastuzumab-TCMC was efficiently labeled with a radiochemical yield of 94 +/− 4% (n = 7) by ITLC and an isolated yield of 73 +/− 3 % (n = 7).
Conclusions
The results show the feasibility of generating radioimmunoconjugates and peptide conjugates for use as in vivo α generator systems in the clinic. The technology holds promise in applications involving the treatment of minimal disease such as micrometastases and residual tumor after surgical debulking, hematological cancers, infections, and compartmental cancers, such as ovarian cancer.
Keywords: Radioimmunotherapy, alpha targeted therapy, Lead-212, trastuzumab, cancer therapy
1. Introduction
Targeted radionuclide therapy is based on the attachment of cytotoxic radionuclides to a suitable vector biomolecule, usually a protein such as a monoclonal antibody or peptide, to permit the specific targeting of pathologic cells. This approach has found success in a number of diseases such as cancer and infection [1–4]. The technology requires the vector (i.e., monoclonal antibodies or peptides) with high specificity and affinity, appropriate pharmacokinetics, a radionuclide with suitable physical characteristics, and the requisite chemistry to attach the radionuclide in a stable manner to the vector. Molecular biology continues to provide high affinity therapeutic monoclonal antibodies that have found success in the clinic [5]. The effector properties of these biotherapeutics can be potentiated and further exploited by coupling them with cytotoxic agents such as conventional cancer therapeutic agents or medically relevant radionuclides [6–9]. Radionuclides used in this latter approach have generally been emitters of β−- or α-radiation, although to a lesser degree, Auger emitters have also been investigated [10, 11]. With respect to β−-emitting nuclides, radioisotopes such as 90Y, 177Lu, 131I, 186Re and 188Re have typically been used to label biomolecular vectors for the therapy of solid and hematologic cancers [8,12]. A limitation of β−–particle radiation is low linear energy transfer (LET) characteristics. For example, the mean LET of the β− particles from 90Y is 0.2 KeV/µm. This means that the particle range is long, thus, the deposition of the energy of the β− radiation in tissue, upon which cell killing depends, occurs along a relatively long path length. Energy deposition is therefore sparse. The longer path length of β− radiation makes β−-radionuclide targeted therapy more applicable to the treatment of larger, non-hematological, solid tumors that are >1 cm in diameter.[8] However, for the same reason of particle path length, a large proportion of the β−-particle energy can be wasted or deposited outside the target tumor volume. This concept has been well defined by the work of O’Donoghue, et al [13]. The longer path length also results in the irradiation of non-target normal tissue causing considerable tissue toxicity, which is referred to as crossfire [14,15]. The limiting factor for the administered dose in these applications is normal tissue toxicity, usually hematological, although other organs can also be dose limiting.
A primary problem in cancer therapy is the presence of minimal disease such as micrometastases or residual tumor tissue left behind after surgical debulking. Another obstacle in cancer therapy is the failure of chemotherapy or traditional external beam radiotherapy using x- or γ-rays. The development of resistance or survival of cancer cells following regimens of either of these modalities is a consequence of tumor biology, i.e., tumor cell heterogeneity, accessibility, asynchronous cell cycle, zones of hypoxia, etc. Efforts to improve therapeutic efficacy by increasing the radiation dose or chemotherapeutic agent(s), or the inclusion of radiosensitizing agents tends to be only partially effective at best. In order to improve therapeutic efficacy, α-particle radiation has been proposed as an answer to some of these problems. Targeted therapy with α-particle radiation has been shown to induce apoptosis, activate apoptosis pathways, abrogate G2 phase cell cycle arrest and block DNA damage repair, among other effects on cancer cells [16–20]. The approach has been bolstered by a recent study by Friesen et al. [17] which showed that targeted α-particle therapy overcomes resistance to apoptosis, chemotherapy with doxorubicin, γ- and β−-radiation in cancer cells. Alpha therapy has also been proposed for use to overcome multidrug resistance in infection.
A highly desirable goal in the therapy of cancer is the ability to target pathological cells while sparing normal cells in the vicinity of the target cell. If significant differential targeting can be achieved by the vector, then a toxic payload on the vector can deliver a lethal dose primarily to those cells containing higher concentrations of the target molecule while sparing nearby normal cells. The short path length of the α-particle complements this concern and as indicated above reverses resistance to chemotherapy or conventional radiotherapy [16,17] The short path length of α-particles also renders them suitable for the treatment and management of patients with minimal disease such as micrometastases or residual tumor after surgical debulking, hematological cancers, infections, and cancers such as ovarian cancer or neoplastic meningitis that present as single layers or sheets of cells on compartment surfaces. Several pre-clinical and now clinical studies have shown the therapeutic efficacy of monoclonal antibodies labeled with α-particle radionuclides cancers as well as infections [1,21–27]. Most of the applications thus far have involved intraperitonial injections to treat compartmentalized disease.
An α-particle is a naked helium-4 nucleus, therefore, it is relatively heavier than other subatomic particles emitted from decaying radionuclides and nuclear reactions such as electrons (β−- and β+-emission, Auger and conversion electrons), neutrons and protons. With a +2 charge, α-particles are more effective in causing ionization, have a high LET (in the range of 100 KEV/µm) and are highly efficient in depositing energy in tissue. For the same amount of particle energy, an α-particle deposits ≥ 500 times more energy per unit path length than an electron or β−-particle. Unlike low LET radiation, the cytocidal efficacy of α–particle radiation is indifferent to dose fractionation, dose rate or hypoxia.
Approximately 100 α-emitting radionuclides are known and are limited to the higher atomic numbers (atomic number ≥ 82) [28]. Due to problems of availability and production, half-life, cost and the ability to chemically and stably incorporate them into a suitable vector, only a few of these radionuclides are medically relevant and available for potential clinical use. These include 211At, 212Bi, 213Bi, 225Ac, 223Ra, 212Pb, 227Th, and 149Tb [28].
