Abstract
Necrosis and apoptosis are well established as two primary cell death pathways. Mixed neuroglial cultures are commonly used to study cell death mechanisms in neural cells. However, the ages of these cultures vary across studies and little attention has been paid to how cell death processes may change as the cultures mature. To clarify whether neuroglial culture age affects cell death mechanisms, we treated 1- and 3-week-old neuroglial cultures with either the excitotoxic stimulus, N-methyl-d-aspartate (NMDA), or with the oxidative stressor, hydrogen peroxide (H2O2). Although NMDA is known to be toxic only in cultures that are at least 2 weeks old, H2O2 is toxic in cultures of all ages. Here, we confirm that, in 1-week-old neuroglial cultures, NMDA does not induce toxicity, whereas H2O2 induces both calpain-mediated and caspase-mediated neuronal death. In 3-week-old cultures, both NMDA and H2O2 trigger calpain-mediated, but not caspase-mediated, neuronal death. Further, we observed a decrease in caspase-3 levels and an increase in calpain levels in untreated neuroglial cultures as they aged. The findings presented here show that neuronal cell death mechanisms vary with culture age and highlight the necessity of considering culture age when interpreting neural cell culture data.
Keywords: calpain, caspase, neuronal death
Introduction
Neurons undergo cell death during both development and disease. Classically, cell death falls under two major categories. Apoptosis, considered a programmed cell death, is classified by specific cellular morphological changes such as membrane blebbing and nuclear fragmentation [1]. The second major form of cell death is necrosis, classically defined as a passive and unregulated event [1]. However, some reports have suggested that necrosis can also occur as part of a coordinated cell death program comparable with apoptosis and is not an uncontrolled event [1,2].
Caspases, a family of aspartate-specific cysteine proteases, are activated through proteolytic cleavage and are well characterized for their role in apoptosis [3]. Caspases fall into one of two groups: initiator caspases (8, 9, and 10) and their downstream targets, executioner caspases (3, 6, and 7). Activation of the initiator caspases serves to initiate apoptosis and can be triggered either extracellularly through stimulation of the Fas receptor and tumor necrosis factor receptor or intracellularly through the release of cytochrome c from mitochondria [3]. The initiator caspases function to activate caspase-3 or another executioner caspase, which then executes apoptosis [3].
Calpain is a ubiquitously expressed Ca2+-dependent neutral protease. Although apoptosis is classically considered to be under the purview of the caspase family, calpain is now believed to play a role in apoptosis under certain conditions [4]. Calpain is also activated in neurological diseases in instances of both necrotic and apoptotic cell death [5,6]. Calpain is maintained as an inactive proenzyme until a spike in cytosolic free Ca2+ concentration triggers its activation. Cleavage targets of activated calpain comprise many enzymatic, signaling, and cytoskeletal proteins, including the neurofilament proteins, tau and tubulin [7]. Importantly, calpain activity contributes toward neuronal death in multiple neuropathological conditions, such as spinal cord injury [7], cerebral ischemia [8], and Alzheimer disease [9].
Attempts at uncovering the relative roles of caspases and calpains in neuronal death have been complicated by the postmitotic nature of neurons. Unlike cycling cells, cultured neurons differentiate and mature as they age. Indeed, it is well documented that the expressions of certain proteins change as neuronal cultures age in vitro. For example, N-methyl-d-aspartate (NMDA) glutamate receptor expression is absent until approximately 14 days in vitro (DIV) and its subunit composition changes between 14 and 21 DIV [10]. In previous studies of neuronal death, culture age has varied widely and the contributions of these age variations were not considered. Here, we examine the involvement of caspases and calpains in 1- and 3-week-old neuroglial cultures responding to two different toxicities: NMDA, a toxicity known to depend on culture age, and hydrogen peroxide (H2O2), which is toxic to neuronal cultures irrespective of age [11].
Materials and methods
Preparation of primary neuronal cultures
Primary rat neuroglial cortical cultures were prepared from embryonic day 17 Sprague–Dawley rat fetuses as described previously [12]. Cells were plated in dishes precoated with poly-l-lysine (Peptides International, Louisville, Kentucky, USA) at a density of 6 × 106 cells/100 mm dish or 4 × 104 cells/well in 96-well plates and were maintained at 37°C, 5% CO2 in neurobasal media (Invitrogen, Grand Island, New York, USA) with B27 supplement (Invitrogen). Neurons make up 90% of the total cells under these culture conditions [13].
