Abstract
Mpumalanga Province, South Africa has one of the highest HIV/AIDS diagnosis rates among pregnant women (~29.4%). This study sought to enhance male involvement in pregnancy to increase HIV disclosure, sexual communication, HIV knowledge and reduce unprotected sex. Participants attending Antenatal Clinics (ANC) completed HIV counselling and testing and were enrolled with male partners (n = 239 couples, 478 individuals). Twelve ANCs were randomly assigned to provide a prevention of mother-to-child transmission (PMTCT) intervention or the standard of care, health education sessions plus PMTCT. Participants were assessed at baseline and post-intervention (approximately 6–8 weeks post-baseline) on demographics, sexual behaviour, HIV-related knowledge, and conflict resolution strategies. Experimental participants increased HIV knowledge, use of negotiation, and decreased intimate partner violence. Additionally, they were more likely to have increased condom use from baseline to post-intervention (OR = 5.1, 95% CI = (2.0, 13.3)). Seroconversions in the control condition exceeded experimental (6 vs. 0). HIV serostatus disclosure to partner did not increase over time for men or women within the experimental or control condition. Male involvement in pregnancy may be an important strategy to reduce sexual risk behavior and HIV transmission. Results support the utility of group interventions to enhance communication and HIV knowledge among pregnant couples.
Keywords: HIV, PMTCT, male involvement, pregnancy, sexual risk reduction
Introduction
South Africa has the highest HIV/AIDS rates among women worldwide (UNAIDS, 2011). An estimated 29.4% of women aged 15 – 49 years in antenatal care are diagnosed with HIV/AIDS during pregnancy (Maman, Moodley, & Groves, 2011). Women in South Africa spend a significant portion of their reproductive years pregnant, and unprotected sex during attempts to conceive and the ensuing pregnancy greatly increases the risk of HIV transmission (Mugo et al., 2011). HIV incidence is four times higher among women in late stage pregnancy (Moodley, Esterhuizen, Pather, Chetty, & Ngaleka, 2009), and in South Africa, the risk of seroconversion during pregnancy is 29% and 22% among women less than 24 and 24 to 35 years of age, respectively, and increases as the pregnancy progresses (Wand & Ramjee, 2011). HIV transmission to men also increases with HIV positive pregnant partners in comparison with non-pregnant women (Mugo et al., 2011). While HIV re-testing during the latter stages of pregnancy is essential (Chen et al., 2010; Kinuthia et al., 2010) and a component of the prevention of mother to child transmission (PMTCT) protocol, many HIV negative women do not re-test (Department of Health, 2010). These high rates of HIV transmission and seroconversion during pregnancy necessitate increased prevention strategies targeting sexual risk behavior and engagement of pregnant HIV-positive and negative women and their partners during antenatal care to reduce the transmission risk between partners and to unborn children.
Women’s vulnerability to HIV infection increases during pregnancy, due to in part to the reduced use of condoms as birth control (Mugo et al., 2011; Onoya et al., 2010) and social norms restricting their use (Langen, 2005). Gender-based power dynamics, e.g., intimate partner violence (IPV), loss of financial support, and social discrimination (Peltzer & Mlambo, 2010) limit safer sex negotiation and disclosure of HIV status (Dunkle et al., 2004). In fact, despite the risk of HIV and STIs (Gray et al., 2005; Onoya et al., 2010), low rates of condom use are reported in steady relationships (Manenti et al., 2011). Women’s non-disclosure of HIV-seropositive status during pregnancy may not only increase male partners’ HIV-risk, but also reduce women’s engagement in antenatal care and place unborn children at increased risk of infection (Visser, Neufeld, de Villiers, Makin, & Forsyth, 2008).
Increasing male involvement in PMTCT programs is a potential method to increase HIV testing and disclosure of HIV status, safer sex and PMTCT uptake (WHO & UNICEF, 2007) and efforts to stimulate involvement has met with modest success throughout sub-Saharan Africa (Aluisio et al., 2011; Becker, Mlay, Schwandt, & Lyamuya, 2010; Farquhar et al., 2004). This study evaluated an intervention to enhance male involvement in pregnancy in rural South Africa. The intervention was designed to enhance sexual communication and HIV knowledge, decrease the potential for IPV, increase safer sex and encourage HIV testing and mutual disclosure of HIV status. It was theorized that enrolling men in a prevention intervention to increase involvement in pregnancy would reduce sexual risk behavior, enhance mutual HIV testing and disclosure, decrease HIV transmission and optimize uptake of PMTCT strategies (Auvinen, Suominen, & Valimaki, 2010; Conkling et al., 2010; Peltzer, Mlambo, Phaswana-Mafuya, & Ladzani, 2010).
