Abstract
Background
We reported that obesity was associated with increased arterial compliance in children, possibly due to accelerated vascular maturation. Here we explored the additional burden of type 2 diabetes (T2DM) on vascular function in children.
Methods
50 normal weight (BMI 25-75%), 58 obese (BMI≥95%), and 34 children with type 2 diabetes diagnosed by ADA criteria ages 10-18y were studied. Large and small artery elasticity (LAEI and SAEI, respectively) were measured by diastolic pulsewave contour analysis.
Results
SAEI was 27% higher in children with T2DM compared to normal weight children (p=0.005). Mean LAEI for those with T2DM not different from either group. In the group with T2DM, both SAEI and LAEI increased with age up to age 16 years, but declined thereafter. The strongest multivariable model predicting SAEI in children with T2DM combined lean mass, systolic blood pressure, and glucose (r2=0.59); for predicting LAEI, the strongest model included height, systolic blood pressure and LDL-cholesterol (r2=0.61).
Conclusion
The lower arterial compliance in older adolescents with T2DM compared to that of their peers without diabetes may indicate a premature maturation of the vascular system, however, follow up will clarify whether these vascular changes portend an early increase in diabetes associated cardiovascular disease risk.
Introduction
The prevalence of type 2 diabetes (T2DM) in youth has steadily risen in tandem with the childhood obesity epidemic.(1-3) According to Framingham risk assessment, T2DM adds the same negative impact on cardiovascular disease risk as an increase in age of 10 years.(4) When combined with any other risk factor, including hypertension, T2DM increases the risk of cardiovascular disease by three to four-fold.(5)
The increased risk of cardiovascular disease in patients with T2DM is potentially mediated in part through an increase in arterial stiffness.(5) Several studies of adults have shown that T2DM is associated with increased large artery stiffness, measured by central pulse wave velocity or central pulse pressure, compared to people without diabetes.(6)
The purpose of the current investigation was to determine the effect of T2DM on arterial compliance in children with type 2 diabetes compared to normal weight and obese peers. Since we previously showed that obesity was associated with increased compliance in children (7), we tested the hypothesis that T2DM may reverse that effect as a result of glycemic or inflammatory stress on the vasculature.
Methods
Subjects
One hundred forty-two children, ages 10 to 18 years old participated, including 50 normal weight children, 58 obese children, and 34 children with T2DM. Primary outcome data from the normal weight and obese group were previously published.(7) The current report includes a subset of the previously published data; we selected normal weight and obese children who matched the age distribution of the children with T2DM. While type 2 diabetes is slightly more prevalent in girls than boys(8), we have previously shown that in obese children, arterial compliance did not differ between the sexes (9), and therefore, did not match for sex between the T2DM and non-diabetic groups in the present study. The criterion for inclusion in the normal weight group was a body mass index (BMI) between the 25-75th percentile based on 2000 Centers for Disease Control (CDC) growth charts. Children in the obese group had a BMI greater than the 95th percentile based on CDC growth charts. The children in the normal weight and obese groups were free of diabetes, cardiovascular disease, or other chronic disease. The children in the T2DM group were diagnosed according to the American Diabetes Association guidelines.(10) None of the participants with diabetes used insulin at the time of the study, and the duration of diabetes for all participants was less than 5 years (average duration of diagnosis 1.9±1.7 years). While 7 children in the obese group and 27 children with T2DM took metformin at the time of the study, we confirmed that none of the seven obese children met the ADA criteria for a diagnosis of diabetes. None of the participants in the normal weight or obese groups were on lipid lowering medication. One participant in the T2DM group took fish oil.
Informed written consent and assent were obtained in accordance with the guidelines of the University of Oklahoma Health Sciences Center Institutional Review Board for Human Subjects. Each subject filled out a brief questionnaire regarding family history and ethnicity. Each subject also completed the “Modifiable Activities Questionnaire for Adolescents” to subjectively quantify physical activity over the past 12 months (11). Volume of physical activity was calculated as metabolic equivalents of task (METs) and expressed as MET-hours/week.
Anthropometric measures
A pediatrician completed a medical history and physical examination on each participant and determined pubertal status using Tanner staging. Height and weight were measured, and BMI calculated from these measures and expressed as a percentile using CDC norms for the child’s age and sex. Blood pressure was measured using an appropriate sized cuff. Body composition was quantified using dual energy X-ray absorptiometry (DXA, GE iDXA, Fairfield, CT).