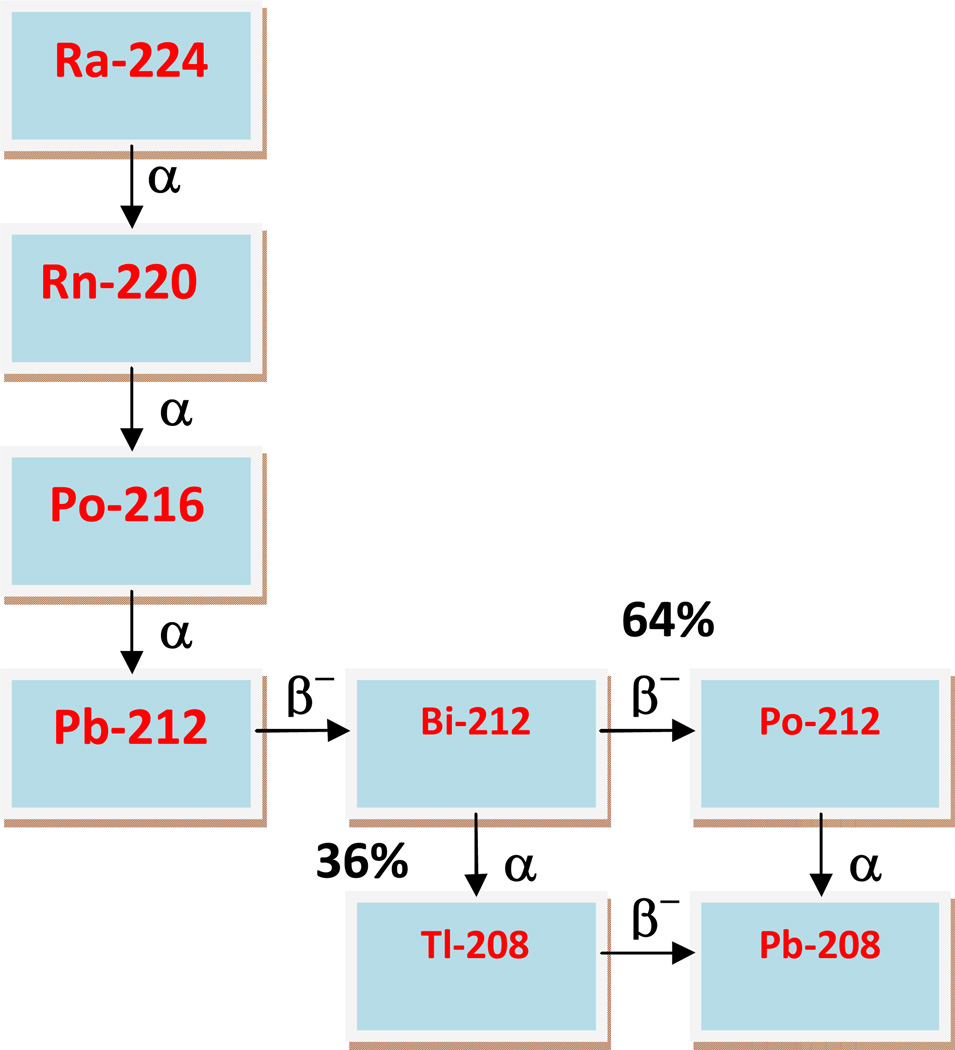
Of radionuclides proposed for applications in targeted α–particle therapy, 212Pb, part of the 228Th decay chain (Figure 1), is highly appealing. 212Pb is available from a 224Ra-based generator that has a convenient half-life of 3.6 days and can provide clinical grade material in enough quantities for administration to patients. Furthermore, the associated photon radiation from the 224Ra/212Pb system can be attenuated with shielding material to avoid high radiation dose to personnel. The parental 224Ra is produced from 228Th which is in turn produced from 232Th or 232U. 212Pb is a 10.6 h half-life β−-emitter that decays to 212Bi, which is a short half-life (1.06 h) α-emitter. By incorporating 212Pb into bioactive vectors, the radionuclide functions as an in vivo generator of 212Bi to overcome the disadvantages of the shorter half-life of the 212Bi. 212Pb used in this manner has distinct advantages over the shorter half-life bismuth radionuclides, 212Bi and 213Bi. Other than the convenience of the 10.6 h half-life that offers a reasonable dose preparation and administration schedule, 212Pb can deliver >10 times the dose per unit administered activity of the Bi radionuclides to tumor. However, the decay of 212Pb results in the recoil of a significant proportion (30–36%) of the 212Bi daughter, a consequence of the conversion electrons from the accompanying γ-emission [29, 30]. For this reason, it has been suggested that efforts be made to counter the delivery of free 212Bi to the kidneys by, for example, co-injecting chelating agents such as DTPA or EDTA with the injectate to facilitate the rapid excretion of released 212Bi [30]. These problems notwithstanding, many pre-clinical studies have shown that 212Pb labeled antibodies are effective as single agents or in combination with chemotherapeutics in cancer therapy in select applications where this loss of 212Bi is of minimal concern, e.g., intraperitoneal administration to treat local metastatic or residual disease [18, 25, 31–35].
Figure 1.
Decay Scheme of 224Ra
With the advent of the first clinical trail that employs 212Pb as the therapeutic agent (targeted by trastuzumab [36]), this radionuclide has finally reached a landmark position in the family of α-emitters suitable for such applications [28]. The radiochemical protocols for elution of 212Pb from the 224Ra generator have undergone considerable evolution and modification over the span of the >2 decades long chemical and pre-clinical development of 212Pb; the radiolabeling protocols have undergone a similar process as well. Furthermore, both of these critical protocols were subject to revalidation for their inclusion in the IND filing for the clinical trial. However, despite all of the pre-clinical studies in the literature, there is no single complete fully detailed literature report of these operations that would facilitate the use of 224Ra generators in those laboratories that would want to use this radionuclide. As such, provided herein is the full, detailed methodology that permits the use of 224Ra generators to create 212Pb radiolabeled proteins and peptides.
2. Methods
2.1 Materials
Phosphate buffered saline (PBS) (Lonza 17-516F), ascorbic acid (Fluka 95209), ammonium acetate (NH4OAc) (Mallinckrodt Chemicals, 3272 -04), hydrochloric acid 37% (Fisher Scientific, Optima, 7647-01-0), nitric acid (Fisher Scientific, Optima, A467–500), Chelex 100 Resin Sodium form (Bio-Rad 200–400 mesh 142–2842), or equivalents were purchased and used as described (vide infra). Water (18 MOhms) was obtained from an Ultra Pure Water System (Hydro Service and Supplies Inc., Gaithersburg, MD). The specific laboratory supplies that these processes used were 50 mL conical polypropylene tubes (Becton Dickinson 4-2098-1), 15 mL conical polypropylene tubes (Cellstar 188281), 20 mL borosilicate glass vials (Kimble Chase 74500-20), 250 mL polyethylene bottles (Corning 430281), 3 mL syringes (Becton Dickinson 309585), 12 mL syringes (Monoject 8881-512878), 10 µL pipet tips (Denville Scientific), 200 µL pipet tips (Denville Scientific P3020), 1000 µL pipet tips (Denville Scientific P1123) pH paper 2.0–9.0 (EMD Chemicals 9578), PD-10 columns (GE Healthcare 17-0851-01), Teflon coated magnetic stir bars (Fisher Scientific, 14-513-57SIX), and Poly-Prep Column (Bio-Rad 731-1550). NH4OAc buffer (5.0 M, pH 7), and ascorbic acid (220 mg/mL) were prepared as described below. Hydrochloric acid (2 M), nitric acid (8 M and 0.1 M), were prepared using ultrapure reagents and 18 MOhms water as specified above. Other reagents prepared were 10 mM EDTA in 0.15 M NH4OAc solution, and 10 mM NaOH in normal saline for ITLC
2.1.1 Acid washed vials
A teflon coated magnetic stir bar was added to a 20 mL borosilicate glass vial and the vial was filled with 8 M nitric acid. The vial is stirred overnight and the acid discarded. The vial is washed thoroughly with water (18 MOhms), filled with 0.1 M nitric acid, and again stirred for 1 h. The acid was again discarded, and the vial and stir bar thoroughly rinsed with water (18 MOhms).
2.1.2. Ascorbic Acid Solution
Ascorbic acid (2.20 g), was added to H2O (10 mL) (18 MOhms) in a 50 mL conical polypropylene (PP) tube and vortexed vigorously until dissolved. The solution was demetalized using Chelex 100 resin (pre-swollen in H2O). The resin was transferred into a Poly-prep chromatography column to make a 2 mL column and washed with H2O (100 mL). The ascorbic acid solution was loaded onto the column and allowed to flow through the column by gravity. The first 5 mL of ascorbic acid eluate were discarded. The remaining ascorbic acid eluate was transferred into a 15 mL conical PP tube, capped and wrapped in aluminum foil to protect from light. The solution was stored at room temperature.
2.1.3. 5.0 M NH4OAc, pH 7
NH4OAc (38.54 g) was dissolved in H2O (100 mL) (18 MOhms) in a metal free 250 mL Corning polyethylene (PE) bottle. The solution was demetalated using Chelex 100 resin (pre-swollen in H2O). The resin was transferred into a Poly-prep chromatography column to make a 2 mL column and washed with H2O (100 mL). The NH4OAc solution was loaded onto the column and allowed to flow through the column by gravity. The first 5 mL of eluate were discarded. The remaining eluate was collected into a clean PE bottle; the pH of the eluate was checked with pH paper. The solution was stored at room temperature.