MAP2 cell-based ELISA assay
Primary rat cortical neuroglial cultures were plated in 96-well plates. Neuronal death and damage were assessed using a microtubule-associate protein 2 (MAP2) cell-based enzyme-linked immunosorbent (ELISA) assay, as described previously [13]. The reliability of the ELISA as an indicator of cell death was verified by traditional hand counting of MAP2-positive cells [13].
Western blotting of primary cell cultures
Whole-cell extracts were prepared from primary rat cortical cultures and were subjected to western blotting. Antibody dilutions are as follows: calpain-cleaved spectrin, 1 : 15 000 (gift from David Lynch, University of Pennsylvania, Philadelphia, Pennsylvania, USA); μ-calpain, 1 : 500 (#2556; Cell Signaling Technologies, Danvers, Massachusetts, USA), caspase-3, 1 : 500 (#06735; Millipore, Billerica, Massachusetts, USA); caspase-9, 1 : 500 (#04443; Millipore); caspase-8, 1 : 250 (AB1879; Millipore); and cleaved caspase-3, 1 : 250 (#9661; Cell Signaling Technologies).
Statistical analysis
Differences between the test groups were examined by one-way analysis of variance, with a threshold for significance of P value less than 0.05.
Results
Calpain, not caspase, mediates NMDA-induced neurotoxicity in 3-week-old cultures
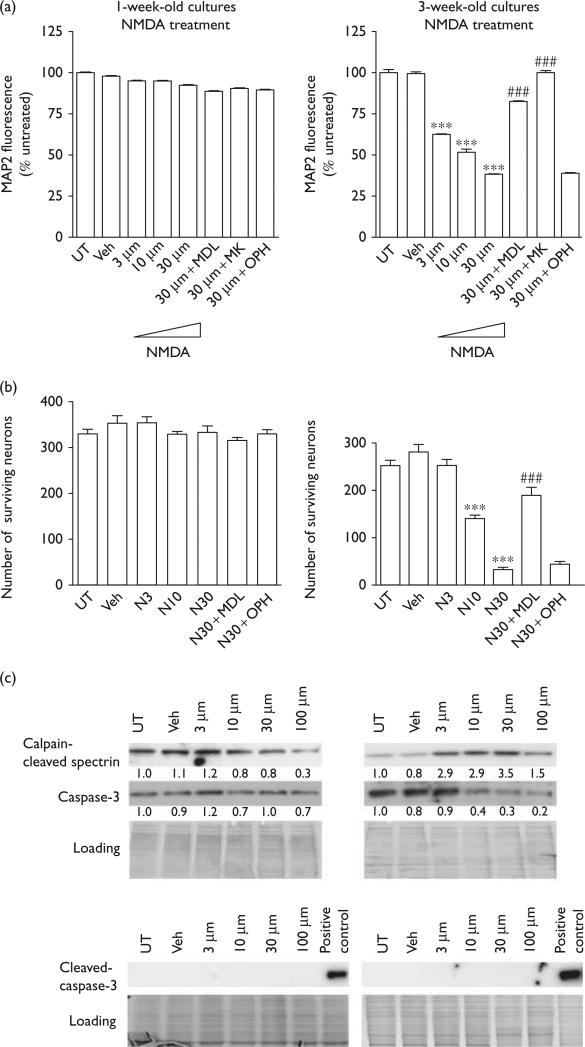
To elucidate age-related differences in neuronal death processes, we treated 1- and 3-week-old mixed rat cortical cultures with varying doses of NMDA, the chemical agonist of the glutamate NMDA receptor. First, we examined cultures for neuronal damage and death using a cell-based (CB)-ELISA to detect the neuronal marker protein, MAP2. We found that, as expected, 1-week-old cultures showed no loss of MAP2 fluorescence when treated with NMDA, whereas 3-week-old cultures showed a significant decrease in MAP2 levels at all NMDA dosage levels (Fig. 1a). This decrease in MAP2 fluorescence was rescued by pretreatment with the calpain inhibitor MDL28170 (MDL), but not the pancaspase inhibitor qVD-OPH (OPH). As a positive control for protection, we also pretreated control cultures with the NMDA receptor antagonist, MK801, which completely blocks NMDA-induced toxicity. The result was confirmed on counting the number of MAP2-positive cells (Fig. 1b).