Arterial Compliance
Arterial compliance of the large and small arteries was measured using diastolic pulse-wave analysis (HDI/ Pulsewave CR-2000, Hypertension Diagnostics, Inc, Eagan, MN). After 10 minutes of rest, an upper arm blood pressure cuff was placed on one arm while the opposite wrist was immobilized with a stabilizer to minimize movement of the tonometric sensor placed over the radial artery. Once optimal arterial waveforms and a stable baseline were achieved, the waveforms were recorded at 200 Hz for 30 seconds. An automated software program calculated small and large artery elasticity (SAEI and LAEI respectively) from the digitized waveforms. The average of three replicates for each participant was used for data analyses. The intraclass correlation coefficients for LAEI and SAEI in children using this device are reported to be 0.90 and 0.88, respectively (12).
Reactive hyperemia peripheral arterial tonometry (RH-PAT)
RH-PAT (Endo-PAT2000, itamar, Franklin, MA) was performed to assess each participant’s vascular endothelial function as previously described.(7) Plethysmographic sensors placed on the index finger of each hand recorded pulse pressure before, during and after successive 5-minute periods of pre-occlusion baseline, brachial artery occlusion, and hyperemic recovery. The RH-PAT index is calculated as the increase in post-occlusion pulse response relative to baseline, corrected for changes in systemic vascular tone based on measurements from the finger on the nonoccluded arm.
Serum Analysis
A fasting blood sample was collected for measurement of serum glucose, insulin, C-reactive peptide (CRP) and lipids. Glucose was measured by the glucose oxidase method (YSI 2300 STAT plus, Yellow Springs, OH). Insulin was measured using Human Insulin ELISA kit from Millipore (St. Charles, MO). Glucose and insulin values were used to calculate fasting insulin resistance using the HOMA-IR model (13). Serum triglycerides and total-, HDL- and LDL-cholesterol, and C-reactive protein were measured at the Clinical Chemistry Laboratory of the Oklahoma Veterans Administration Hospital (Oklahoma City) using validated enzymatic assays (Synchron Systems, Beckman Coulter, Brea, CA).
Statistical Analysis
All outcomes were inspected for normality of distribution. Outcomes that were notably skewed were log transformed for statistical analysis. Separate analysis of variance models identified differences among the normal weight, obese and T2DM groups in anthropometric, serum, and arterial function measurements. Univariate modeling established correlations between each of the three measures of arterial function and the anthropometric and serum data in the group with T2DM. Variables that were significantly correlated with the outcomes were then combined to identify the multivariable regression models that best predicted arterial function in the group with T2DM. SAS version 9.1 (Cary, NC) was used to calculate the regression models. Because the study explored novel hypotheses, we did not adjust for multiple comparisons, but considered any association significant that yielded a p-value less than 0.05.
Comparisons of mean SAEI and LAEI between age groups were performed using a contrast from a two factor analysis of variance model. Additional analyses across the entire range of participants’ ages were performed to estimate the age of transition at which an increase in arterial compliance reversed and became a decreasing trend. Estimates of the age of transition in LAEI were generated from nonlinear regression models. When nonlinear models did not produce congruent results for SAEI, we explored a series of linear piecewise regression models that compared values for the age of transition from 15 to 17 years. To prevent unusual observations from unduly influencing the model’s predictions, we employed a weighted analysis using robust regression techniques.
Results
Anthropometric Measures
The normal weight and obese groups were comprised of approximately equal numbers of boys and girls, but 75% of the group with T2DM was girls. The children with T2DM were also more ethnically diverse than the children in the normal weight and obese groups. The racial/ethnic distribution of the children with type 2 diabetes was: 12 Caucasians, 14 Hispanic, 4 African American, 2 Native American, 1 Asian, and 1 other. As shown in table 1, the group with T2DM had higher values for body weight (p<0.001), lean mass (p<0.001), fat mass (p<0.001), and systolic blood pressure (SBP) (p<0.001) compared to the normal weight group. The group with T2DM generally did not differ from the obese group except for fat mass, which was greater in the group with T2DM (p=0.046). Reported physical activity did not differ between the normal weight and obese groups. However, the T2DM group was less active than the obese group (p=0.040) and tended to be less active than the normal weight peers (p=0.057).
Table 1.