2.2 Safety
212Pb emits β−-radiation while 212Bi, its daughter product, emits α-radiation. These particular radiations are hazardous if internalized and care must be taken to avoid ingestion or exposure to open skin wounds. Of particular concern, with respect to external irradiation, is the highly energetic γ-ray from 208Tl (Eγ = 2.61 MeV) decay. This γ-ray is highly penetrating, and as such work with 212Pb must be performed in either an appropriately shielded hot cell such as presented here, or with adequate shielding using 6” lead bricks. In lieu of using the manipulator arms of the hot cell to aid in the elution of the generator, a length of PEEK tubing fitted with a male Luer-Lok adaptor at one end can be connected to the generator inlet. The other end of the PEEK tubing can be fitted with a female Luer-Lok adaptor to which the syringe containing the generator elution acid can be connected from outside the Hot Cell or beyond other shielding.
2.3 The 224Ra/212Pb Generator
The generator was manufactured by Pacific Northwest National Laboratories (Richland, WA) and sold as a 224Ra/212Pb generator through AlphaMed Inc. (Sheldonville, MA). Generators of up to 20 mCi 224Ra have been obtained from the vendor. The generator consists of 224Ra (T1/2 = 3.6 days) adsorbed on a cation ion-exchange resin, AG-MP50 (BioRad; Hercules, CA) which is fitted with inlet and outlet tubing to facilitate the attachment of a Luer-Lok syringe at the inlet for elution. The resin is shielded with lead (the inlet and outlet tubing lead from the resin to the outside of the lead shielding). The generator is mounted in a Hot Cell (Von Gahlen International Inc, Chatsworth, GA). The Hot Cell (72 × 60 in) has 6” lead shielding and is fitted with two manipulator arms (Model GM 1100, Inter Control Technology, Germany), two lead shield doors (one on either side) and a retractable 18” × 24” × 12” leaded glass (6” lead equivalent) window. The generator is eluted daily with 2 M ultrapure HCl (4 mL) contained in a syringe fitted to the inlet at a flow rate of ~ 1 mL per minute using the manipulator arms of the Hot Cell.
2.4 Equipment and Instrumentation
The following laboratory equipment (or equivalent) was employed: Scientech analytical balance (Model SA 210), Heater/Stir plate (Sybron/Thermolyne, Model Nuova II), Dose Calibrator (Capintec, Model CRC-127R), Heat Lamp (Fisher Scientific, # 11-505-50), Eppendorf thermomixer (Model 5436). Size exclusion HPLC (SE-HPLC) was performed on a Waters HPLC Breeze System equipped with a Binary HPLC Pump (Model 1525), an Autosampler (Model 717 Plus), a Dual Wavelength Absorbance detector (Model 2487), a two channel Waters Bus SAT/IN interphase for a Beckman Radioisotope Detector (Model 170) and a TSK SW3000 size-exclusion column (TosoHas Bioscience). The HPLC system was controlled by a Compaq Deskpro EN computer running a Waters Breeze software version 3.2. The HPLC was run at 0.5 mL/min using PBS as solvent.
Assaying 212Pb (µCi amounts) was performed exploiting the 238.6 KeV peak utilizing a pre-calibrated Princeton Gammatec High Purity Germanium Detector cooled with a PGT Lab Cooler and fitted with an Ortec EG&G HVPS, an Ortec EG&G Pulser 480, an Ortec EG&G Bias supply 459, an Ortec EG&G Bin 4001A, and an Ortec EG&G Spectroscopy 672. The instrument is run by Ortec EG&G GammaVision Software, A66-B32 version 6.1, installed on a Dell Dimension XPS T 450 computer running on Windows NT. The high purity germanium detector was calibrated for efficiency and energy by accumulating counts for 50,000 s using a mixed gamma standard source (Eckert and Ziegler, Atlanta, GA) and the calibration wizard of the Gammavision software. 212Pb was measured using the 238.6 keV gamma peak (43.6%). Sub-µCi quantities of 212Pb were counted using γ-counter (WizardOne, Perkin Elmer)
2.5 Generator Elution and Radioconjugate Preparation
2.5.1 Pre-Elution Preparations
The controls for the stirrer/hot plate, heating and stirring, in the Hot Cell were both set to 3.5. The 250 watt heating lamp was set adjacent to the stirrer/hot plate and turned on. A trio of 15 mL PP tubes were labeled HNO3 “A”, HNO3 “B”, and HNO3 “C”, respectively, and placed in a test tube rack. The plunger of a 5 mL Luer-Lok syringe was drawn to the 2 mL mark and 8 M HNO3 (1.5 mL) was then drawn into the syringe. The syringe and contents were placed into the 15 mL PP tube labeled HNO3 “A”. A second and third 5 mL syringe were each similarly loaded and placed in the PP 15 mL tubes labeled HNO3 “B” or HNO3 “C”, respectively.
2.5.2 Generator Elution and Protein/Peptide Labeling
With the generator in place in its shielded environment, both the inlet and outlets were first wiped with an alcohol swab. An acid washed 20 mL borosilicate glass vial equipped with a small acid washed Teflon coated magnetic stir bar and a cap were weighed and the weight recorded. The vial with stir bar was situated in a secure position in front of the generator and the outlet of the generator was placed into the vial. No 0.22 micron filter was used at the outlet. The plunger of a 12 mL Luer-Lok syringe was withdrawn to the 5 mL mark, and thereafter 2 M HCl (4.0 mL) was taken up into the syringe. This syringe was then fitted onto the inlet of the generator and slowly emptied of the contents onto the generator, including the air in the headspace, at a rate of ~1 mL/min. The generator outlet was removed from the borosilicate glass vial and placed into an empty clean 50 mL PP tube. The borosilicate glass vial was capped and weighed. The volume of the generator eluate contained in the borosilicate glass was noted by comparison of the weights of the borosilicate glass vial with and without the eluate. The activity of the generator eluate in the borosilicate glass vial was then assayed in the dose calibrator. The borosilicate glass vial was placed on a stir plate and stirred moderately for 30 sec. A sample of the generator eluate (2 µL) was removed from the vial, placed into a microfuge vial, and assayed for activity of 212Pb using the high purity germanium (Ge) detector from which the total eluted activity was calculated and recorded at that time. The total activity was calculated by multiplying the activity per µL measured with the high purity Ge detector with the total volume of the 212Pb eluate from the generator. The cap was removed from the borosilicate vial containing the 212Pb eluate and using a manipulator arm of the Hot Cell, the vial containing the 212Pb eluate was gently placed on the stirrer/hot plate with the 250 watt heating lamp shining directly on the vial. The 212Pb eluate in the borosilicate glass was evaporated completely with gentle stirring. The borosilicate glass vial was removed from the stirrer/hot plate, placed in a lead pig, and allowed to cool for 5 min. Using a manipulating arm of the Hot Cell, the syringe in the 15 mL PP tube labeled HNO3 “A” was picked up making sure to hold the syringe at the barrel. Using the other manipulating arm of the Hot Cell, the contents of the syringe (8 M HNO3) were emptied into the vial containing the 212Pb. The syringe was returned to the 15 mL PP tube labeled HNO3 “A”. The vial was returned to the stirrer/hot plate making sure that the 250 watt heating lamp was shining directly on the vial. This process was then repeated using the syringes in the 15 mL PP tube labeled HNO3 “B” and “C”. An empty capped PP test tube (12 × 75 mm) was labeled “reaction vial” and tared. The activity in the 20 mL borosilicate vial was measured with the dose calibrator. The digested 212Pb residue was extracted with 0.1 M HNO3 (200 µL) and transferred to the tared test tube. The extraction process was repeated using two additional portions of 0.1 M HNO3 (150 µL) adding them to the test tube. The residual activity in the vial was assayed with a dose calibrator after completing the extractions. The extracted 212Pb was briefly vortexed and then 2 µL transferred to a microfuge tube for subsequent analysis. The activity of the 2 µL aliquot was measured using the High purity Ge detector. The test tube with remaining contents was capped and weighed. The total activity of 212Pb extracted was calculated by again multiplying the activity per µL as assayed on the High purity Ge detector by the calculated volume of the extract. Ascorbic acid solution (50 µL, 220 mg/mL) was added to the 212Pb solution in the “reaction vial”. For every 10 µL of 212Pb solution, 5 M NH4OAc (1 µL) was added into the “reaction vial”. The test tube labeled “reaction vial” was picked up using a manipulating arm of the Hot Cell, the contents of the vial mixed on the vortex mixer (3 sec), and the vial returned to a stand close to the Hot Cell access door. The pH of the reaction mixture was tested with pH paper (range 2 – 9) and verified to be 5 – 5.5. The antibody chelate conjugate, TCMC-trastuzumab [25, 32–35] was added to the 212Pb (add 200 µg of immunoconjugate per 1000 or more µCi of 212Pb activity) in the “reaction vial”, capped and vortexed for 3 sec. Thereafter, the reaction mixture was moved to the Eppendorf Thermomixer set at 37 °C where the reaction was incubated for 1 h. The vial was then returned to a test tube rack near the Hot Cell door and the reaction was halted the by addition of 0.1M EDTA (4 µL) to the vial and vortexed again for 3 sec. A 2 µL sample of the reaction mixture was removed to be assayed on the High Purity Ge detector. Total volume and activity of the reaction mixture was assayed and recorded. The total preparation time is 3.5 h.