Methods
This manuscript presents data drawn from a larger trial conducted September 2010 to March 2012. University of Miami Miller School of Medicine Institutional Review Board, Human Sciences Research Council Research Ethics Committee, and the Mpumalanga Provincial Department of Health approvals were obtained prior to study onset. All procedures were in accordance with the ethical standards of the review committees and the Helsinki Declaration of 1975, as revised in 2000.
Study Population and Setting
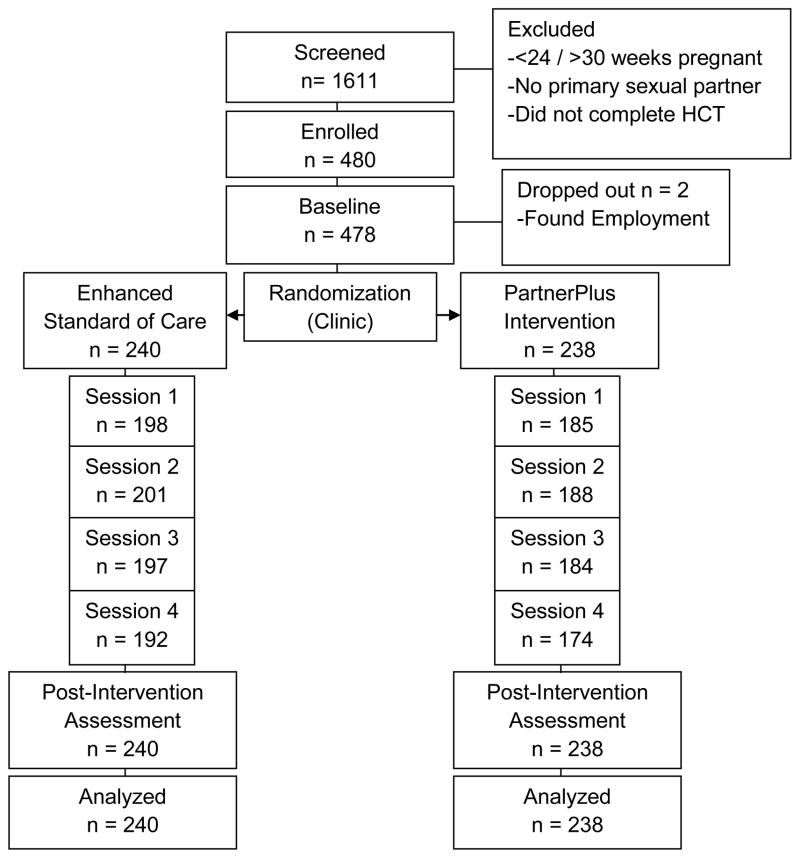
Recruitment was conducted from 12 antenatal clinics (ANCs) in Gert Sibande and Nkangala districts of Mpumalanga Province, South Africa; ANC HIV prevalence rates ranged from 32.5 and 38.2%, respectively (Peltzer, Jones, Weiss, & Shikwane, 2011). Pregnant women who completed HIV counselling and testing (HCT) were invited to participate and provided an invitation to their male partners; women were recruited as the primary participant. Inclusion criteria were as follows: 1) 18 years of age, 2) completed HCT, 3) between 24 – 30 weeks gestation, and 4) agreed to enroll with their male partners. Among those women who were screened (n = 1611), 239 were enrolled with their male partners following written informed consent and screening to ensure primary sexual partner status (N = 478 individuals, 239 couples). Those excluded were < 24 or >30 weeks gestation, were single or had partners unavailable to participate.
In accordance with South African guidelines, women testing HIV-negative at entry into antenatal care were given the option to re-test at 32 weeks pregnant. HIV seropositive women were referred for evaluation of CD4 count and liver function; those with CD4 count less than 350 cells/mm3 were referred for ARV therapy.