Participant characteristics
| Normal Weight (N=50) | Obese (N=58) | Type 2 Diabetes (N=34) | |
|---|---|---|---|
| Sex (F/M) | 24/26 | 31/27 | 25/9 |
| Age (years) | 14.3±2.3 (13.7-15.0) | 14.3±2.2 (13.7-14.9) | 14.9±2.4 (14.1-15.7) |
| Ethnicity (% Caucasian) | 74% | 66% | 35% |
| BMI (kg/m2)*‡ | 19.6±1.9 (19.1-20.1) | 32.4±5.9 (30.9-33.9) | 37.2±9.0 (34.2-40.2) |
| BMI SDS* | 0.05±0.41 (−0.06-0.16) | 2.13±0.33 (2.05-2.21) | 2.33±0.48 (2.17-2.49) |
| Tanner Stage | 3.3±1.1 (3.0-3.6) | 3.6±1.0 (3.3-3.9) | 3.8±1.3 (2.4-4.2) |
| Lean Mass (kg)* | 33.5±9.0 (31.0-36.0) | 44.7±10.6 (42.0-47.4) | 47.1±12.8 (43.1-51.7) |
| Fat Mass (kg)*‡ | 12.6±4.4 (11.4-13.8) | 38.0±13.7 (34.5-41.5) | 45.4±18.3 (39.3-51.6) |
| Systolic Blood Pressure (mmHg)* | 110±7 (108-112) | 118±8 (116-120) | 118±12 (114-122) |
| Diastolic Blood Pressure (mmHg) | 59±5 (58-60) | 60±7 (58-62) | 61±8 (58-64) |
| Metabolic Equivalents (MET-h/wk)‡ | 32.6±28.2 (24.8-40.4) | 37.2±47.4 (25.0-49.4) | 21.5±23.6 (13.6-29.4) |
All results are reported as mean ± SD (95%CI).
denotes p<0.05 comparing obese and T2DM groups to normal weight groups
denotes p<0.05 comparing T2DM group to the obese group
Metabolic Equivalents (METs) is a unit used to estimate the amount of oxygen used by the body during physical activity.
Serum Measures
As shown in table 2, the group with T2DM had higher fasting glucose, insulin, and HOMA-IR than either of the non-diabetic groups. The lipid analyses showed that the group with T2DM had lower total LDL-, and HDL-cholesterol but higher TG compared to the obese group. However, compared to the normal weight group, the group with T2DM had higher LDL-, lower HDL-cholesterol and higher TG. The groups did not differ in total cholesterol. CRP in the T2DM group was higher than in the normal weight group (p<0.001) but similar to the obese group.
Table 2.
Serum biochemical outcomes
| Normal weight | Obese | Type 2 Diabetes | |
|---|---|---|---|
| Glucose (mmol/L)‡+ | 4.6±0.3 (4.5-4.7) | 4.7±0.4 (4.6-4.8) | 6.9±2.6 (6.0-7.8) |
| Insulin (pmol/L)*‡+ | 47.64±37.29 (37.30-57.98) | 167.03±133.27 (132.73-201.33) | 247.03±166.68 (191.00-303.06) |
| HOMA-IR*‡+ | 1.41±0.08(1.39-1.43) | 5.14±4.89 (3.88-6.40) | 10.39±8.24 (7.62-13.16) |
| Triglycerides (mmol/L)*‡ | 0.84±0.37 (0.74-0.94) | 1.25±0.68 (1.07-1.43) | 1.41±0.63 (1.20-1.62) |
| Cholesterol (mmol/L)*# | 4.14±0.73 (3.94-4.34) | 5.02±1.92 (4.53-5.51) | 4.14±.83 (3.86-4.42) |
| HDL (mmol/L)†# | 1.25±0.28 (1.17-1.33) | 1.24±0.35 (1.15-1.33) | 0.89±0.23 (0.81-0.97) |
| LDL (mmol/L)*‡# | 2.41±0.66 (2.23-2.59) | 3.28±1.72 (2.84-3.72) | 2.73±0.71(2.49-2.97) |
| CRP (nmol/L)*‡ | 7.62±13.71 (3.82-11.42) | 31.52±47.62 (19.26-43.78) | 53.05±59.62 (33.01-73.09) |
All results are presented as mean ± SD (95%CI).
p<0.05 with normal weight < obese
p<0.05 with normal weight < T2DM
p<0.05 with obese < T2DM
p<0.05 with T2DM < normal weight
p<0.05 with T2DM < obese
Vascular Measures
As shown in figure 1, Reactive Hyperemia Index (RHI) did not differ among the three groups or vary with age. However, glucose was negatively correlated with RHI in the children with T2DM (R2=0.21, p=0.007). In a multiple variable model that controlled for height, glucose and insulin were both independently and negatively associated with RHI for the group with T2DM (R2=0.30, p=0.005). Holding insulin and height constant, RHI decreased 0.0067 for every 1mg/dL increase in glucose. Alternatively, holding glucose and height constant, RHI decreased 0.0093 for every 1μU/mL increase in insulin. RHI was not associated with either insulin or glucose in either of the non-diabetic groups.