Fig. 1.
Calpain mediates NMDA-induced neurotoxicity in 3-week-old cultures. (a) MAP2 cell-based ELISA was performed in 1- or 3-week-old rat cortical cultures in response to 3, 10, and 30 μM NMDA. NMDA had no effect on the MAP2 fluorescence signal strength in 1-week-old rat cortical cultures. In 3-week-old rat cortical cultures, NMDA induced a dose-dependent decrease in the fluorescence signal strength in MAP2 cell-based ELISA, and the decrease is blocked by the calpain inhibitor, MDL28170, and by the NMDA receptor antagonist, MK801, but is not affected by the caspase inhibitor, OPH (***P < 0.001, compared with veh; ###P < 0.001, compared with cells treated with 30 μM NMDA). (b) Neuronal survival as determined by counts of MAP2-positive cells in 1- or 3-week-old rat cortical cultures in response to 3, 10, and 30 μM NMDA. Data represent the average ±SD counts of multiple wells (n = 6; ***P < 0.001, compared with veh; ###P < 0.001, compared with cells treated with 30 μM NMDA). (c) One- and 3-week-old rat cortical cultures were treated with 3, 10, 30, or 100 μM NMDA, and the expression levels of calpain-cleaved spectrin, caspase-3, and cleaved caspase-3 were detected (n = 3) by western blot. The fold change in protein expression compared with that of the control (1.0) is specified beneath the respective bands in each blot. Each sample was subjected to SDS-PAGE and stained with coomassie blue to show equal loading of samples. ELISA, enzyme-linked immunosorbent; MAP2, microtubule-associate protein 2; NMDA, N-methyl-d-aspartate; PAGE, polyacrylamide gel electrophoresis; veh, vehicle.
To verify our pharmacological inhibitor data, we examined the levels of calpain-cleaved spectrin and executioner caspase-3 in our treated cultures to assess the activation of each protease. As expected, we observed no increase in cleaved caspase-3 levels or in calpain-cleaved spectrin levels in 1-week-old cultures following treatments with 3, 10, 30, or 100 μM NMDA (Fig. 1c). In 3-week-old cultures, NMDA treatment induced an increase in calpain-cleaved spectrin at all treatment doses. However, there was no increase in cleaved caspase-3 levels at any dose of NMDA treatment. Taken together, these data suggest that calpain, rather than caspase-3, mediates NMDA-induced neuronal death in 3-week-old cultures.
Both caspase and calpain activities contribute toward H2O2-induced toxicity in 1-week-old cultures, whereas toxicity in 3-week-old cultures is mediated through calpain alone
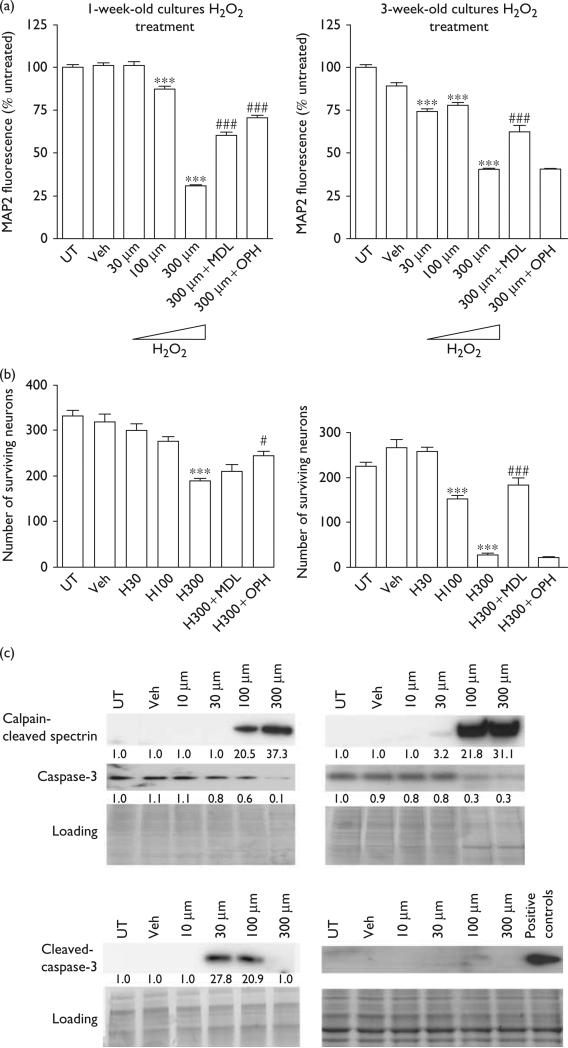
We next treated 1- and 3-week-old cultures with H2O2 to examine neuronal death processes following an insult known to induce toxicity in neuronal cultures irrespective of age [11]. Specifically, we treated cortical neuroglial cultures with 30, 100, or 300 μM H2O2 and examined neuronal death and damage using the MAP2 CB-ELISA. In 1-week-old cultures, we observed significant MAP2 loss following treatment with either 100 or 300 μM H2O2; in 3-week-old cultures, all three doses of H2O2 induced a significant reduction in MAP2 (Fig. 2a). The decreased MAP2 fluorescence was rescued by pretreatment with MDL and OPH in 1-week cultures, but only by MDL in 3-week cultures. The result was confirmed on counting the number of MAP2-positive cells (Fig. 2b).