2.5.3 PD-10 Purification
A PD-10 column was pre-equilibrated by eluting with PBS (25 mL). The reaction mixture was loaded onto the top of the pre-equilibrated PD-10 column and allowed to flow through the PD-10 column. PBS (500 µL) was added to the column. Additional PBS was added to make the total volume through the column to be 2500 µL. The receiving PP tube of the PD-10 column was replaced with a product vial containing 0.1 M EDTA (5 µL), and 20 µL of human or mouse serum albumin. The PD-10 column was eluted with PBS (1700 µL) into the product receiving vial. Thereafter, 2 – 5 µL was transferred to a microfuge tube for subsequent analysis. The product mixture (2 µL) was removed for assay on the High Purity Ge detector. The total isolated activity was calculated by multiplying the activity/µL with the total volume of the product. Specific activity of isolated product was 825 +/− 225 mCi/µmol (5.5 +/− 1.5 mCi/mg) protein (n = 7).
2.5.4 ITLC Assay Procedure
The stationary phase for ITLC was ITLC™ SG (Pall Life Sciences, P/N 61886): Instant Thin Layer Chromatography Silica Gel Impregnated Glass Fiber Sheets or the equivalent. The mobile phases were: (A) 10 mM EDTA in 0.15 M NH4OAc and (B) 10 mM NaOH in normal saline.
Two 15 mL PP tubes per sample were labeled Solvent A and Solvent B, respectively. Into each tube was pipetted 1 mL of the respective mobile phase solution. The ITLC sheets were cut into 1 × 10 cm strips, marked 1 cm from one end with a pencil to indicate the solvent front, and marked 1.5 cm from the other end to indicate the origin. Two ITLC strips were spotted with 1 – 2 µL of the test Pb2+ solution at the origin. Two ITLC strips were spotted with 1 – 2 µL of the quenched reaction mixture at the origin. Two ITLC strips were spotted with 1 – 2 µL of the PD-10 purified antibody radioimmunoconjugate at the origin. One strip from each pair of the strips prepared was inserted into the PP tubes labeled Solvent A and the other strip from each pair was inserted into the PP tubes labeled Solvent B making sure that the end marked for the origin touches the solvent, but the spot was above the solvent. After the solvent reaches the marked solvent front, the strips were transferred to an appropriately labeled empty 15 mL PP tube. After 5 min, the ITLC strips were cut in half and each half was placed separately into an appropriately labeled 20 mL scintillation vial. The scintillation vials were counted with a high purity Ge detector. After at a minimum of 10 h, the scintillation vials were counted using the γ-detector. The percent (%) count at the origin half and solvent front half was calculated. The % free 212Pb was determined from the % activity at the solvent front of the ITLCs in solvent A. The % radiochemical yield by ITLC was determined from the % activity at the origin for the quenched reaction mixture ITLC strips. The purity of the product was determined from the % activity at the origin for the PD-10 purified product sample.
3. Results and Discussion
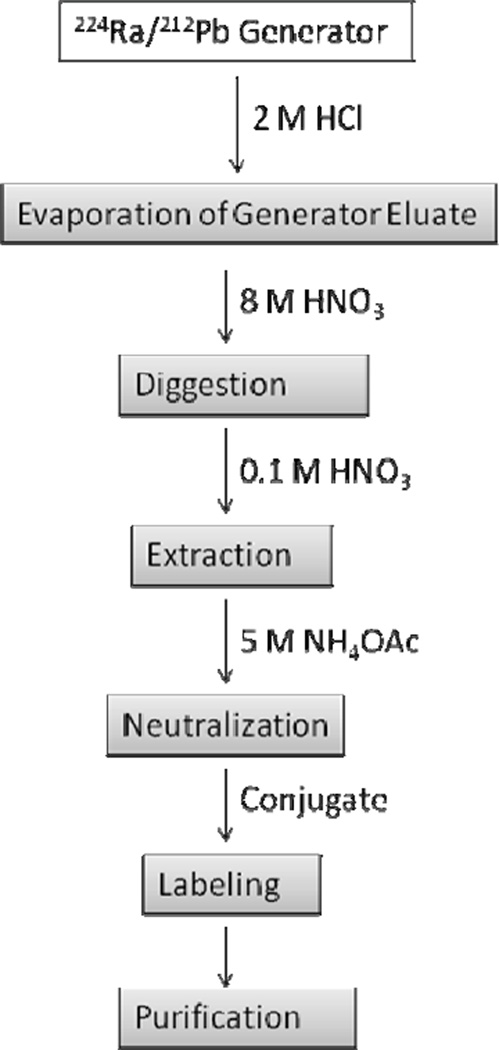
Figure 2 shows a flow chart of the 212Pb labeling process from generator elution to the purification of the radioconjugate. When the generator is eluted daily, > 90% of the expected 212Pb activity can be eluted. Typically, a 2 mCi generator at time of receipt will yield ~1.8 mCi of 212Pb on the first day. Generators of greater activity (e.g., up to 20 mCi at time of receipt) are routinely available from AlphaMed Inc. Dependent upon transit time and the level of activity on the column, it may be necessary to wash the generator with 4 mL HCl (2 M) as soon as it is received. This will wash the generator, removing debris brought about by radiolysis. It is also imperative at this time to assay for 224Ra breakthrough. The high purity Ge detector assay of the generator eluate can indicate if breakthrough is excessive. Regardless, an aliquot of the first eluate should be saved for further breakthrough analysis after most of the 212Pb contained therein has decayed (between 48 and 72 h) and a new equilibrium is established between 224Ra and its daughters. The saved aliquot is re-assayed on the high purity Ge detector. At this stage, a daily activity measurement of the total activity with a γ-counter can be made to determine the effective half-life of the radioactivity in the aliquot. A half-life significantly greater than 10 h will indicate 224Ra breakthrough. No assays of organic material in the eluate have been performed. The generator has been used for up to a week after receipt without any problems. To our knowledge, no IND’s have been issued to date using the generator described here. On the second day after receipt of the generator the amount of 212Pb eluted from the generator will be 65 – 75% of the activity from the first day. Subsequent elutions of the generator will yield about 80% of the previous day’s 212Pb activity. These are > 90% of the available 212Pb on the column.
Figure 2.
Flow chart for labeling conjugates with 212Pb
After evaporation of the generator eluate, the residue is digested with 8 M HNO3. The color of the solution after the addition of HNO3 is usually an indicator of radiolytic debris eluted from the generator. A dark brown color is indicative of extensive radiolysis and a high amount of debris and may indicate need for more digestions beyond the 3 recommended in this protocol. Approximately, 75 – 90% of the 212Pb is usually extracted into the HNO3 (0.1 M).