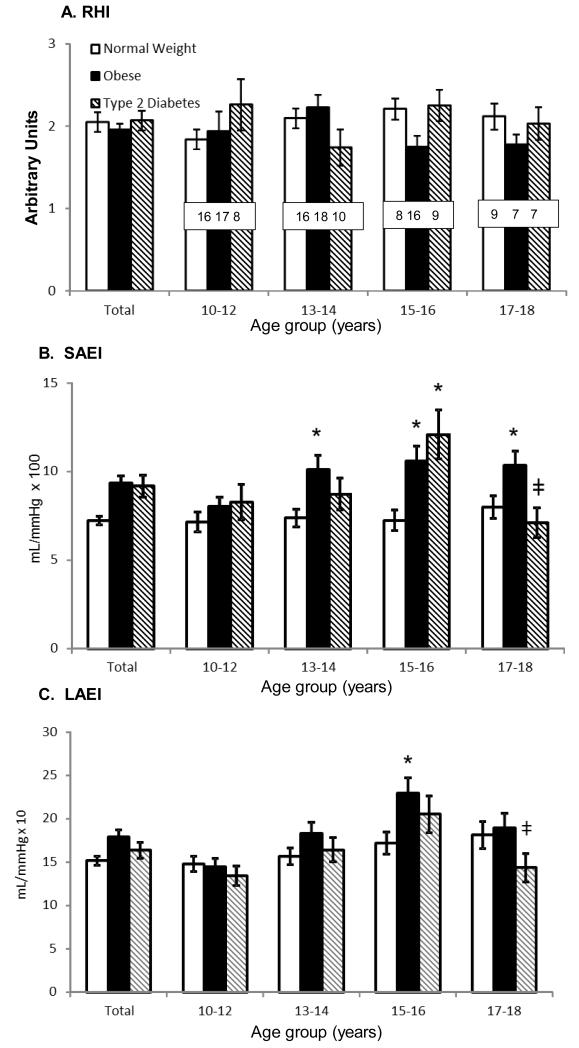
Figure 1.
A. Reactive Hyperemia Index (RHI) – RHI represented as the comparison of normal weight, obese and T2DM for all subjects and broken down into 2 year age groups. B. Small Artery Elasticity Index (SAEI) – SAEI represented similar to RHI but *denotes p<0.05 differences compared to the normal weight group. ‡ denotes p<0.05 difference in SAEI of the 15-16 year group with T2DM compared to the 17-18 year group. C. Large Artery Elasticity (LAEI) – LAEI represented similar as above.
Across all age groups, the mean SAEI for the group with T2DM was 24% higher (p=0.010) than in the normal weight group, but not different compared to the obese group (Figure 1). Individual variables that were positively correlated with SAEI in the T2DM group included total body lean mass (R2=0.33, p=0.0004) and fat mass (R2=0.22, p=0.0048), insulin (R2=0.20, p=0.0096), and height (R2=0.15, and p=0.0218). Variables that were negatively associated included diastolic blood pressure (DBP) (R2=0.21, p=0.007), systolic blood pressure (SBP) (R2=0.17, p=0.0156), cholesterol (R2=0.13, p=0.0390), and glucose (R2=0.12, p=0.0478). In a multiple variable model, lean mass, SBP, and glucose together best explained the variation in SAEI (R2=0.59). In this model lean mass and SBP were both independent predictors of SAEI. In a model that controlled for glucose and SBP, SAEI increased 0.17 mL/mmHg×100 for every 1kg increase in lean mass. Alternatively, controlling for glucose and lean mass, SAEI decreased 0.14mL/mmHg×100 for every 1mmHg increase in SBP.
Figure 1 depicts that, across all age groups, mean LAEI in the T2DM group (95%CI: 14.57-18.15 mL/mmHg × 10) was not different from that of the normal weight group (95%CI: 14.95-17-13 mL/mmHg × 10, p=0.768) and was nonsignificantly lower than that of the obese group (95%CI: 16.88-20.14 mL/mmHg × 10, p=0.086). In the group with T2DM, LAEI was positively associated with height (R2=0.45, p<0.0001) and lean mass (R2=0.26, p=0.0021) and negatively associated with cholesterol (R2=0.13, p=0.0356). In a multiple variable model, a combination of height, SBP and LDL best predicted LAEI (R2=0.61). Controlling for LDL-cholesterol, both height and SBP were independently associated with LAEI (p<0.001 and p=0.0036, respectively). Controlling for LDL-cholesterol and SBP, LAEI increased 0.38 mL/mmHg×10 for every 1cm increase in height. Controlling for LDL-cholesterol and height, LAEI decreased 0.16 mL/mmHg×10 for every 1 mmHg increase in SBP.
