Abstract
Background
Mass spectrometric assays have the potential to replace protein immunoassays in basic science, clinical research, and clinical care. Previous studies have demonstrated the utility of assays using multiple-reaction monitoring mass spectrometry (MRM-MS) for the quantification of proteins in biological samples and many examples of the accuracy of these approaches to quantify spiked analytes have been reported. However, a direct comparison of multiplexed assays using liquid chromatography-tandem mass spectrometry with established immunoassays to measure endogenous proteins has not been reported.
Methods
We purified the HDL from the plasma of 30 human subjects enrolled in a clinical nutrition research study and used label-free shotgun proteomics approaches to analyze each sample. We then developed two different 6-plex assays that used isotope dilution MRM-MS: one assay used stable isotope labeled peptides and the other used stable isotope labeled apolipoprotein A-I (apoA-I), the most abundant protein in HDL, as internal standards to control for matrix effects and mass spectrometer performance. The shotgun and MRM-MS assays were then compared with commercially available immunoassays for each of the six analytes.
Results
Quantification by shotgun proteomics approaches correlated poorly with the six protein immunoassays. However, the MRM-MS approaches that used internal standard peptide or a single internal standard protein correlated well. In addition, MRM-MS approaches had good repeatability (<10% CV) and linearity.
Conclusions
Multiplexed MRM-MS assays correlate well with immunochemical measurements and have acceptable operating characteristics in complex samples. Our results support the proposal that MRM-MS could be used to replace immunoassays in a variety of settings.
Keywords: Mass spectrometry, multiple reaction monitoring, endogenous, proteins, high density lipoprotein, targeted proteomics
Multiplexed accurate quantification of proteins is becoming increasingly important in both basic biology and in clinical biomarker studies. Multiplexing immunoassays can be problematic (1) and migration of clinical protein assays from immunoassay platforms to mass spectrometric platforms could result in great benefits to patients and laboratories.(2) It is currently unknown how well multiplexed quantification of endogenous proteins by mass spectrometry agrees with traditional immunoassays, the current standard of care, but simultaneous measurement of two apolipoproteins in plasma using multiple reaction monitoring-mass spectrometry (MRM-MS) has shown great promise.(3)
Until recently, proteomic studies have focused primarily on the global identification of as many proteins as possible. This paradigm has shifted and the precise, simultaneous quantification of relative protein abundance of many proteins has become an important goal for many applications. Targeted proteomics experiments based on MRM-MS, a method that has been used clinically for many years for small molecule quantification, may provide precise relative quantification of known proteins in complex mixtures. In targeted proteomics peptides produced by protease digestion—typically with trypsin—serve as surrogate markers of protein abundance. MRM-MS assays offer many advantages over traditional immunoassays used for protein quantification in biological samples; in particular MRM-based methods can be rapidly developed and validated. Furthermore, such assays are readily multiplexed for quantification of many proteins (>50) in a single analysis over wide range of relative concentrations without cross-reacting interferences often found in multiplexed immunoassays.(4) Combined with isotope dilution using stable isotope-labeled peptides as internal standards (ISpep), MRM has been established as the most promising approach to precise relative protein quantification.(5-8)
A recent multi-laboratory study has demonstrated good reproducibility of MRM-based assays across several laboratories.(9) However, this study also underscored the importance of the reproducibility of the enzymatic digestion of proteins, a critical step in MRM-based assays. More specifically, the CV of measurements that included the digestion step were more than 2-fold higher than the CV of measurements using pre-digested samples.(10) Importantly, digestion reproducibility cannot be corrected for by using ISpep. For quantification of a specific endogenous protein, this limitation can be largely overcome when a stable-isotope labeled protein analog (ISprot) is included as the internal standard.(11 up to 25-fold differences between the two approaches.(12) We therefore aimed to compare MRM-MS with more clinically relevant immunoassays.
As an example of a complex mixture of proteins, we chose HDL because it is an important plasma protein-lipid complex directly involved in cardiovascular disease. Elevated plasma HDL cholesterol is one of the only known negative risk factors for cardiovascular disease. HDL may mitigate atherosclerosis by multiple mechanisms including cholesterol efflux and antioxidant and anti-inflammatory properties, and its in vitro activity correlates with atherosclerosis.(13) Although a much simpler proteome compared to plasma, over 80 proteins have been identified in HDL with concentrations spanning four orders of magnitude,(14) HDL poses a major analytical challenge due to the very high content of lipids (50% by weight), especially phospholipids (30%), which are well-recognized ionization suppressants during atmospheric pressure ionization.(15) Importantly, we have recently shown that HDL from subjects with cardiovascular disease carries a unique ensemble of proteins which is modulated by lipid-lowering therapy.(16, 17) Precise quantification of the concentration of proteins in HDL could potentially serve as a clinical diagnostic tool and as a test of therapeutic efficacy.
