Abstract
Exosomes are 30–150 nm vesicles secreted by a wide range of mammalian cells that can contain microRNA (miRNA). To test if marrow stromal cell (MSC) exosomes could be used as a vehicle for delivery of anti-tumor miRNAs, we transfected MSCs with a miR-146b expression plasmid, and harvested exosomes released by the MSCs. Intra-tumor injection of exosomes derived from miR-146-expressing MSCs significantly reduced glioma xenograft growth in a rat model of primary brain tumor.
1. Introduction
Each miRNA can target numerous mRNAs, and some are potent tumor suppressors [4]. miRNAs are abundant in extracellular exosomes, and can be transferred from cell-to-cell by exosome release and uptake, resulting in cross-cellular gene-regulation [11]. Cultured MSCs release significant amounts of exosomes which contain functional miRNAs [23]. Here, we tested if MSC exosomes could be used as a delivery vehicle for anti-tumor miRNA therapy in a rodent model of malignant glioma.
Aberrant gene expression is a key mechanism of miRNA dysfunction in cancer, including glioblastoma multiforme (GBM), and miRNAs are differentially expressed in GBM relative to normal tissue [21]. Human mir-146b is located on chromosome 10 within 10q24–26 (10q24.32, 104186259–104186331+), a region lost in a majority of these tumors [18]. mir-146b reduces glioma cell motility and invasion, and EGFR mRNA is a binding-target for miR-146b silencing [13]. EGFR gene amplification occurs in approximately 40% of all GBMs and increased EGFR correlates with glioma invasiveness and malignancy [14].
Transfection with miR-146b reduces glioma cell invasion, migration, viability, and expression of EGFR [13]. The EGFR signaling network is an attractive target for anti-tumor therapy, and antibodies, tyrosine kinase inhibitors, and vaccines have been employed to inhibit the receptor [9]. As miR-146b inhibits EGFR expression, and suppresses the malignant phenotype in glioma cells, we hypothesized that miR-146b could function as an anti-glioma miRNA in vivo. We have reported that miR-146b expression is significantly lower in human GBM cells compared to non-tumor human astrocytes [13]. Similarly, we found that 9L gliosarcoma miR-146b expression was 0.07 ± 0.01 fold of miR-146 expression in primary rat astrocytes. Here, we employed a 9L xenograft model of primary brain tumor.
2. Materials and Methods
2.1 Tumor implantation
Male Fischer rats (250–275 g) were used for this study. A 2 mm diameter craniotomy was made on the right hemisphere anterior to the coronal suture. Using a Hamilton syringe, cells were injected 3.5 mm deep, 3.0 mm to the right and 1.0 mm anterior of the bregma. Rats were implanted with 2.5 × 105 9L cells (5 μl PBS) over a 15-minute interval. The craniotomy was covered with Horsley’s bone wax, and the incision was closed with 4-0 silk suture (Ethicon). Animals were weighed at tumor implantation, treatment, and prior to sacrifice. No statistical difference in weights was detected between treatment groups. Rats were sacrificed 10 days after implantation under anesthesia with i.p. administration of ketamine (100 mg/kg) and xylazine (10 mg/kg). Animals were perfused with 10% formalin following vascular washout with 0.9% saline. Brains were removed, fixed and cut into 2 mm thick blocks which were embedded in paraffin. Sections were stained with hematoxylin and eosin. To measure tumor volume, in each coronal section, the area of the tumor was measured using MCID software (InterFocus Imaging) by tracing the demarcation of the tumor, and the section volume was determined by multiplying the traced area by the section thickness.
2.2 Plasmids and MSC transfection
Cel-miR-67 and hsa-miR-146b expression plasmids (GenScript) were used. MSC transfection was performed using electroporation. 2 × 106 MSCs were suspended in 150 μl of Ingenio Electroporation Solution (Mirus) with 2 μg of plasmid DNA. Program A-33 was used for electroporation in an Amaxa Nucleofector Device. Transfected cells were resuspended in 10 ml complete culture medium, centrifuged, and then plated for exosome production.
2.3 Exosome preparation and harvest
2 × 106 MSCs were seeded in 10 ml Dulbecco’s Modified Eagle Medium supplemented with 20% Fetal Bovine Serum. After 48 hours, exosomes were isolated from the MSC medium using ExoQuick-TC (System Biosciences, CA). Exosome pellets were resuspended in sterile PBS at a total protein concentration of 10 μg/μl. Exosome suspensions were placed on ice and administered to animals within 6 hours following harvest. Exosomes visualized by electron microscopy were fixed in glutaraldehyde (5 μg/μl) for 30 minutes.