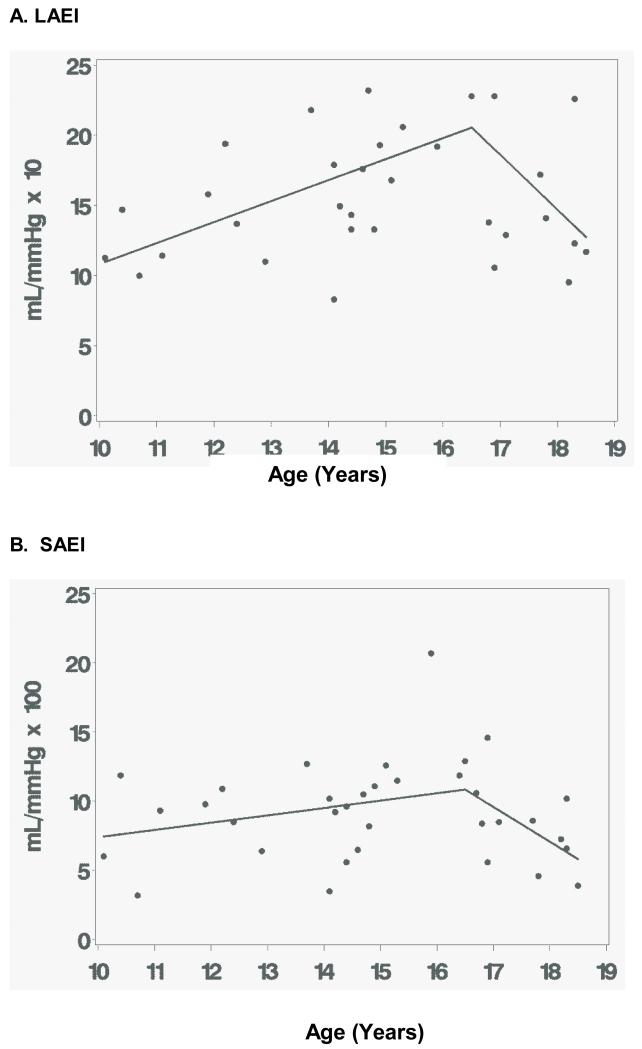
As shown in Figure 1, among children with T2DM, LAEI and SAEI were lower in the 17-18 year old age group compared to the 15-16 year old group. Within the T2DM group, LAEI was 30% lower for the 17-18 year old subgroup than the 15-16 year old subgroup (14.3±4.3 versus 20.5±6.4 mL/mmHg × 10, respectively; 95% CI for the difference = 1.3, 11.0; p=0.0128). Similar to LAEI, within the T2DM group, SAEI was 41% lower for 17-18 year olds compared to 15-16 year olds (7.1±2.3 versus 12.1±4.2 mL/mmHg × 100; 95%CI for the difference = 2.3, 7.7; p=0.0004). Further analyses were performed using nonlinear regression models to estimate the age at which an age-associated increase in arterial compliance reversed and became a decreasing trend. This modeling approach predicted that LAEI increased 1.50 mL/mmHg × 10 per year (95% CI: 0.45, 2.55 mL/mmHg × 10 per year; p=0.0066) from age 10 until age 16.5 years (95% CI: 15.4, 17.6 years; p=0.0001) (Figure 2). For children with T2DM older than 16.5 years, LAEI decreased by 3.9 mL/mmHg × 10 a year (95% CI: 0.03, 7.79 mL/mmHg × 10 per year; p=0.0483) until age 18.5.
Figure 2.
A. Large Artery Elasticity (LAEI) – Representation of nonlinear regression model depicting change in association of LAEI with older adolescents. B. Small Artery Elasticity (SAEI) – Representation of linear piecewise depicting change in association of SAEI with older adolescents.
Because a similar nonlinear model did not produce congruent results for SAEI, we explored separate linear piecewise regression models that set the age of transition at a series of values from 15 to 17 years. A model that stipulated the age of transition at 16.5 years best fit the observed values for SAEI, as judged by the R-square statistic (R2=0.1541 at 16.5 years). This model predicted little change in SAEI from age 10 to age 16.5 (95%CI: −0.09 mL/mmHg per year, 1.15 mL/mmHg × 100 per year; p=0.0934). The model predicted that the trend changes after age 16.5 years (p=0.0107) so that SAEI declined between ages 16.5 and 18 years, by 2.51 mL/mmHg × 100 per year.