Fig. 2.
Both caspase and calpain activity contribute toward hydrogen peroxide (H2O2)-induced toxicity in 1-week-old cultures, whereas toxicity in 3-week-old cultures is mediated through calpain. (a) MAP2 cell-based ELISA was performed in 1- or 3-week-old rat cortical cultures in response to H2O2. In 1-week-old rat cortical cultures, H2O2 induced a dose-dependent decrease in the fluorescence signal strength in MAP2 cell-based ELISA, and the decrease was blocked by the calpain inhibitor, MDL28170, and the caspase inhibitor, OPH. In 3-week-old rat cortical cultures, H2O2 induced a dose-dependent decrease in the fluorescence signal strength in MAP2 cell-based ELISA, and the decrease was blocked by MDL28170, but not by OPH (***P < 0.001, compared with veh; ###P < 0.001, compared with cells treated with 300 μM H2O2). (b) Neuronal survival as determined by counts of MAP2-positive cells in 1- or 3-week-old rat cortical cultures in response to H2O2. Data represent the average ±SD counts of multiple wells (n = 6; ***P < 0.001, compared with veh; #P < 0.05, ###P < 0.001, compared with cells treated with 300 μM H2O2). (c) One- or 3-week-old rat cortical cultures were treated with different concentrations of H2O2 and the expression levels of calpain-cleaved spectrin, caspase-3, and cleaved caspase-3 were determined (n = 3). The numerical values specified beneath the respective bands of western blots represent the fold change in protein expression as compared with that of the control (1.0). Each sample was subjected to SDS-PAGE and stained with coomassie blue to show equal loading of samples. ELISA, enzyme-linked immunosorbent; MAP2, microtubule-associate protein 2; NMDA, N-methyl-d-aspartate; PAGE, polyacrylamide gel electrophoresis; veh, vehicle.
When we immunoblotted lysates from H2O2-treated cultures for markers of protease activation, we found high levels of calpain-cleaved spectrin in both 1- and 3-week-old cultures treated with 100 and 300 μM H2O2 and moderate levels of calpain-cleaved spectrin in 3-week-old cultures treated with 30 μM H2O2 (Fig. 2c). In support of our pharmacological inhibitor data, we observed increased levels of cleaved caspase-3 in 1-week-old cultures treated with 100 μM H2O2, but no increases in cleaved caspase-3 in 3-week-old cultures subjected to any doses of H2O2 (Fig. 2c). Interestingly, we observed increased levels of cleaved caspase-3 in 1-week-old cultures treated with 30 μM H2O2, even though we had not observed MAP2 loss in these cultures. Further, we observed no increases in cleaved caspase-3 in 1-week-old cultures treated with 300 μM H2O2, even though OPH protected against MAP2 loss induced at this dose. These findings suggest that in 1-week-old mixed cortical cultures, caspase-3 plays a role in H2O2-induced toxicity, particularly at lower doses, but does not contribute toward H2O2-mediated cell death in 3-week-old cultures. In contrast, calpain appears to mediate H2O2-induced cell death in both 1- and 3-week-old cultures.