Intervention
The PartnerPlus intervention combined key elements of two evidence-based interventions (The Partner Project: a couples’ behavioral HIV risk reduction intervention plus an intervention designed to enhance PMTCT uptake) into a comprehensive couples-based PMTCT intervention. The intervention consisted of four weekly 90 to 120 minute sessions emphasizing cognitive-behavioral skill building to improve communication, sexual negotiation, conflict resolution, STI/HIV prevention, PMTCT, use of male and female condoms, and gender-relevant issues. Intervention sessions were closed, structured, gender-concordant group sessions limited to 10 participants per group; male and female groups were conducted concurrently. Sessions were led by two gender-matched, trained HIV counselors who did not administer assessments. Participants in the control condition received the standard of care (PMTCT) and four time-matched sessions (health-related videos). All participants were provided with male and female condoms at the close of each session. Audiotapes of all intervention sessions were transcribed and reviewed biweekly to ensure fidelity to the intervention.
Study Design and Data Collection
This study was a group-randomized controlled trial using a 2 × 2 comparison (Condition × Time) registered at clinicaltrials.gov, number NCT01448512. To avoid contamination between conditions, ANCs (n = 12) were randomly assigned, using a remote secure randomization service, to provide either the PartnerPlus PMTCT intervention (6 sites) or time matched health education sessions plus PMTCT (6 sites). HIV prevalence rates were comparable between experimental and control clinics at baseline (Chen et al., 2010). Each ANC recruited an average of 2 cohorts over 6 months, beginning November 2010. Treatment protocols were in accordance with the 2010 South African Clinical Guidelines for PMTCT, as detailed in Peltzer et al., 2011.
Assessments were translated into local languages by certified translators and back-translated to ensure fidelity, and were interviewer-administered by trained assessors. All participants (both men and women) completed all assessments which, with the exception of demographic information, were collected at study entry and within a 1–3 week window following the final group session. Participants were reimbursed for their travel during assessments and sessions with food vouchers (R75 per visit, ~ US$10). The retention rate for post-intervention assessments was 100%.
Measures
Demographics and HIV serostatus
Demographics included age, education, employment status, income, locality (urban or rural), marital status and number of children. HIV serostatus of women was obtained from clinic records; women who tested negative at entry to ante-natal care were given the option to re-test at 32 weeks gestation according to South African PMTCT guidelines. Men were invited, but not required, to test for HIV at study entry and at post-intervention. Additionally, participants reported their partner’s serostatus, from which analyses of disclosure were drawn. “Accurate disclosure” here indicates those whose partner correctly identified their serostatus; “total disclosure” includes those whose partner provided an inaccurate serostatus.
Sexual Activities Questionnaire
This measure was adapted from the Sexual Risk Behavior Assessment Schedule (SERBAS) (Meyer-Bahlburg, Ehrhardt, Exner, & Gruen, 1990) and used to assess frequency of multiple partnering and sexual barrier use with non-primary partners over the past month.
Sexual Diary
Sexual activities each day of the previous week and use of condoms, if any, was assessed using a sexual diary, and the percentage of the time a condom (male or female) was used was computed. Due to its highly skewed distribution, condom use was dichotomized into consistent (100% of the time in the past week) and inconsistent (<100%). For longitudinal analysis, change in condom use was calculated by subtracting baseline percentage of condom use from post-intervention percentage of condom use and dichotomized into improved (post-intervention minus baseline > 0) or not improved/decreased (post-intervention minus baseline ≤ 0).
Conflict Tactics Scale
A modified version of the Conflict Tactics Scale (Straus, 1979) was used to assess conflict resolution strategies, grouped into three subscales: negotiation (e.g., discussing problems, working out solutions), verbal aggression (e.g., insulting, sulking), and physical violence (e.g., throwing objects, hitting, assault with a weapon). Due to the skewed distribution of verbal aggression and violence, both were dichotomized into experience of at least one act in the previous month or none.
HIV-related knowledge
Knowledge concerning HIV transmission, PMTCT and AIDS was assessed using 13 items adapted from an AIDS-related knowledge scale (Cronbach’s alpha (α) ranged from .75 to .89 in validation samples, Carey & Schroder, 2002). All questions were answered with “yes,” “no,” or “don’t know.” HIV knowledge was scored as the proportion of correct responses, with “don’t know” responses scored as incorrect.
Statistical Analyses
Analyses with dichotomous variables utilized chi-square tests of independence and McNemar’s tests for repeated measures. Continuous variables were assessed with t-tests and repeated measures ANOVA. Condom use was analyzed using generalized estimating equations (GEE) due to non-independence within clinic. All tests were performed with a 2-tailed significance level of 0.05 using IBM SPSS version 19.0.