Discussion
The goal of the current study was to determine the impact of childhood T2DM on arterial compliance and endothelial function. In children with T2DM, small arterial compliance was higher than normal weight children but similar to that of obese children, while large artery compliance was intermediate and not significantly different from values for normal weight or obese peers. Anthropometric measures such as height and lean and fat mass were positive predictors of compliance in children with T2DM, similar to what we reported previously for normal weight and obese non-diabetic children (7). However, a novel finding, unique to the group with T2DM, was that blood pressure, cholesterol, and glucose all were negatively correlated with arterial compliance. Fasting glucose was also negatively associated with endothelial function (RHI-PAT), but only in the group with T2DM, suggesting T2DM has small but potentially important effects on vascular function in youth, independent of and additive to those of obesity.
In adults, obesity without concomitant diabetes is positively correlated with cardiovascular disease mortality (14) and arterial stiffness (15), an early marker of cardiovascular disease. The effect of childhood obesity on arterial compliance is less clear. In some studies central compliance is reduced in obese children, similar to what is reported in obese adults.(16-18) In contrast, we recently showed that arterial compliance is increased in obese youth compared to their normal weight peers and is associated with greater lean and fat mass in the obese children.(7) Our findings are consistent with results reported by Dangardt and colleagues (19), who found that pulse wave velocity measured at the radial artery was reduced (compliance was increased) in obese children compared to their normal weight peers. Chalmers and colleagues (12) also reported increased arterial compliance in obese children using pulse-wave velocity, the method used in the current study. Both of the latter studies (12, 19) measured compliance at the radial artery whereas other studies, which reported reduced compliance in obese children, made measurements at the carotid artery (16-18). It is therefore possible that the reported variation in findings among studies may relate to the location of the vessel. More peripheral vessels such as the radial artery may be more dilated and compliant than central vessels.
Endothelial dysfunction is known to be one of the earliest signs of cardiovascular disease, and adults with impaired glucose tolerance and T2DM have been shown to have impaired endothelial function.(20) Even acute hyperglycemia has been shown to impair endothelial function in adults with DM and impaired glucose tolerance.(21) However, while the association of hyperglycemia and impaired endothelial function is well established, the mechanism underlying the impairment is not clear. Either hyperglycemia or excursions in circulating glucose concentration may adversely affect the function of vascular endothelia, potentially as the result of oxidative stress.(22) Endo-PAT has been shown to a reliable tool to assess endothelial function in adolescents.(23) Although RHI did not differ among the groups, our finding that fasting glucose concentration was negatively associated with RHI in children with T2DM could therefore be evidence that hyperglycemia is an early marker of cardiovascular risk.
To our knowledge, this is the first study to report that small artery compliance is increased in children with T2DM compared to normal weight peers. Similar to the results previously reported in obese children (7), lean mass was significantly associated with small arterial compliance in children with T2DM. Since lean mass is typically comprised of tissue that is more vascularly dense than adipose tissue (24), our findings may simply reflect the impact of larger body size of children with obesity and obesity combined with T2DM. The relatively low correlation between LAEI and SAEI (r2=0.41) suggests that different factors may regulate SAEI and LAEI. In adults with T2DM hyperglycemia, dyslipidemia, hypertension, oxidative stress, and diabetes duration have all been shown to be negatively associated with arterial compliance.(6) We found that SAEI was negatively associated with blood pressure, cholesterol, and glucose, and therefore that the negative effects of those outcomes on arterial compliance appear to be similarly important in children with T2DM as they are in adults.
T2DM in children is highly associated with obesity. However, because children with T2DM are less well studied than adults, it is not yet clear whether obesity and T2DM simultaneously and independently affect vascular function in children with the disease. In a study that included both children and young adults (mean age 18 years old) with T2DM, Urbina and colleagues demonstrated that carotid intima media thickness, a marker of future disease risk, was increased in young people with T2DM compared to normal weight peers.(18) Gungor and colleagues reported that pulse wave velocity was increased (and therefore arterial compliance was decreased), in children with T2DM compared to normal weight peers.(25) Both of those studies performed measurements on the carotid artery, whereas the radial artery was the site of measurement in the current study; it is unclear if measurement site or other methodological or physiological differences among studies accounts for the differences in outcomes. In the study by Gungor, the duration of diabetes is not mentioned and some of the subjects are on multiple medications. Participants in our study may have had disease of shorter duration. Moreover, the use of a single agent, metformin, suggests they may have been in relatively better glycemic control.