Calpain expression increases whereas caspase-3 expression decreases as neuroglial cultures age
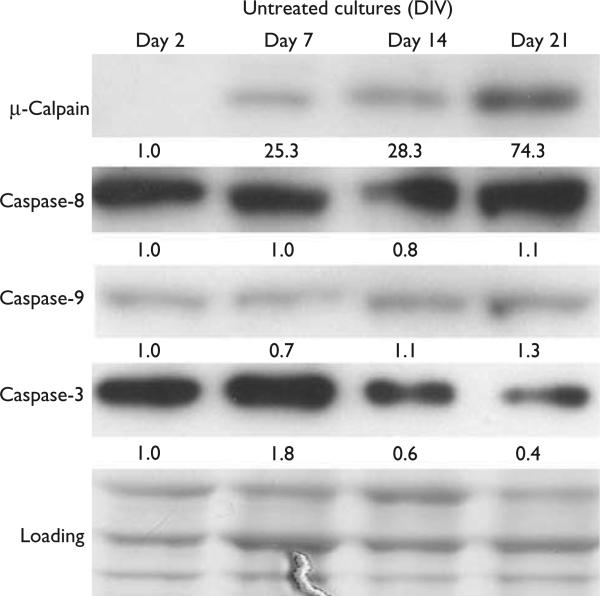
We next sought to determine whether 1- and 3-week-old cultures showed any difference in the expression levels of calpains and caspases to determine whether our observed differences in protease responses to toxicities might occur solely at the cleavage/activation level or whether differences in expression levels might also contribute. Thus, through immunoblot, we examined the levels of μ-calpain, caspase-3, and of the initiator caspases, caspase-8 and caspase-9 in 2-, 7-, 14-, and 21-day-old mixed cortical cultures. We found that the levels of calpain increased with age and that full-length procaspase-3 decreased (Fig. 3). We observed no change in the levels of full-length caspase-8 or caspase-9.
Fig. 3.
Representative blot showing age-dependent expressions of calpain and of full-length caspases: 3, 8, and 9. Expression levels of calpain, caspase-3, caspase-8, and caspase-9 were detected in days 2, 7, 14, and 21 rat cortical cultures (n = 3). The numerical values specified beneath the respective bands of western blots represent the fold change in protein expression as compared with that of the control (1.0). Each sample was subjected to SDS-PAGE and stained with coomassie blue to show equal loading of samples. DIV, days in vitro; PAGE, polyacrylamide gel electrophoresis.
Discussion
In the present study, we highlight how the age of primary neuronal cultures influences the respective contributions of calpain and caspase to neuronal death. We investigated the role of calpain and caspase activation in two toxicity models, NMDA and H2O2, using rat primary cortical neurons aged for 1 and 3 weeks in culture. We found that 1-week-old cultures treated with NMDA showed no significant calpain-mediated or caspase-mediated toxicity, whereas NMDA treatment of 3-week-old neuronal cultures triggered calpain-dependent neurotoxicity. H2O2 treatment of 1-week-old cultures produced both calpain and caspase-3 activation as well as calpain-mediated and caspase-mediated neurotoxicity. In contrast, H2O2 treatment of neuronal cultures at 3 weeks of age primarily produced calpain-mediated toxicity. To examine the possible mechanisms for these age-dependent responses, we measured the expression of μ-calpain and caspase-3, caspase-8, and caspase-9 in primary neuronal cultures over time. We found that μ-calpain is expressed at higher levels as cultures age. In contrast, caspase-3 expression shows a decrease in protein levels as the cultures age. Together, our findings suggest that caspase-triggered death may play a major role in younger cultures, but that a calpain-mediated death pathway predominates as cultured neurons mature.
Caspase activation has been reported to play an instrumental role in neurotoxicity caused by Aβ peptides, glutamate, and 6-hydroxydopamine, as well as that caused by serum withdrawal [14–17]. However, the primary neuronal cultures used in each of these studies have been aged for a week or less in vitro. The presence of caspase-dependent neuronal death in 1-week-old cultures and its absence in 3-week-old cultures indicate that caspase involvement in neuronal death is greatest when the cells are immature and highlight the importance of culture age in studies examining death mechanisms in neurons, such as those mentioned above.