2.4 Exosome treatment
For treatment, 5 μl of the M67-exo or M146-exo suspension was injected in each animal via intra-tumor injection at 5 days after tumor implantation. Using a Hamilton syringe, exosome suspension or PBS vehicle was injected at the same coordinates as the tumor implant, over a 5-minute interval. Real-time PCR was used to determine relative cel-miR-67 expression and miR-146b expression in M67-exo and M146-exo used for treatment. miR-146b was 7.3 ± 1.7 fold higher in M146-exo compared to M67-exo. Cel-miR-67 was not detected in M146-exo, but was detected with a CT value of 33.2 ± 2.3 in M67-exo.
2.5 Real-time PCR
To analyze miRNA expression, MSC cells, 9L cells or MSC exosomes were lysed in Qiazol reagent and total RNA was isolated using the miRNeasy Mini kit (Qiagen). Reverse transcription was performed with the miRNA Reverse Transcription Kit (Applied Biosystems), and cDNA was amplified with TaqMan miRNA assays (Applied Biosystems), which are specific for mature miRNA sequences. For detection of EGFR mRNA, cDNA was prepared using oligo(dT), dNTP mix, DTT, First-Strand Buffer, RNaseOUT, and Superscipt III (Invitrogen). PCR was performed using the SYBR Green real-time PCR system (ABI7000, Applied Biosystems). 40 amplification cycles were performed. The 2-ΔΔCT method was used to determine relative mRNA or miRNA expression. If a CT was not reached in 40 amplification cycles, the measured RNA was considered to be undetected.
2.6 Western blot
2 × 105 9L cells were seeded in a 6-well plate and cultured overnight. M67-exo or M146-exo (16.7 μg/ml total protein of exosome pellet) in 5 μl PBS was added to the culture medium. 24 hours later 9L cells were lysed and Western blot was used to detect EGFR and β-actin (Santa Cruz Biotechnology). Protein concentration was quantified using a BCA protein assay kit (Pierce). SuperSignal West Pico Chemiluminescent Substrate (Pierce) and Kodak X-omat film (Kodak) exposure were used for visualization.
2.7 Growth assay
2500 cells primary rat cortical astrocytes or 9L gliosarcoma cells were plated in each well of a 96-well plate containing DMEM with FBS at a concentration of 20% or 10%, respectively. MSC exosomes, M67-exo, or M146-exo were added at a concentration of 16.7 μg/ml (total protein of exosome pellet). Every 24 hours, total adherent and non-adherent cells in each well were quantified using a hematocytometer. Cell counts of three wells per time point per group were averaged.
2.8 Statistical analysis
Data are shown as mean ± sem. P-values were calculated using one-way ANOVA or Student’s t test. A p-value of 0.05 or less was considered statistically significant.
3. Results
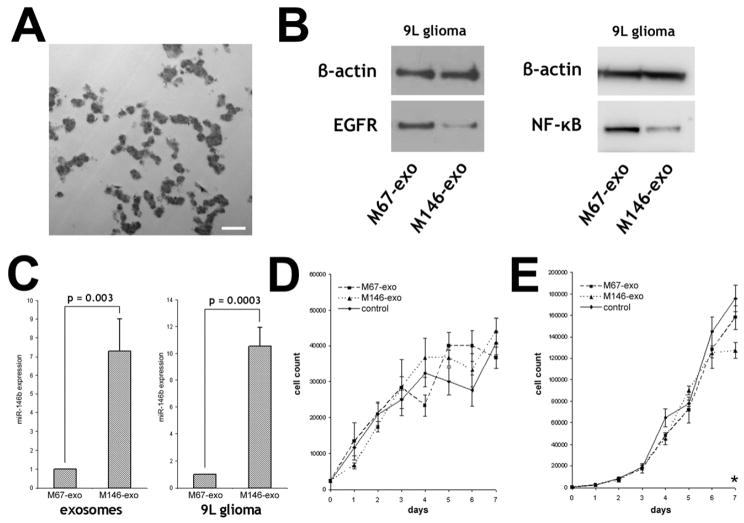
To test if MSCs package miR-146b into secreted exosomes, we transfected MSCs from rats with a plasmid encoding for miR-146b, or for cel-miR-67, which has no known mRNA binding targets in rat. 48 hours after transfection, exosomes were isolated from the medium, and miR-146b and cel-miR-67 were measured in MSCs and extra-cellular exosomes. miR-146b was 7.1 ± 3.7 fold higher in MSCs and 7.3 ± 1.7 fold higher in MSC exosomes after miR-146b expression plasmid transfection, compared to miR-146 levels in cel-miR-67 plasmid transfected MSCs and their exosomes, respectively (Fig. 1C). Cel-miR-67 was not detected in miR-146b plasmid transfected MSCs or their exosomes (M146-exo), but was detected in cel-miR-67 plasmid transfected MSCs and their exosomes (M67-exo). These data demonstrate that plasmid-expressed miRNA is efficiently packaged into MSC exosomes by endogenous mechanisms.