In the children with T2DM, it is plausible that hyperglycemia, hypertension, and dyslipidemia each negatively impact arterial compliance, thereby offsetting the positive effect of the obesity-related increase in lean body mass. First, as with endothelial function, hyperglycemia may promote arterial stiffening through oxidative stress and inactivation of nitric oxide synthase.(22) Reduced availability of nitric oxide to smooth muscle cells limits vasodilation response.(22) Furthermore, free radicals produced from glucose autoxidation activate prostaglandin and thromboxane receptors resulting in direct vasoconstriction.(22) Second, hypertension alters vascular structure, leading to smooth muscle hypertrophy, increased arterial wall thickness, and decreased vessel diameter.(26) There is evidence that youth with T2DM have early development of thickening of the intima media in the carotid artery.(18) Although the current study did not measure vessel thickness, the implications are that children with T2DM have thicker vessel walls which could result in increased peripheral resistance. Third, hypercholesterolemia has been shown to be associated with impaired vasodilation, independent of the effects of the endothelium, which suggests that it impairs smooth muscle dilation.(27) The potential mechanism for this effect may be altered smooth muscle membrane potential, which inhibits hyperpolarization and relaxation, resulting in heightened responsiveness to vasoconstriction.(27) Although our study was not designed to determine the mechanism through which glycemia, blood pressure or lipids affect arterial compliance, we found these variables to be negatively associated with arterial compliance. We also found that SAEI and LAEI tended to be lower in the oldest youth with T2DM suggesting these factors may contribute to the deterioration of SAEI and LAEI in children and young adults with T2DM.
A decline in arterial compliance was seen in the T2DM group in the teen years. Reports have shown that arterial compliance in healthy individuals increases to the age of 30, plateaus from 30 to 50 years of age, then declines with advancing age, with an especially rapid decline after the age of 70.(28) It has been estimated that diabetes mellitus has the effect of adding 10-15 years of chronological age to the structure and function of arteries, along with the other associated metabolic risks.(5) It is possible that a similar early maturation effect causes the significant decline in arterial compliance observed among the oldest children with T2DM in this study.
In our study, most (80%) of the children with T2DM were taking metformin. Metformin has been shown to improve endothelial function in adults with metabolic syndrome and T2DM.(29, 30) In women with polycystic ovarian syndrome, metformin improves endothelial function and reduces arterial stiffness.(31) It is plausible that the arterial compliance in children with T2DM may be affected by metformin, and could potentially reduce potential differences between children with T2DM compared to normal weight controls.
This study’s cross-sectional design limited our ability to determine long term effects of hyperglycemia and T2DM diagnosed in childhood on vascular function. Moreover, the study is primarily descriptive with limited mechanistic data. While associations with vascular function have been determined, they do not prove causation. A third limitation is that females predominate in the group with T2DM; however we have previously reported that in obese children, arterial compliance does not differ in males and females (9). A final limitation is that the groups were not matched for racial/ethnic distribution. Arterial compliance reported to be lower in American Indians compared to age-matched non-Hispanic white youth (32). However, our study was not designed to identify differences among racial/ethnic groups.
In conclusion, we have shown that small artery compliance is increased in children with T2DM compared to their normal weight peers, but is similar to their obese peers. Increased small artery compliance may be mediated through the positive effects of lean mass and the negative effects of glucose, cholesterol, and blood pressure. Large artery compliance in children with T2DM was not significantly different from either group. In multivariable models, lean mass and fat mass predicted large artery compliance in non-diabetic children. Lean mass, but not fat mass, predicted LAEI in the T2DM group. Finally, a significant decline in arterial compliance was observed in 17 and 18 year olds with T2DM, suggesting that the increased compliance in the earlier ages may be the result of early maturation of the vascular system. This early maturation may hasten the onset of the normal effects of aging on vascular stiffness and on cardiovascular disease risk; this last finding signals a need for interventions to reverse this trend.