Further, although studies had suggested previously that an excitotoxic challenge can trigger caspase-mediated apoptotosis in cultured neurons [5], a recent growing body of evidence has indicated that calpain pathways and not caspase pathways are predominantly responsible for excitotoxic death [10,13,18]. Our observation that NMDA-mediated calpain activation and excitotoxic death only manifest in older neuronal cultures has been similarly supported by several studies highlighting the developmental regulation of NMDA receptor expression in culture. These studies show (a) that NMDA-induced toxicity occurs selectively in mature neuronal cultures and that it can be blocked by the application of the NMDA receptor antagonist MK801, which blocks open channels on the cell surface, and (b) that excitotoxicity-sensitive NR2A and NR2B subunits are highly expressed between 14 and 21 DIV, but are almost undetectable at 7 DIV [10,19]. Consequently, the presence of NR2-containing NMDA receptor subtypes leads to an increased excitotoxic death in older cultures.
The increase in μ-calpain protein expression along with the decrease in caspase-3 expression as our cultures age may partially explain the absence of caspase-dependent neurotoxicity following toxic treatments of our 3-week-old primary neuronal cultures. Interestingly, these converse patterns of calpain and caspase-3 protein expression have been reported previously by others using primary rat cortical cultures aged between 5 and 20 DIV [20]. A number of in-vivo studies have also examined the temporal expression of these proteases during rat brain development. They similarly found that procaspase-3 protein and mRNA levels decrease markedly in the cortex and in brain homogenates during central nervous system maturation [21–23]. These reports are accompanied by functional studies reporting a progressively reduced ability to activate caspases in neurons as they mature in culture and in vivo [21,23]. In contrast, upregulation of calpain mRNA and protein levels has been observed repeatedly as brains age and undergo neurodegeneration, with abnormally high levels observed in the neocortex of Alzheimer's Disease patients and substantia nigra and locus coeruleus of Parkinson's Disease patients [9,22,24].
Although 1-week-old cultures treated with H2O2 showed an accumulation of cleaved caspase-3, 3-week-old cultures treated with either NMDA or H2O2 showed no caspase-3 activation. Procaspase-3 levels were nonetheless detectable in our 3-week cultures and were noticeably reduced by higher concentrations of NMDA and H2O2. Interestingly, this reduction was typically accompanied by calpain activation observed by increased calpain-cleaved spectrin, suggesting that 3-week-old neuronal cultures may utilize calpain-mediated proteolysis or degradation of caspase-3 as a mechanism to silence caspase signaling. Indeed, cross-talk between the calpain and the caspase pathways has been reported in several instances, with calpain typically acting upstream of caspases, cleaving caspase-3 as well as caspase-7, caspase-8, and caspase-9 at noncanonical sites, rendering them inactive [25].
Our results strongly suggest that the caspase-dependent death pathway is active in developing neuronal cultures, but is replaced by calpain as the predominant death pathway when cultured neurons mature. We suggest two potential mechanisms that underlie this change: (a) calpain-mediated inactivation of caspases (Figs 1 and 2 and (b) downregulation of procaspase expression as neurons age (Fig. 3). Both mechanisms may be at play as neurons age. Given our findings, in-vitro studies of caspase involvement in neuronal death should be interpreted with careful consideration given to the age of culture.
Acknowledgements
The authors thank Dr David Lynch for generously providing us with the calpain-cleaved spectrin antibody (AB38). They also thank Dr Marc A. Dichter and Margaret A. Maronski for providing us with primary cultures.