Typically, elution of the generator takes 4–5 min. Evaporation of the generator eluate and digestion of the residue takes ~45 min. Extraction of the 212Pb with 0.1 M HNO3 takes ~20 min. Labeling of the protein/peptide and subsequent purification requires ~80 min. Thus, the entire process requires ~3.5 h to prepare an injectable dose.
Of a critical nature is the removal of all trace metal contaminants in the preparation of all reagents and materials. Thus, all glassware should be acid treated as described above, prepared just prior to use, and all other labware should be made from metal-free polyethylene or polypropylene. The importance of utilizing aqueous solutions devoid of trace metals can not be overemphasized. For aqueous acids this is ensured by utilizing ultra-pure reagents as recommended in the reagents list and 18 MOhm water. The rigorous demetalization of all other aqueous solutions by using Chelex 100 resin as outlined in the preparation of the 5 M NH4OAc and ascorbic acid solutions can not be overlooked.
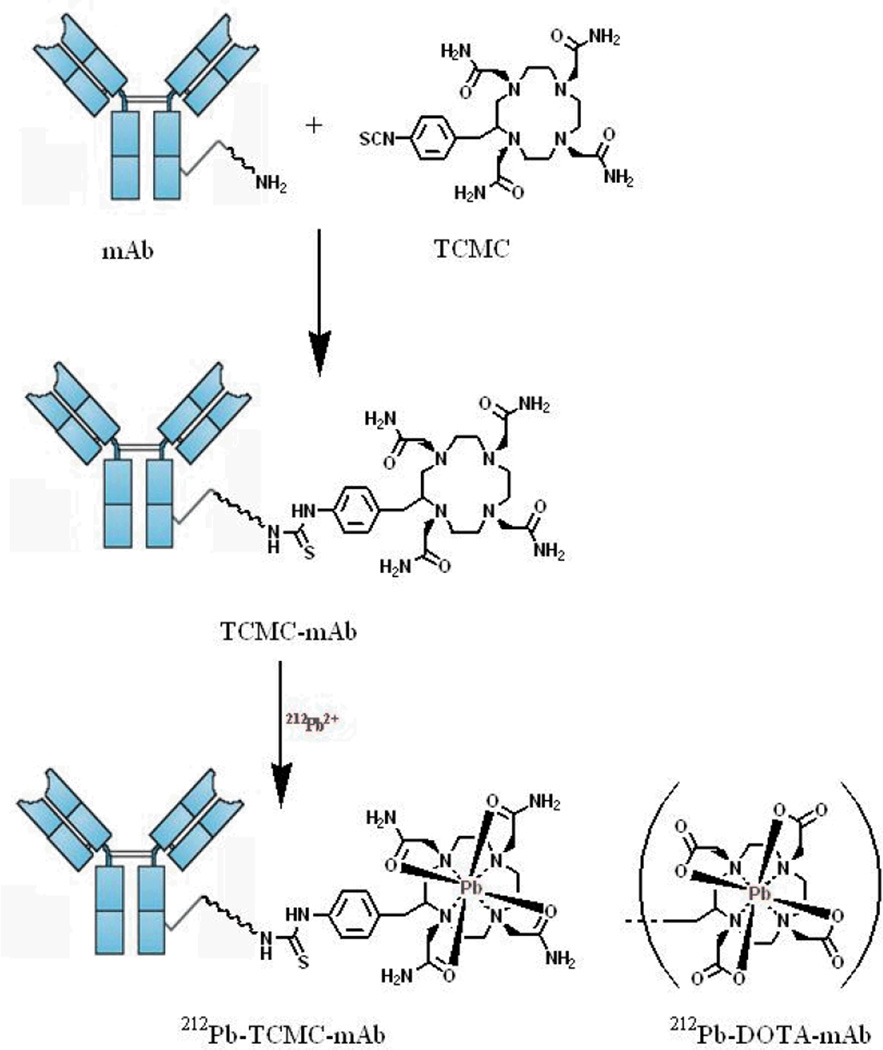
Two chelating systems have been used successfully to form conjugates for labeling with 212Pb, namely, the macrocycles DOTA and TCMC (2-(4-isothiocyanatobenzyl-1,4,7,10-tetraaza-1,4,7,10,tetra-(2-carbamonylmethyl)-cyclododecane). [30–38]. Figure 3 shows a schematic of the conjugation and labeling reactions. The preparation and storage of these bifunctional chelating agents as well as their respective conjugates is published [25, 30–38]. The TCMC chelate was specifically developed for chelating Pb while DOTA is multipurpose and has been used for chelating other metals as well. TCMC appears to form a more stable complex with Pb and as such has proven superior for in vivo use [37–40].
Figure 3.
Schematic of the conjugation of TCMC or DOTA to monoclonal antibodies and subsequent 212Pb labeling.
In general, the conjugation of proteins with the TCMC chelator is routinely performed with molar ratios of chelate to protein/peptide ranging from 5–20:1 of the chelate to protein/peptide [25,32–35]. The ratio is determined empirically for each protein/peptide. All reagents utilized for the conjugation are demetalated using Chelex-100. Following the conjugation reaction, the conjugate is dialyzed extensively to remove all free TCMC chelator from the solution. The product is then assayed for the amount of protein and the number of TCMC chelates present in the solution [41]. The average number of TCMC chelates per protein is determined by dividing the chelate concentration by the protein concentration. The final product is then aliquoted and stored at the temperature appropriate for the specific protein/peptide.
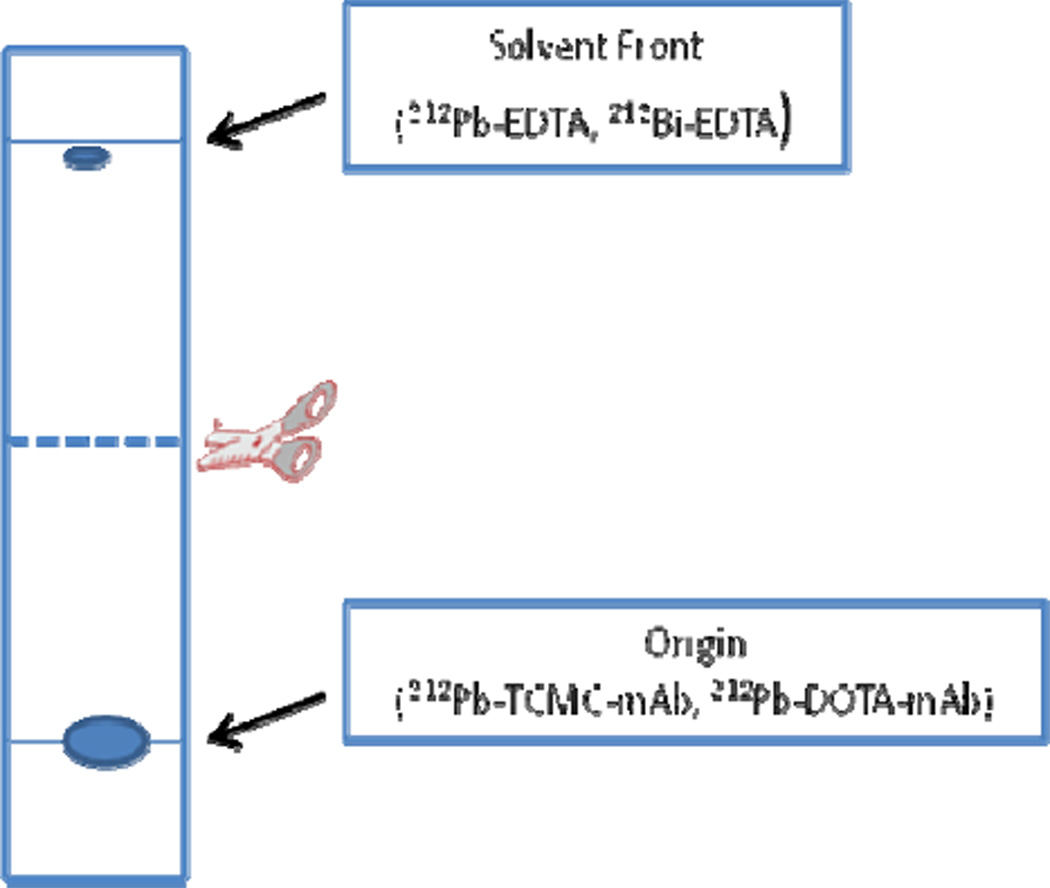
The radiochemical yield of 212Pb labeled substrate using TCMC as the chelating agent is 94 +/− 4% (n = 7) by ITLC as a percent of total 212Pb. This can be measured directly with the high purity Ge detector which allows for the differential measurement of 212Pb in the presence of its radioactive decay products. The same results can also be obtained at least 10 h after the development of the ITLC when the daughter radionuclides originally present on the ITLC strip at the time of development, which have shorter half lives than the 212Pb parent, have decayed. The ITLC solvent used depends on the substrate. For protein conjugates, two solvent systems (Solvent A and Solvent B) can be used. The Rf of the labeled protein is zero in either system (Figure 4). Because DTPA or EDTA solution is used to quench the reaction, unbound 212Pb will be chelated as the DTPA or EDTA complex. Under these circumstances the non-protein bound 212Pb as well as free recoil 212Bi (also existing as the DTPA or EDTA complexes) moves with the solvent (Rf = 1.0).