Acknowledgements
Statement of financial support: Funding for this study was provided by the Endocrine Fellows Foundation Marilyn Fishman Grant for Diabetes Research, the Lawson Wilkins Pediatric Endocrine Society Clinical Scholars Award, the University of Oklahoma Health Sciences Center Department of Pediatric Diabetes and Endocrinology and NIH Grant Number P20 RR 024215 from the COBRE Program of the National Center for Research Resources
References
- 1.Dabelea D, Bell RA, D’Agostino RB, Jr., et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 2.Kempf K, Rathmann W, Herder C. Impaired glucose regulation and type 2 diabetes in children and adolescents. Diabetes Metab Res Rev. 2008;24(6):427–37. doi: 10.1002/dmrr.869. [DOI] [PubMed] [Google Scholar]
- 3.Skilton MR, Celermajer DS. Endothelial dysfunction and arterial abnormalities in childhood obesity. Int J Obes. 2006;30(7):1041–9. doi: 10.1038/sj.ijo.0803397. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 5.Cameron JD, Cruickshank JK. Glucose, insulin, diabetes and mechanisms of arterial dysfunction. Clin Exp Pharmacol Physiol. 2007;34(7):677–82. doi: 10.1111/j.1440-1681.2007.04659.x. [DOI] [PubMed] [Google Scholar]
- 6.Woodman RJ, Watts GF. Measurement and application of arterial stiffness in clinical research: focus on new methodologies and diabetes mellitus. Med Sci Monit. 2003;9(5):RA81–9. [PubMed] [Google Scholar]
- 7.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity (Silver Spring) 2012;20(1):165–71. doi: 10.1038/oby.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liese AD, D’Agostino RB, Jr., Hamman RF, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510–8. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 9.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Sex differences in vascular compliance in normal-weight but not obese boys and girls: the effect of body composition. Int J Pediatr. 2012;2012:607895. doi: 10.1155/2012/607895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes A Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142(2):191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers LJ, Copeland KC, Hester CN, Fields DA, Gardner AW. Paradoxical increase in arterial compliance in obese pubertal children. Angiology. 2011;62(7):565–70. doi: 10.1177/0003319711399117. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Cooney MT, Cooney HC, Dudina A, Graham IM. Total cardiovascular disease risk assessment: a review. Curr Opin Cardiol. 2011;26(5):429–37. doi: 10.1097/HCO.0b013e3283499f06. [DOI] [PubMed] [Google Scholar]
- 15.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42(4):468–73. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 16.Sakuragi S, Abhayaratna K, Gravenmaker KJ, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53(4):611–6. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 17.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–4. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 18.Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation. 2009;119(22):2913–9. doi: 10.1161/CIRCULATIONAHA.108.830380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging. 2008;28:287–93. doi: 10.1111/j.1475-097X.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 20.Metzig AM, Schwarzenberg SJ, Fox CK, Deering MM, Nathan BM, Kelly AS. Postprandial endothelial function, inflammation, and oxidative stress in obese children and adolescents. Obesity (Silver Spring) 2011;19(6):1279–83. doi: 10.1038/oby.2010.318. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Park KW, Kim YS, et al. Effects of acute hyperglycemia on endothelium-dependent vasodilation in patients with diabetes mellitus or impaired glucose metabolism. Endothelium. 2003;10(2):65–70. doi: 10.1080/10623320303362. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 23.Selamet Tierney ES, Newburger JW, Gauvreau K, et al. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr. 2009;154(6):901–5. doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Ripoll E, Sillau AH, Banchero N. Changes in the capillarity of skeletal muscle in the growing rat. Pflugers Arch. 1979;380(2):153–8. doi: 10.1007/BF00582151. [DOI] [PubMed] [Google Scholar]
- 25.Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005;28(5):1219–21. doi: 10.2337/diacare.28.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DH, Neutel JM. Change in arterial compliance as an early manifestation of hypertension. J Cardiovasc Risk. 1997;4(4):267–70. [PubMed] [Google Scholar]
- 27.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86(1):228–34. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner AW, Parker DE. Association between arterial compliance and age in participants 9 to 77 years old. Angiology. 2010;61(1):37–41. doi: 10.1177/0003319709339588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey CJ. Metformin: effects on micro and macrovascular complications in type 2 diabetes. Cardiovasc Drugs Ther. 2008;22(3):215–24. doi: 10.1007/s10557-008-6092-0. [DOI] [PubMed] [Google Scholar]
- 30.de Aguiar LG, Bahia LR, Villela N, et al. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care. 2006;29(5):1083–9. doi: 10.2337/diacare.2951083. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal N, Rice SP, Bolusani H, et al. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95(2):722–30. doi: 10.1210/jc.2009-1985. [DOI] [PubMed] [Google Scholar]
- 32.Gardner AW, Parker DE. Arterial elasticity in American Indian and Caucasian children, adolescents, and young adults. Vasc Med. 2011;16(4):275–83. doi: 10.1177/1358863X11415569. [DOI] [PMC free article] [PubMed] [Google Scholar]