Figure 1.
Exosomes from MSCs transfected with miR-146b or cel-miR-67 expression plasmids. A, Electron micrograph of MSC exosomes isolated from MSC culture medium. Scalebar = 500 nm. Data are mean ± sem.; comparison is two-tailed t-test. B, Western blot for beta;-actin, EGFR and NF-κB protein expression in 9L cells treated with M67-exo and M146-exo. C, Real-time PCR detection of miR-146b expression in M67-exo and M146-exo (n = 7), and in 9L cells treated with M67-exo or M146-exo (n = 3). D, Growth assay of primary cortical rat astrocytes treated with MSC exosomes, M67-exo or M146-exo. E, Growth assay of 9L glioma cells rat treated with MSC exosomes, M67-exo or M146-exo (*p = 0.027).
To determine if MSC exosomes carrying miR-146b deliver the miRNA into tumor cells, we exposed 9L cells to M146-exo or M67-exo in vitro. After 24 hours, miR-146b detected in M146-exo-treated 9L cells was 10.5 ± 1.4 times higher compared to M67-exo-treated cells (Fig. 1C), whereas cel-miR-67 was detected in M67-exo-treated 9L cells, but not detected M146-exo-treated cells. Thus, MSC exosomes can deliver plasmid-expressed miRNAs into tumor cells in vitro. To test if M146-exo could alter target protein expression in tumor cells, we exposed 9L cells to M146-exo in vitro, and after 24 hours, EGFR and NF-κB protein levels were lower in M146-exo-treated 9L cells compared to M67-exo-treated 9L cells (Fig. 1B). EGFR mRNA levels were not significantly different between M67-exo- or M146-exo-treated cells (p > 0.05). Finally, to test if M146-exo affected glioma growth in vitro, we performed a growth assay using primary rat astrocytes or 9L glioma cells treated with normal MSC exosomes, M67-exo or M146-exo (Fig. 1D,E). We found that the growth of non-tumor astrocytes was not significantly altered by M146-exo; however after 7 days, in vitro growth of M146-exo-treated 9L cells was significantly less than normal MSC exosome-treated control. These findings suggest that miR-146b, delivered via MSC exosomes, is functionally active in the acceptor tumor cells.
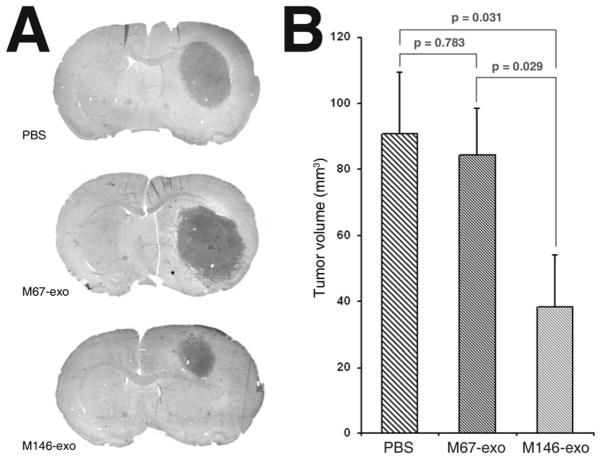
Finally, to determine if M146-exo had an anti-tumor effect in vivo, we administered M146-exo or M67-exo (50 μg total protein in 5μl volume) to Fischer rats bearing 9L gliosarcoma. We found that one intra-tumor injection of M146-exo 5 days after intracranial tumor xenograft implantation lead to a significant reduction in tumor volume at 10 days post-implant compared M67-exo or vehicle treated control (Fig. 2). These data suggest that M146-exo elicits an anti-tumor effect in the rat brain.
Figure 2.