Figure 4.
Schematic of the ITLC of the labeling mixture at the end of a labeling reaction.
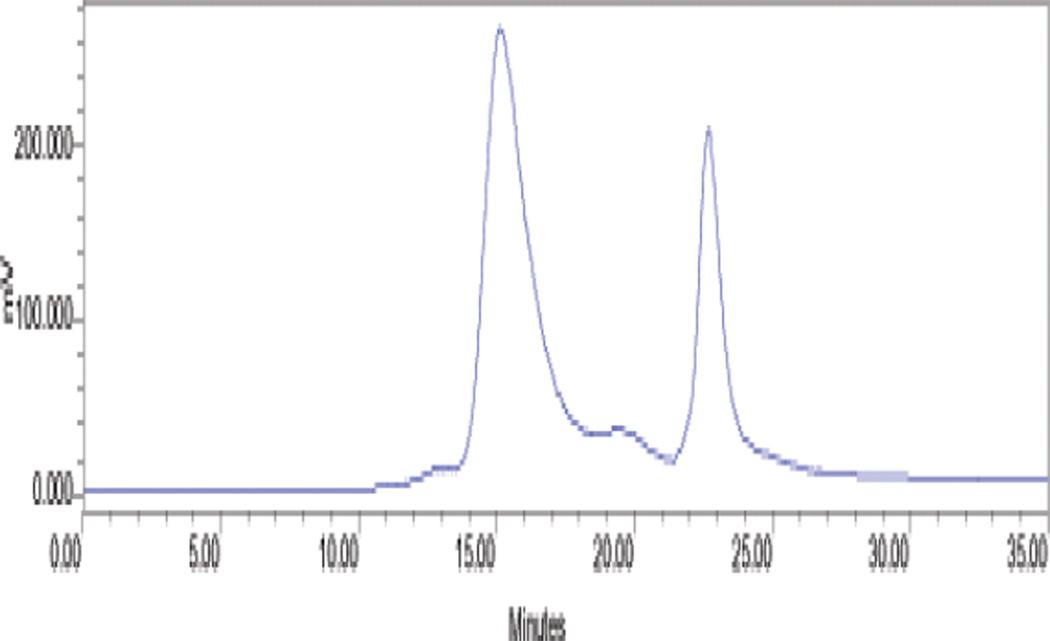
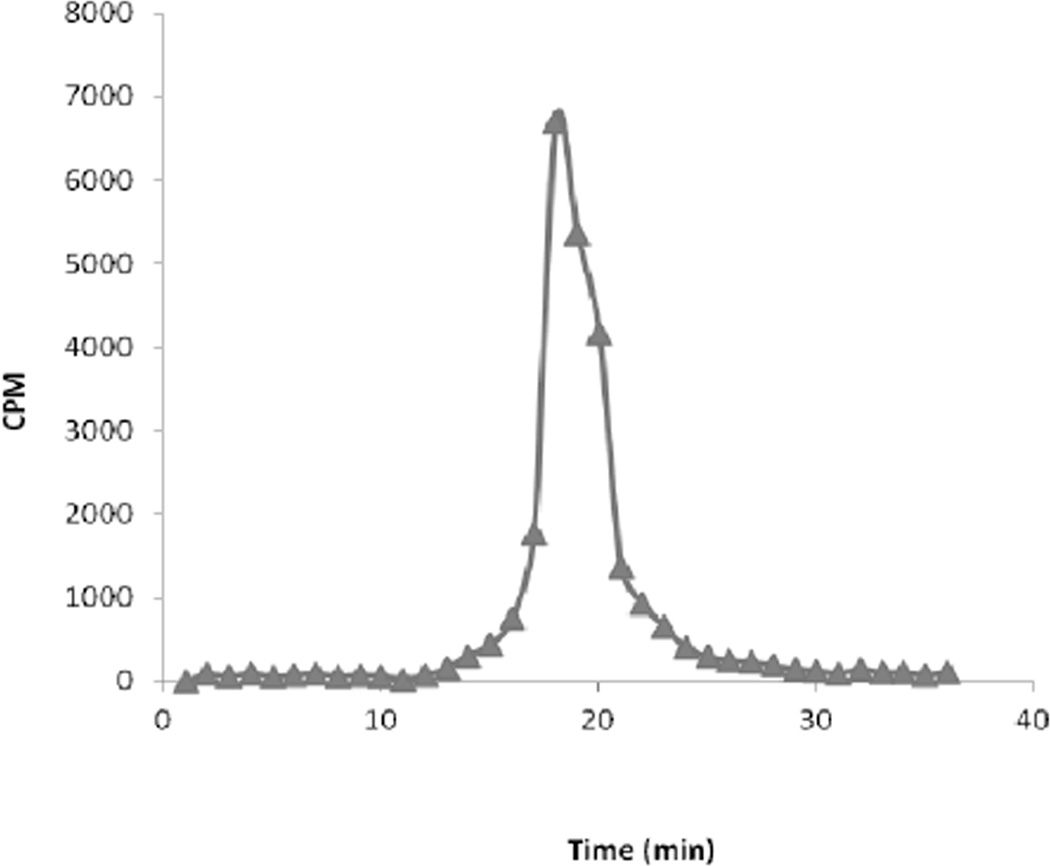
Dependent on the biomolecule being labeled, purification of the 212Pb labeled radioconjugate can be accomplished either by HPLC or by use of a simple desalting column such as a PD-10 column. The latter is generally sufficient when radiolabeling proteins such as antibodies. After quenching the reaction with DTPA or EDTA solution, the mixture is loaded onto the pre-equilibrated PD-10 column. After 2.5 mL of eluate has passed through the column, the protein conjugate is eluted in the next 1.7 mL of eluate. Using TCMC-trastuzumab as the protein conjugate, isolated yields by PD-10 column purification are 73 +/− 3 % (n = 7). After purification it is advisable to add a small amount of EDTA or DTPA (e.g., 0.1 M, 5 µL) to scavenge recoil 212Bi. It is also advisable to add albumin to limit radiolysis of the radioconjugate (31). Figure 5 shows a representative HPLC chromatograph of PD-10 purified 212Pb-TCMC trastuzumab. Since EDTA solution was added to the product before HPLC was performed, a small molecule peak is routinely observed at a retention time of 23 min representing the 212Bi-EDTA complex. Upon collection of HPLC fractions and allowing decay to occur overnight, a plot of the activity in the collected fractions as a function of time yields Figure 6 in which the small molecule peak is absent.