This work was supported by National Institutes of Health Grants NS41202, NS056885, and by NRSA F31 fellowship awards NS074942 and NS071787.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Wyllie AH. Apoptosis: an overview. Br Med Bull. 1997;53:451–465. doi: 10.1093/oxfordjournals.bmb.a011623. [DOI] [PubMed] [Google Scholar]
- 4.O'Donovan CN, Tobin D, Cotter TG. Prion protein fragment PrP-(106-126) induces apoptosis via mitochondrial disruption in human neuronal SH-SY5Y cells. J Biol Chem. 2001;276:43516–43523. doi: 10.1074/jbc.M103894200. [DOI] [PubMed] [Google Scholar]
- 5.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heron A, Pollard H, Dessi F, Moreau J, Lasbennes F, Ben-Ari Y, et al. Regional variability in DNA fragmentation after global ischemia evidenced by combined histological and gel electrophoresis observations in the rat brain. J Neurochem. 1993;61:1973–1976. doi: 10.1111/j.1471-4159.1993.tb09843.x. [DOI] [PubMed] [Google Scholar]
- 7.Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- 8.Rami A. Ischemic neuronal death in the rat hippocampus: the calpain–calpastatin–caspase hypothesis. Neurobiol Dis. 2003;13:75–88. doi: 10.1016/s0969-9961(03)00018-4. [DOI] [PubMed] [Google Scholar]
- 9.Nixon RA, Saito KI, Grynspan F, Griffin WR, Katayama S, Honda T, et al. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer's disease. Ann N Y Acad Sci. 1994;747:77–91. doi: 10.1111/j.1749-6632.1994.tb44402.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci. 2006;26:981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamichi N, Kambe Y, Oikawa H, Ogura M, Takano K, Tamaki K, et al. Protection by exogenous pyruvate through a mechanism related to monocarboxylate transporters against cell death induced by hydrogen peroxide in cultured rat cortical neurons. J Neurochem. 2005;93:84–93. doi: 10.1111/j.1471-4159.2005.02999.x. [DOI] [PubMed] [Google Scholar]
- 12.Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 13.White MG, Wang Y, Akay C, Lindl KA, Kolson DL, Jordan-Sciutto KL. Parallel high throughput neuronal toxicity assays demonstrate uncoupling between loss of mitochondrial membrane potential and neuronal damage in a model of HIV-induced neurodegeneration. Neurosci Res. 2011;70:220–229. doi: 10.1016/j.neures.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Bales KR, Dodel RC, Hamilton-Byrd E, Horn JW, Czilli DL, et al. Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons. Proc Natl Acad Sci USA. 1997;94:11657–11662. doi: 10.1073/pnas.94.21.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovanni A, Keramaris E, Morris EJ, Hou ST, O'Hare M, Dyson N, et al. E2F1 mediates death of B-amyloid-treated cortical neurons in a manner independent of p53 and dependent on Bax and caspase 3. J Biol Chem. 2000;275:11553–11560. doi: 10.1074/jbc.275.16.11553. [DOI] [PubMed] [Google Scholar]
- 16.Hou ST, Cowan E, Dostanic S, Rasquinha I, Comas T, Morley P, et al. Increased expression of the transcription factor E2F1 during dopamine-evoked, caspase-3-mediated apoptosis in rat cortical neurons. Neurosci Lett. 2001;306:153–156. doi: 10.1016/s0304-3940(01)01909-7. [DOI] [PubMed] [Google Scholar]
- 17.Marks N, Berg MJ, Guidotti A, Saito M. Activation of caspase-3 and apoptosis in cerebellar granule cells. J Neurosci Res. 1998;52:334–341. doi: 10.1002/(SICI)1097-4547(19980501)52:3<334::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Nimmrich V, Reymann KG, Strassburger M, Schoder UH, Gross G, Hahn A, et al. Inhibition of calpain prevents NMDA-induced cell death and beta-amyloid-induced synaptic dysfunction in hippocampal slice cultures. Br J Pharmacol. 2010;159:1523–1531. doi: 10.1111/j.1476-5381.2010.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eugenin EA, King JE, Hazleton JE, Major EO, Bennett MV, Zukin RS, et al. Differences in NMDA receptor expression during human development determine the response of neurons to HIV-tat-mediated neurotoxicity. Neurotox Res. 2011;19:138–148. doi: 10.1007/s12640-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MJ, Oh SJ, Park SH, Kang HJ, Won MH, Kang TC, et al. Neuronal loss in primary long-term cortical culture involves neurodegeneration-like cell death via calpain and p35 processing, but not developmental apoptosis or aging. Exp Mol Med. 2007;39:14–26. doi: 10.1038/emm.2007.3. [DOI] [PubMed] [Google Scholar]
- 21.Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Bondada V, Joshi A, Geddes JW. Calpain 1 and Calpastatin expression is developmentally regulated in rat brain. Exp Neurol. 2009;220:316–319. doi: 10.1016/j.expneurol.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yakovlev AG, Ota K, Wang G, Movsesyan V, Bao WL, Yoshihara K, et al. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21:7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouatt-Prigent A, Karlsson JO, Agid Y, Hirsch EC. Increased M-calpain expression in the mesencephalon of patients with Parkinson's disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience. 1996;73:979–987. doi: 10.1016/0306-4522(96)00100-5. [DOI] [PubMed] [Google Scholar]
- 25.Chua BT, Guo K, Li P. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]