Results
Demographics and Attendance
Participants (n = 478) averaged 28 years old with 1 child, were primarily rural, mostly unemployed, had less than twelve years of education, and reported an average income of 888 Rand per month. Study session attendance was higher in the control condition than the experimental condition and similar between genders (m (men) = 3.2, m (women) = 3.2, t = .61, p = .55). Variables in which experimental and control participants differed were not related to outcome variables. Table 1 details demographics and attendance by condition.
Table 1.
Demographics
| Total | Experimental | Control | t/χ2 | p | |
|---|---|---|---|---|---|
| Measure | N (%) or Mean (SD) | n=238 | n=240 | ||
| Age | 28.2 (7.1) | 28.3(6.8) | 28.1(7.4) | .27 | .79 |
| Personal Income (Rand, per month) | 888 (1758) | 761.8(1418.0) | 1014.7(2036.8) | 1.6 | .12 |
| Employment | .57 | .45 | |||
| Unemployed | 344 (72) | 175(74) | 169(70) | ||
| Employed | 134 (28) | 63(27) | 71(30) | ||
| Education | 2.7 | .10 | |||
| < Grade 12 | 253 (53) | 135(57) | 118(50) | ||
| Grade 12 or more | 225 (47) | 103(43) | 122(51) | ||
| Locality | 8.3 | .004 | |||
| Rural | 340 (71.1) | 155(65) | 185(77) | ||
| Settlement/Urban | 138 (28.9) | 83(35) | 55(23) | ||
| Number of study sessions attended | 3.2 (.83) | 3.1 (.85) | 3.3(.80) | 3.2 | .002 |
HIV serostatus and disclosure
At study entry, thirty-two percent of female participants (n = 76) were confirmed seropositive. Eighty three percent of HIV-negative women in the experimental condition and 77% in the control condition elected to re-test at 32 weeks gestation (no difference between conditions, χ2 = .83, p = .36). Of those, six women (4%) had seroconverted. Control participants had a 9.7% seroconversion rate (6/62 HIV- women) in comparison with 0% in the experimental condition (0/68 HIV- women). The small sample size precluded statistical testing.
Of the 76% (n = 182) of males who elected to test at study entry, 21% (n = 38) were confirmed seropositive, with no difference in the proportion electing to test by condition (χ2 = .04, p = .85). Men were again invited to test at post-intervention, and an additional 26 men agreed, 5 of whom were seropositive. More men in the experimental condition elected to test at post-intervention than the control condition (15% of experimental condition vs. 7% of control condition, χ2 = 4.4, p = .04). Table 2 details results of HIV testing.
Table 2.
HIV serostatus
| Men N (%) | Women N (%) | |||||
|---|---|---|---|---|---|---|
| HIV- Negative | HIV- Positive | Did Not Test | HIV- Negative | HIV- Positive | Did Not Re-test | |
| Baselinea | 144(60) | 38(16) | 57(24) | 163(68) | 76(32) | n/a |
| 32 Weeks Pregnancy/Post- Interventionb | 165(69) | 43(18) | 31(13) | 124(52) | 82(34) | 33(14) |
At baseline, all women had completed HIV counseling and testing (HCT); HIV status was extracted from clinic records. Men were invited to take an HIV test.
Eighty percent (n = 130) of HIV-negative women elected to re-test at 32 weeks. Men were again invited to test post-intervention, and 26 elected to do so.
At baseline, 26 couples were serodiscordant (11%); 19% of women and 2% of men reported not knowing if their partner had been tested for HIV. Disclosure of HIV serostatus is presented in table 3. In each cell, number accurately disclosed divided by the total number disclosed is presented. Accurate disclosure to partner did not increase over time (McNemar’s test, p > .05).
Table 3.
Disclosure of HIV serostatus
| Accurate Disclosurea of Serostatus | Experimental (Accurate Disclosure/Total Disclosure)(%) | Control (Accurate Disclosure/Total Disclosure)(%) |
|---|---|---|
| Men | ||
| Baseline | 60/70 (86%) | 44/61 (72%) |
| Post-Intervention | 76/92 (83%) | 46/58 (80%) |
| Women | ||
| Baseline | 95/119 (80%) | 83/119 (70%) |
| Post-Intervention | 96/118 (81%) | 76/117 (65%) |
”Accurate disclosure” indicates participants whose partner correctly identified their HIV serostatus, and “total disclosure” includes those whose partner provided an inaccurate serostatus.