Figure 5.
HPLC chromatograph of PD-10 purified 212Pb-TCMC trastuzumab
Figure 6.
HPLC chromatograph of PD-10 purified 212Pb-TCMC trastuzumab: Activity in eluate fractions 24 h after sample collection.
Operationally, various problems can arise at different stages of the process. Significant detectable breakthrough of 224Ra in the generator eluate is an unusual but potential real problem to encounter that routine assay of generator eluates should detect. A viable solution to the detection of 224Ra breakthrough is to add an extra column of generator resin (about the same size as the generator column) such that the eluate from the primary generator passes through the second column before any further processing takes place to capture the breakthrough. It is important to conduct the generator elution slowly (no more than 1 mL/min) in order to help prevent breakthrough. Excessive back pressure during generator elution can occur. If this occurs, check the flow lines to make sure there are no kinks in the line. High back pressure can also be the result of the generator frit being clogged. This problem cannot be resolved without disassembling the generator. This can result in excessive radiation dose to personnel and is not recommended.
Extensive discoloration of the 8 M HNO3 digest, wherein a dark brown color is observed is indicative of extensive radiolysis and a high amount of resin debris being eluted from the generator. The higher the activity of the 224Ra on the generator, the greater the amount of debris is possible, especially from the first few elutions. Additional 8 M HNO3 digestions will help degrade debris. In the event that the protein labeling step fails or very low radiolabeling yield results, there may be small molecule chelate contaminants in the protein-chelator conjugate solution. Re-dialyze the conjugate solution over 48 h or re-purify the conjugate solution using a PD-10 column as described for the purification of the radiolabeled conjugate using a Chelexed solution of 0.1 M NH4OAc as the eluent. Additionally, washing the 224Ra generator with 2 M HCl (~10 mL) and allow the generator to re-equilibrate for 24 hours before the next elution will help clean up the generator of any contaminants that would deleteriously affect labeling.
At the time of injection of purified 212Pb labeled immunoconjugates, 212Pb exists with its daughters 212Bi, 212Po. Depending on the time between purification and injection of the radioimmunoconjugate, 212Pb and its daughters may or may not be in secular equilibrium, which takes ~4 h to establish after final purification. Thus, it is advisable to inject as soon as possible in order to present as much undecayed 212Pb in the injectate as possible to the target to limit formed daughters. It is probably better to use internalizing molecules as vectors because once internalized both the Pb and its daughters are expected to residualize in the cell. As such, even the 212Bi that may be lost from the chelate may be retained within the cell. If the 212Pb decays before binding to the cell, in which case up to 36% of the Bi could be lost from the chelate, the remaining chelate bound 212Bi would still be bound to the cell, and if internalized, would still be residualized together with its daughters. Loss of the 212Bi appears not to be particularly problematic if the radioimmunoconjugate is injected intraperitoneally. Most of the dose delivered to the cell comes from the α-decays of 212Bi and 212Po [42]. Of the total dose, only 1.8% is attributable to parent 212 Pb radiations, 6.6% from electron and photon contributions, 26.1% from the 6.0 MeV 212Bi α–particle emission, and 67.3% from the 8.8 MeV 212Po α-particle emission. Hence, 93.4% of the deposited energy is directly attributable to the high-LET α-particle emissions. It must be noted that the distribution of radioactivity in vivo is a composite of the multiple decay of 212Pb and its daughters that makes it is a challenge to address because not all the activity may be antibody associated. Utilizing 212Pb intraperitoneally presents a best case scenario of containing the unchelated radioactivity. The short lived nature of the daughters also makes containment in the peritoneal compartment more feasible.
4. Conclusions
The in vivo α-particle generator 212Pb can be produced on-site from a convenient 224Ra/212Pb generator that can produce clinical grade material in substantial activity. Labeling with the radionuclide to produce an injectable product can be performed in ~3.5 h, a time frame that is amenable to the 10.2 h half life of the radioisotope. Currently, two bifunctional chelates, DOTA and TCMC, can be used for labeling with 212Pb. Application of 212Pb in labeling protocols will enhance the use of α targeted therapy to treat various malignant diseases dependent upon appropriate scale and accessibility [1]. The ongoing first clinical use of 212Pb should promote and define the value of this radionuclide as a component of the therapy armamentarium. Attention is drawn to the fact that clinical impact remains to be fully defined, in any overarching sense, regarding the use of all α emitters given the very limited number of clinical trials that have been executed or are ongoing. More specifically with respect to 212Pb, the lone Phase 1 clinical trial has been open less than a year and any determination of actual efficacy upon which real clinical impact will be determined probably will not be made until completion of a phase 2 trial.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Dadachova E, Casadevall A. Antibodies as delivery vehicles for radioimmunotherapy of infectious diseases. Expert Opin Drug Deliv. 2005;2:1075–1084. doi: 10.1517/17425247.2.6.1075. [DOI] [PubMed] [Google Scholar]
- 2.Aarts F, Bleichrodt RP, Oyen WJ, Boerman OC. Intracavitary radioimmunotherapy to treat solid tumors. Cancer Biother Radiopharm. 2008;23:92–107. doi: 10.1089/cbr.2007.0412. [DOI] [PubMed] [Google Scholar]
- 3.Govindan SV, Goldenberg DM. New antibody conjugates in cancer therapy. ScientificWorld Journal. 2010;10:2070–2089. doi: 10.1100/tsw.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner M, Neri D. Antibody-radionuclide conjugates for cancer therapy: historical considerations and new trends. Clin Cancer Res. 2011;17:6406–6416. doi: 10.1158/1078-0432.CCR-11-0483. [DOI] [PubMed] [Google Scholar]
- 5.Milenic DE. Antibody engineering – Optimizing the delivery vehicle. In: Reilly RM, editor. Monoclonal Antibody and Peptide Target Radiotherapy of Malignancies. Hoboken, NJ: John Wiley and Sons; 2010. pp. 1–38. [Google Scholar]
- 6.Ricart AD, Tolcher AW. Technology Insight: Cytotoxic drug immunoconjugates for cancer therapy. Nat Clin Pract Oncol. 2007;4:245–255. doi: 10.1038/ncponc0774. [DOI] [PubMed] [Google Scholar]
- 7.Carter PJ, Senter PD. Antibody-drug conjugates for cancer therapy. Cancer J. 2008;14:154–169. doi: 10.1097/PPO.0b013e318172d704. [DOI] [PubMed] [Google Scholar]
- 8.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nat Rev Drug Discov. 2004;3:488–499. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 9.Milenic DE. Monoclonal antibody-based therapy strategies: providing options for the cancer patient. Curr Pharm Des. 2002;8:1749–1764. doi: 10.2174/1381612023393963. [DOI] [PubMed] [Google Scholar]
- 10.Wester HJ, Kessler H. Molecular targeting with peptides or peptide-polymer conjugates: just a question of size? J Nucl Med. 2005;46:1940–1945. [PubMed] [Google Scholar]
- 11.Boswell CA, Brechbiel MW. Auger electrons: Lethal, low energy, and coming soon to a tumor cell nucleus near you. J Nucl Med. 2005;46:1946–1947. [PubMed] [Google Scholar]