Intra-tumor injection of M146-exo reduced 9L glioma growth in rat brain. A, Representative H&E-stained coronal sections from rats sacrificed 10 days after tumor implantation. B, Volumetric measurement of 9L xenograft tumors 10 days after tumor implant, and 5 days after PBS, M67-exo, or M146-exo treatment (n = 8 per group). ANOVA: p = 0.042. Data are mean ± sem. Post hoc multiple comparisons are two-tailed t-tests.
4. Discussion
Despite advances in diagnostics, chemotherapeutics, and surgical techniques, the prognosis for GBM remains poor [24]. Here, we have demonstrated a novel treatment whereby therapeutic miRNA that is produced in MSCs and loaded into extra-cellular exosomes by endogenous mechanisms, is used to treat tumor.
Interest is growing in using exosomes as biological delivery vehicles. Exosomes are taken up by acceptor cells, whereby cellular processes can be altered [11]. There is evidence that exosomes do not elicit acute immune rejection, and as they are non-viable, they do not risk tumor formation [3, 11]. Furthermore, exosomes can be manufactured at scale in culture, possibly using autologous cells, and exosome-producing cells could incorporate multiple therapeutic miRNAs, enabling personalized treatment [3]. Our in vitro experiments indicate that miRNA packaged into MSC exosomes are incorporated by tumor cells in culture. For in vivo treatment, we delivered exosomes directly by intra-tumor injection. Previously, we demonstrated that functional miRNAs are transferred between glioma cells, suggesting that therapeutic miRNAs may distribute throughout the tumor [12]. Here, we directly injected exosomes into the tumor. Viral vectors, proteins, and cells have been administered by intratumoral injection or injection into the brain adjacent to the tumor cavity during surgical resection [5, 6, 15, 17]. It is possible that exosomes could be similarly administered. Additional work is needed to characterize the delivery of exosomal miRNA in tumor in brain.
EGFR and miR-146b expression are inversely correlated in GBMs, and a role for EGFR in glioma initiation and malignancy is established [20]. Still, EGFR inhibitors have largely failed to induce GBM regressions clinically, even where the relationship between genotype and drug response is observed in other cancers [16]. GBMs display a variety of genetic aberrations, and it has been suggested that the broad-signaling of miRNAs may be more therapeutically effective in GBM, when compared to the alteration of just one gene [2, 19, 22, 24]. Previously, we demonstrated that EGFR mRNA is a binding target for miR-146b [13]. Indeed, in addition to EGFR, miR-146 inhibits NF-κB, as well as its upstream regulator TRAF6, and this effect reduces invasion in breast cancer cells [1]. Here, we demonstrated that M146-exo decreased EGFR and NF-κB protein in 9L glioma cells in vitro. miR-146b has also been identified to inhibit SMAD4, the loss of which is a independent indicator of poor outcome in glioma [7, 10]. Therefore, it is likely that concurrent inhibition of factors in addition to EGFR underpins the anti-tumor effect of M146-exo.
Treatment of with M146-exo significantly reduced growth of 9L glioma cells after 7 days in vitro. This modest effect of M146-exo upon in vitro cell growth is similar to what we previously reported in glioma cells transfected with miR-146b [13]. However, reduction of 9L tumor volume we observed in vivo was significant 5 days after M146-exo treatment. This observed difference may be due to dissimilarities in the kinetics of M146-exo delivery in culture and in brain. However, it is also possible that the brain microenvironment may play a role in the response of 9L to M146-exo treatment.
Interestingly, Guduric-Fuchs et al. found that in HEK293T cells, expression of miR-146a enhanced export of miR146a, but relative expression of endogenous intra-cellular and extra-cellular vesicle miRNA was largely unaffected, suggesting that selective miRNA export is tightly regulated [8]. Even so, we surmise that regulation of miRNA export may differ between cell types, within different extra-cellular vesicles, and may be differentially affected by other miRNAs. In addition, expression of miR-146b may alter the non-miRNA characteristics of MSC exosomes as well. As each of these factors could change the therapeutic potential of M146-exo, the effect of miR-146b expression upon MSC exosomes warrants further study.
Here, one intra-tumor injection of 50 μg M146-exo significantly reduced glioma xenograft growth in rat brain. These findings suggest that export of specific therapeutic miRNA into MSC exosomes represents a new treatment strategy for malignant glioma.