Sexual risk behavior
At baseline, 355 participants reported sexual activity in the past week, decreasing to 251 participants at post-intervention. Sixty-nine percent of control condition and 66% of experimental condition participants reported inconsistent condom use (<100% of the time) within the past week (no difference between conditions, χ2 = .21, p = .76). Nine percent of experimental condition participants (n = 22) and 18% of controls (n = 44) reported sex with multiple partners in the past month (χ2 = 8.3, p = .004). Among those, 38% of experimental condition participants (n = 8) and 34% of controls (n =15) reported inconsistent condom use while having sex with non-primary partners (χ2 = .1, p = .79).
A total of 191 participants were sexually active at baseline and post-intervention; the following analysis regarding condom use is restricted to those participants. Non-independence of change in condom use within clinics was confirmed (Intra-class correlation = .26, Wald = 2.6, p = .009). Adjusting for the cluster effect of clinic, GEE analysis estimated 5.1 greater odds of increased condom use during the study for a participant in the experimental condition, in comparison with control condition [b=1.632, Wald = 11.2, p = .001, OR = 5.1, 95% CI (OR) = (2.0, 13.3)].
Across the study, there was a non-significant decrease in the number of participants endorsing multiple partners (experimental, n = 17, 7%; control, n = 29, 12%; McNemar’s test, p > .05 for both conditions). Among those with multiple partners, there was no change in inconsistent condom use (experimental n = 11, 65%; control n = 16, 56%; McNemar’s test, p > .05).
HIV-related knowledge
Baseline mean HIV-related knowledge scores did not differ between experimental and control condition participants (t (476 df) = .94, p = .35). Across the study, HIV-related knowledge increased in the experimental condition but did not change in the control condition (F(1, 476) = 13.9, p < .001) Table 4 presents means and standard deviations for knowledge in both conditions.
Table 4.
Knowledge and conflict resolution
| Knowledge | Experimental Mean (SD) n=478 | Control Mean (SD) n=478 | Fa | p |
|---|---|---|---|---|
| 13.4* | <.001 | |||
| Baseline | 10.4(1.6) | 10.5(1.8) | ||
| Post-Intervention | 10.9(1.7) | 10.4(1.8) | ||
| Conflict Resolution | Experimental Mean (SD)/N (%) | Control Mean (SD)/N (%) | F | p |
| Negotiation | 5.4 | .02 | ||
| Baseline | 2.8 (1.6) | 3.0 (1.7) | ||
| Post-Intervention | 3.1 (1.7) | 2.9 (1.6) | ||
| Verbal Aggression | ||||
| Baseline | 94 (79%) | 89 (74%) | ||
| Post-Intervention | 76 (64%)b | 84 (70%) | ||
| Violence | ||||
| Baseline | 51 (43%) | 57 (48%) | ||
| Post-Intervention | 21 (18%)c | 46 (38%) |
F value is for interaction term (condition*time)
Reports of at least one act of verbal aggression decreased in the experimental condition (McNemar’s test, p = .01) but not in the control condition (p = .49).
Reports of at least one act of violence decreased in the experimental condition (McNemar’s test, p < .001) but not in the control condition (p = .10)
Conflict resolution and intimate partner violence (IPV)
Baseline dyadic data indicated that couples reported having used negotiation to resolve conflict an average of approximately 3 times over the previous month. Seventy-seven percent of couples reported at least one act of verbal aggression and 45% of couples reported at least one act of violence (IPV) in the last month. Post-intervention, the frequency of negotiation increased in the experimental condition but not among controls (F = 5.4, p = .02). Similarly, reports of at least one act of verbal aggression decreased in the experimental condition (McNemar’s test, p = .01) but not in the control condition (McNemar’s test, p = .49), and reports of at least one act of violence decreased in the experimental condition (McNemar’s test, p < .001) but not in the control condition (McNemar’s test, p = .1). Table 4 presents the results of conflict resolution analyses.
Discussion
This study sought to increase male involvement in pregnancy, reduce unprotected sex and enhance HIV knowledge and sexual communication. The intervention was successful in decreasing unprotected sex and no women in the intervention seroconverted between entry in antenatal care and 32 weeks gestation. Additionally, HIV knowledge increased among those in the intervention. Finally, the intervention successfully increased the use of negotiation strategies for conflict resolution and decreased the use of negative communication and IPV among couples.