- 12.Wu AM, Senter PD. Arming antibodies: Prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 13.O'Donoghue JA, Bardiès M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995;36:1902–1909. [PubMed] [Google Scholar]
- 14.Azzam EI, de Toledo SM, Little JB. Stress signaling from irradiated to non-irradiated cells. Curr Cancer Drug Targets. 2004;4:53–64. doi: 10.2174/1568009043481641. [DOI] [PubMed] [Google Scholar]
- 15.Prise KM, Folkard M, Michael BD. Bystander responses induced by low LET radiation. Oncogene. 2003;22:7043–7049. doi: 10.1038/sj.onc.1206991. [DOI] [PubMed] [Google Scholar]
- 16.Seidl C, Schröck H, Seidenschwang S, Beck R, Schmid E, Abend M, et al. Cell death triggered by alpha-emitting 213Bi-immunoconjugates in HSC45-M2 gastric cancer cells is different from apoptotic cell death. Eur J Nucl Med Mol Imaging. 2005;32:274–285. doi: 10.1007/s00259-004-1653-3. [DOI] [PubMed] [Google Scholar]
- 17.Friesen C, Glatting G, Koop B, Schwarz K, Morgenstern A, Apostolidis C, et al. Breaking chemoresistance and radioresistance with [213Bi]anti-CD45 antibodies in leukemia cells. Cancer Res. 2007;67:1950–1958. doi: 10.1158/0008-5472.CAN-06-3569. [DOI] [PubMed] [Google Scholar]
- 18.Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. 212Pb-Radioimmunotherapy induces G2 cell cycle arrest and delays DNA damage repair in tumor xenografts in a model for disseminated intraperitoneal disease. Mol Cancer Ther. 2012;11:639–648. doi: 10.1158/1535-7163.MCT-11-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallon M, Seidl C, Blechert B, Li Z, Gilbertz KP, Baumgart A, et al. Enhanced efficacy of combined (213)Bi-DTPA-F3 and paclitaxel therapy of peritoneal carcinomatosis is mediated by enhanced induction of apoptosis and G2/M phase arrest. Eur J Nucl Med Mol Imaging. 2012 Aug 8; doi: 10.1007/s00259-012-2203-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. Sensitization of tumor to 212Pb-radioimmunotherapy by gemcitabine involves initial abrogation of G2 arrest and blocked DNA damage repair by interference with Rad5. Accepted for publication in Int J of Rad Oncol Biol Phy. doi: 10.1016/j.ijrobp.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson H, Cederkrantz E, Back T, Divgi C, Elgqvist J, Himmelman J, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of (211)At-MX35 F(ab')2--a phase I study. J Nucl Med. 2009;50:1153–1160. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 22.Rotmensch J, Atcher RW, Hines J, Grdina D, Schwartz JS, Toohill M, et al. The development of alpha-emitting radionuclide lead 212 for the potential treatment of ovarian carcinoma. Am J Obstet Gynecol. 1989;160:789–797. doi: 10.1016/0002-9378(89)90293-7. [DOI] [PubMed] [Google Scholar]
- 23.Senekowitsch-Schmidtke R, Schuhmacher C, Becker K-F, Nikula TK, Seidl C, et al. Highly specific tumor binding of a 213Bi-labeled monoclonal antibody against mutant E-cadherin suggests its usefulness for locoregional α-radioimmunotherapy of diffuse-type gastric cancer. Cancer Res. 2001;61:2804–2808. [PubMed] [Google Scholar]
- 24.Sgouros G, Ballangrud AM, Jurcic JG, McDevitt MR, Humm JL, Erdi YE, et al. Pharmacokinetics and dosimetry of an α-particle emitter labeled antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia. J Nucl Med. 1999;40:1935–1946. [PubMed] [Google Scholar]
- 25.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, et al. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin Cancer Res. 2004;10:7834–7841. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]
- 26.Milenic D, Garmestani K, Dadachova E, Chappell L, Albert P, Hill D, et al. Radioimmunotherapy of human colon carcinoma xenografts using a 213Bi-labeled domain-deleted humanized monoclonal antibody. Cancer Biother Radiopharm. 2004;19:135–147. doi: 10.1089/108497804323071904. [DOI] [PubMed] [Google Scholar]
- 27.Yong K, Brechbiel MW. Towards translation of 212Pb as a clinical therapeutic; getting the lead in! Dalton Trans. 2011;40:6068–6076. doi: 10.1039/c0dt01387k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y-S, Brechbiel MW. An overview of targeted alpha therapy. Tumor Biol. 2012;33:573–590. doi: 10.1007/s13277-011-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirzadeh S, Kumar K, Gansow OA. The chemical fate of212Bi-DOTA formed by beta-decay of 212Pb(DOTA)2 . Radiochim.. Acta. 1993;60:1–10. [Google Scholar]
- 30.Su FM, Beaumier P, Axworthy D, Atcher R, Fritzberg A. Pretargeted radioimmunotherapy in tumored mice using an in vivo 212Pb/212Bi generator. Nucl Med Biol. 2005;32:741–747. doi: 10.1016/j.nucmedbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Horak E, Hartmann F, Garmestani K, Wu C, Brechbiel M, Gansow OA, et al. Radioimmunotherapy targeting of HER2/neu oncoprotein on ovarian tumor using lead-212-DOTA-AE1. J Nucl Med. 1997;38:1944–1950. [PubMed] [Google Scholar]
- 32.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, et al. α-Particle radioimmunotherapy of disseminated peritoneal disease using a 212Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm. 2005;20:557–568. doi: 10.1089/cbr.2005.20.557. [DOI] [PubMed] [Google Scholar]
- 33.Milenic DE, Garmestani K, Brady ED, Albert PS, Abdulla A, Flynn J, et al. Potentiation of high LET radiation by gemcitabine: targeting of HER2 with trastuzumab for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2007;13:1926–1935. doi: 10.1158/1078-0432.CCR-06-2300. [DOI] [PubMed] [Google Scholar]
- 34.Milenic DE, Garmestani K, Brady ED, Albert PS, Wong KJ, Flynn J, et al. Multimodality therapy: potentiation of High-LET radiation with paclitaxel for the treatment of disseminated peritoneal disease Clin. Cancer Res. 2008;14:5108–5115. doi: 10.1158/1078-0432.CCR-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milenic DE, Brady ED, Garmestani K, Albert PS, Abdulla A, Brechbiel MW. Improved Efficacy of α-particle targeted radiation therapy: dual targeting of HER2 and TAG-72. Cancer. 2010;116(S4):1059–1066. doi: 10.1002/cncr.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. http://clinicaltrials.gov/show/NCT01384253.
- 37.Chappell LL, Dadachova E, Milenic DE, Garmestani K, Wu C, Brechbiel MW. Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl Med Biol. 2000;27:93–100. doi: 10.1016/s0969-8051(99)00086-4. [DOI] [PubMed] [Google Scholar]
- 38.Ruble G, Wu C, Squire RA, Gansow OA, Strand M. The use of 212Pb-labeled monoclonal antibody in the treatment of murine erythroleukemia. Int J Radiat Oncol Biol Phys. 1996;34:609–616. doi: 10.1016/0360-3016(95)02119-1. [DOI] [PubMed] [Google Scholar]
- 39.McMurry TJ, Brechbiel MW, Kumar K, Gansow OA. Convenient synthesis of bifunctional tetraaza macrocycles. Bioconjugate Chem. 1992;3:108–117. doi: 10.1021/bc00014a004. [DOI] [PubMed] [Google Scholar]
- 40.Cuenot F, Meyer M, Espinosa E, Bucaille A, Burgat R, Guilard R, et al. New insights into the complexation of lead(II) by 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane (DOTAM): Structural, thermodynamic, and kinetic studies. Eu J Inorg Chem. 2008:267–283. [Google Scholar]
- 41.Dadachova E, Chappell LL, Brechbiel MW. Spectrophotometric method for determination of bifunctional macrocyclic ligands in macrocyclic ligand - protein conjugates. Nucl Med Biol. 1999;26:977–982. doi: 10.1016/s0969-8051(99)00054-2. [DOI] [PubMed] [Google Scholar]
- 42.Howell RW, Azure MT, Narra VR, Rao DV. Relative biological effectiveness of alpha-particle emitters in vivo at low doses. Radiat Res. 1994;137:352–360. [PMC free article] [PubMed] [Google Scholar]