Footnotes
Conflict of Interest Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008 doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgess R, Jenkins R, Zhang Z. Epigenetic changes in gliomas. Cancer Biol Ther. 2008;7:1326–1334. doi: 10.4161/cbt.7.9.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, Padmanabhan J, Lee CN, de Kleijn DP, Lim SK. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47. doi: 10.1186/1479-5876-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillman RO, Duma CM, Schiltz PM, DePriest C, Ellis RA, Okamoto K, Beutel LD, De Leon C, Chico S. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother. 2004;27:398–404. doi: 10.1097/00002371-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Geletneky K, Huesing J, Rommelaere J, Schlehofer JR, Leuchs B, Dahm M, Krebs O, von Knebel Doeberitz M, Huber B, Hajda J. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 12:99. doi: 10.1186/1471-2407-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geraldo MV, Yamashita AS, Kimura ET. MicroRNA miR-146b-5p regulates signal transduction of TGF-beta by repressing SMAD4 in thyroid cancer. Oncogene. 2012;31:1910–1922. doi: 10.1038/onc.2011.381. [DOI] [PubMed] [Google Scholar]
- 8.Guduric-Fuchs J, Camp OCAB, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12:675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He SM, Zhao ZW, Wang Y, Zhao JP, Wang L, Hou F, Gao GD. Reduced expression of SMAD4 in gliomas correlates with progression and survival of patients. J Exp Clin Cancer Res. 2011;30:70. doi: 10.1186/1756-9966-30-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu G, Drescher KM, Chen XM. Exosomal miRNAs: Biological Properties and Therapeutic Potential. Front Genet. 2012;3:56. doi: 10.3389/fgene.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–8263. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katakowski M, Zheng X, Jiang F, Rogers T, Szalad A, Chopp M. MiR-146b-5p suppresses EGFR expression and reduces in vitro migration and invasion of glioma. Cancer Invest. 2010;28:1024–1030. doi: 10.3109/07357907.2010.512596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, Riggins GJ. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–3339. [PubMed] [Google Scholar]
- 15.Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, Nabors LB, Markiewicz M, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Gillespie GY. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellinghoff IK, Lassman AB, Wen PY. Signal transduction inhibitors and antiangiogenic therapies for malignant glioma. Glia. 59:1205–1212. doi: 10.1002/glia.21137. [DOI] [PubMed] [Google Scholar]
- 17.Oshiro S, Tsugu H, Komatsu F, Ohnishi H, Ueno Y, Sakamoto S, Fukushima T, Soma G. Evaluation of intratumoral administration of tumor necrosis factor-alpha in patients with malignant glioma. Anticancer Res. 2006;26:4027–4032. [PubMed] [Google Scholar]
- 18.Rasheed BK, Fuller GN, Friedman AH, Bigner DD, Bigner SH. Loss of heterozygosity for 10q loci in human gliomas. Genes Chromosomes Cancer. 1992;5:75–82. doi: 10.1002/gcc.2870050111. [DOI] [PubMed] [Google Scholar]
- 19.Riemenschneider MJ, Jeuken JW, Wesseling P, Reifenberger G. Molecular diagnostics of gliomas: state of the art. Acta Neuropathol. 2010;120:567–584. doi: 10.1007/s00401-010-0736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao M, Rossi S, Chelladurai B, Shimizu M, Ntukogu O, Ivan M, Calin GA, Matei D. PDGF induced microRNA alterations in cancer cells. Nucleic Acids Res. 2011;39:4035–4047. doi: 10.1093/nar/gkq1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silber J, James CD, Hodgson JG. microRNAs in gliomas: small regulators of a big problem. Neuromolecular Med. 2009;11:208–222. doi: 10.1007/s12017-009-8087-9. [DOI] [PubMed] [Google Scholar]
- 22.Turner JD, Williamson R, Almefty KK, Nakaji P, Porter R, Tse V, Kalani MY. The many roles of microRNAs in brain tumor biology. Neurosurg Focus. 2010;28:E3. doi: 10.3171/2009.10.FOCUS09207. [DOI] [PubMed] [Google Scholar]
- 23.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang XL, Sang X, Zhang ZG, Chopp M. Exosome Mediated Transfer of miR-133b from Multipotent Mesenchymal Stromal Cells to Neural Cells Contributes to Neurite Outgrowth. Stem Cells. 2012 doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng S, Chheda MG, Verhaak RG. Studying a complex tumor: potential and pitfalls. Cancer J. 2012;18:107–114. doi: 10.1097/PPO.0b013e3182431c57. [DOI] [PMC free article] [PubMed] [Google Scholar]