HIV transmission during pregnancy exceeds that among non-pregnant couples (Moodley et al., 2009; Mugo et al., 2011; Wand & Ramjee, 2011). This study identified high rates of unprotected sex during pregnancy as well as 6 seroconversions among control condition women by 32 weeks pregnancy. Furthermore, one fifth of women did not re-test during this critical period. High rates of HIV infection and potential seroconversions underscore the value of intensive male involvement during pregnancy. Protected sex among couples in the intervention increased despite reductions normally associated with pregnancy due to the lack of need for birth control (Moodley et al., 2009; Mugo et al., 2011; Onoya et al., 2010; Wand & Ramjee, 2011), and the lower rates of condom use typical among established couples (Manenti et al., 2011). In addition, sex with non-primary partners decreased, although the decrease was not significant.
While enhancing male involvement in antenatal care requires having men join their female partners at the clinic, male involvement in pregnancy remains an important strategy to reduce sexual risk behavior and HIV transmission. Targeted interventions for men during pregnancy, rather than encouraging ANC attendance with partners, may be more beneficial. Previous couples research has found seronegative male partners to require more intensive sexual counselling in comparison with seropositive men (Jones, Weiss, Bhat, & Bwalya, 2006); this group is especially vulnerable during pregnancy with partners who are HIV seropositive. Future research should also explore interventions for seronegative men that emphasize HIV testing and disclosure.
While gender-based power dynamics and intimate partner violence limit safer sex negotiation (Dunkle et al., 2004; Peltzer & Mlambo, 2010), reductions in IPV and increases in negotiation strategies were achieved by couples in the intervention. Though previous research has not found risk-related knowledge to influence rates of condom use (Gray et al., 2005), this study utilized a comprehensive HIV prevention program that may have contributed to increases in condom use. However, neither condition achieved comprehensive coverage of male HIV testing or mutual serostatus disclosure. This critical failure to increase disclosure of HIV seropositive status during pregnancy represents not only a continued risk to partners of HIV infection, but also a conceivable negation of the potential benefits postulated to be associated with male involvement in antenatal care (Visser et al., 2008). Future studies should continue to explore methods to facilitate the process of HIV serostatus disclosure during this vulnerable period.
This study obtained promising results, but conclusions are limited primarily due to the small number of HIV seropositive women participating. Additionally, this manuscript presents results from baseline and post-intervention data; anticipated follow-up data will demonstrate if gains in condom use and communication are maintained. Finally, sexual behavior data was collected by assessors in person, and responses may have been influenced by social desirability bias. Future studies should utilize alternative methods to reduce bias.
Results support the utility of a group gender concordant intervention for couples to enhance protected sex and positive communication during pregnancy. The importance of safer sexual behaviour during pregnancy by both partners highlights the continued need for not only male involvement but mutual engagement of couples in pregnancy.
Figure 1.
CONSORT Flow Diagram
Acknowledgments
This study was supported by the Miami Center for AIDS Research (CFAR) at the University of Miami Miller School of Medicine which is funded by a grant (P30AI073961) from the National Institutes of Health (NIH). The CFAR program at the NIH includes co-funding from: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, and OAR.
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to disclose.
References
- Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011;56:76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen J, Suominen T, Valimaki M. Male participation and prevention of human immunodeficiency virus (HIV) mother-to-child transmission in Africa. Psychol Health Med. 2010;15:288–313. doi: 10.1080/13548501003615290. [DOI] [PubMed] [Google Scholar]
- Becker S, Mlay R, Schwandt HM, Lyamuya E. Comparing couples’ and individual voluntary counseling and testing for HIV at antenatal clinics in Tanzania: a randomized trial. AIDS Behav. 2010;14:558–566. doi: 10.1007/s10461-009-9607-1. [DOI] [PubMed] [Google Scholar]
- Carey MP, Schroder KEE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002;14:172–182. doi: 10.1521/aeap.14.2.172.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Young A, Brown ER, Chasela CS, Fiscus SA, Hoffman IF, Read JS. Population attributable fractions for late postnatal mother-to-child transmission of HIV-1 in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2010;54:311–316. doi: 10.1097/QAI.0b013e3181d61c2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkling M, Shutes EL, Karita E, Chomba E, Tichacek A, Sinkala M, Allen SA. Couples’ voluntary counselling and testing and nevirapine use in antenatal clinics in two African capitals: a prospective cohort study. J Int AIDS Soc. 2010;13:10–10. doi: 10.1186/1758-2652-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health, S. A. National Antenatal Sentinel HIV and Syphilis Prevalence Survey, 2010: South Africa. 2010 Retrieved August 15, 2012, from http://www.info.gov.za/view/DownloadFileAction?id=155559.
- Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363:1415–1421. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- Farquhar C, Kiarie JN, Richardson BA, Kabura MN, John FN, Nduati RW, John-Stewart GC. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–1626. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, Wawer MJ. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- Jones DL, Weiss SM, Bhat GJ, Bwalya V. Influencing sexual practices among HIV-positive Zambian women. AIDS Care. 2006;18:629–634. doi: 10.1080/09540120500415371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinuthia J, Kiarie JN, Farquhar C, Richardson B, Nduati R, Mbori-Ngacha D, John-Stewart G. Cofactors for HIV-1 incidence during pregnancy and postpartum period. Curr HIV Res. 2010;8:510–514. doi: 10.2174/157016210793499213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen TT. Gender power imbalance on women’s capacity to negotiate self-protection against HIV/AIDS in Botswana and South Africa. Afr Health Sci. 2005;5:188–197. doi: 10.5555/afhs.2005.5.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maman S, Moodley D, Groves AK. Defining male support during and after pregnancy from the perspective of HIV-positive and HIV-negative women in Durban, South Africa. J Midwifery Womens Health. 2011;56:325–331. doi: 10.1111/j.1542-2011.2011.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti SA, Galato J, Junior, da Silveira ES, Oenning RT, Simoes PW, Moreira J, Romao PR. Epidemiologic and clinical characteristics of pregnant women living with HIV/AIDS in a region of Southern Brazil where the subtype C of HIV-1 infection predominates. Braz J Infect Dis. 2011;15:349–355. [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Ehrhardt A, Exner T, Gruen R. Sexual Risk Behavior Assessment Schedule: Adult Manual (SERBAS-A-DF-4) 1990. [Google Scholar]
- Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23:1255–1259. doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, Baeten JM. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25:1887–1895. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoya D, Reddy P, Sifunda S, Lang D, Wingood G, van den Borne B, Ruiter R. Comparing STI risk and sexual behaviour profiles of pregnant versus non-pregnant, HIV negative black south African women. Webmed Central Public Health. 2010;1:WMC001142. [Google Scholar]
- Peltzer K, Jones D, Weiss SM, Shikwane E. Promoting male involvement to improve PMTCT uptake and reduce antenatal HIV infection: a cluster randomized controlled trial protocol. BMC Public Health. 2011;11:778–778. doi: 10.1186/1471-2458-11-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, Mlambo G. Factors determining HIV viral testing of infants in the context of mother-to-child transmission. Acta Paediatr. 2010;99:590–596. doi: 10.1111/j.1651-2227.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- Peltzer K, Mlambo M, Phaswana-Mafuya N, Ladzani R. Determinants of adherence to a single-dose nevirapine regimen for the prevention of mother-to-child HIV transmission in Gert Sibande district in South Africa. Acta Paediatr. 2010;99:699–704. doi: 10.1111/j.1651-2227.2010.01699.x. [DOI] [PubMed] [Google Scholar]
- Straus MA. Measuring intrafamily conflict and violence: The conflict tactics (CT) scales. Journal of Marriage and the Family. 1979;41:75–88. [Google Scholar]
- UNAIDS. UNAIDS World AIDS Day Report. 2011 Retrieved August 15, 2012, from http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf.
- Visser MJ, Neufeld S, de Villiers A, Makin JD, Forsyth BWC. To tell or not to tell: South African women’s disclosure of HIV status during pregnancy. AIDS Care. 2008;20:1138–1145. doi: 10.1080/09540120701842779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand H, Ramjee G. Combined impact of sexual risk behaviors for HIV seroconversion among women in Durban, South Africa: implications for prevention policy and planning. AIDS Behav. 2011;15:479–486. doi: 10.1007/s10461-010-9845-2. [DOI] [PubMed] [Google Scholar]
- WHO, & UNICEF. Guidance on global scale up of the prevention of mother to child transmission of HIV. Towards universal access for women, infants and young children and eliminating HIV and AIDS among children. 2007 Retrieved August 15, 2012, from http://www.unicef.org/aids/files/PMTCT_enWEBNov26.pdf.