We isolated HDL from the plasma of 30 normal subjects that were collected during an IRB-approved clinical research study(18) and performed shotgun proteomics on a high resolution mass spectrometer [see Supplemental Material for an overview (Supplemental Fig. 1A) and complete description of methods] to identify and quantify proteins associated with HDL. Based on these results, we selected six proteins for which commercial immunoassays were readily available and whose range of relative concentrations across the study population (as assessed by shotgun proteomics and spectral counting) was at least 50% of the mean concentration (Supplemental Fig. 1B). The selected set of proteins also had wide range of molecular weights (11-550 kDa; Supplemental Fig. 1C). For each protein, two peptides were then selected for the targeted analysis based on the mean spectral count of each peptide across the study population and the number of observations in the PeptideAtlas database.(19) Two peptide precursor ion m/z-to-fragment ion m/z pairs (known as transitions) were then chosen for each peptide based on fragment intensity in the tandem mass spectra from the shotgun experiment (Supplemental Table 1). Stable-isotopically labeled peptides with a C-terminal labeled arginine or lysine were synthesized for each of the six proteins (ISpep). In parallel we also generated recombinant 15N-labeled apoA-I (ISprot).
Much like albumin in serum, the major constitutive protein in HDL, apolipoprotein A-I (apoA-I), represents ~70% of the total protein content. We first evaluated the accuracy of different mass spectrometric approaches for quantification of apoA-I using as the comparative method a clinically-used nephelometric immunoassay that is calibrated against the WHO standard reference material (Siemens BN-II). We spiked two separate HDL digests with ISpep after trypsin digestion and/or ISprot before trypsin digestion and performed a targeted MRM analysis with ISprot (MRM-protein) and ISpep (MRM-peptide), respectively. In parallel we estimated the abundance of apoA-I from the shotgun analysis using spectral counting and extracted ion chromatogram peak areas (XIC) for two peptides.(20) To quantify relative concentrations of apoA-I across the study population we used following measures: MRM peak area (LC-MRM/MS assay with no internal standard), MRM-peptide ratio (LC-MRM/MS assay with ISpep), MRM-protein ratio (LC-MRM/MS assay with ISprot), normalized MRM-protein ratio (average of the MRM-protein ratio for two peptides in each protein), spectral counting, and XIC peak areas.
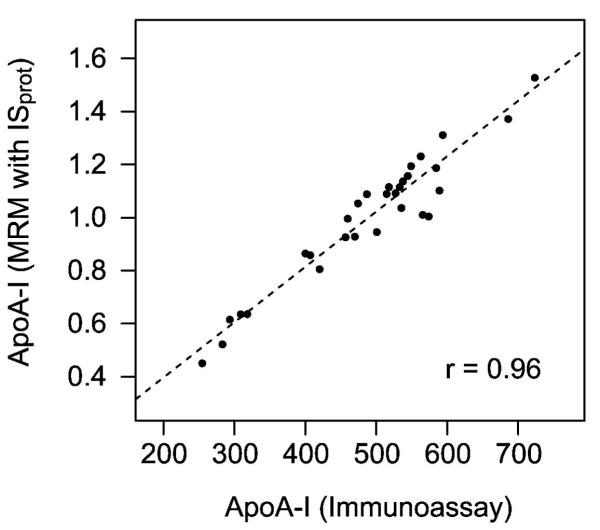
As expected, the MRM peak areas of peptides from the same protein correlated well with one another, as did XIC peak areas (Supplemental Fig. 2). Our results clearly demonstrate that normalization to ISpep internal standard peptides, MRM-peptide ratio, (Supplemental Fig. 2E-H) improves the accuracy of the targeted MRM method over the peak area alone (Supplemental Fig. 2A-D), which is likely due to correction of variable matrix effects across the population, injection volume variability, and deterioration of mass spectrometer performance over the time of analysis. The use of ISprot further improved the accuracy of the MRM-based method (Supplemental Fig. 2I-L), providing excellent correlation with the clinical immunoassay (r = 0.96, Fig. 1). Strikingly, spectral counting and XIC, which are commonly used as quantitative measures in shotgun proteomics experiments in basic science research, correlated poorly with the immunoassay (Supplemental Fig. 2M-P), demonstrating that these two label-free shotgun proteomics approaches yield only a rough semi-quantitative measure of relative protein abundance.(17) These initial results confirmed that the normalized MRM-protein ratio approach was the most accurate for the quantification of a single protein by MRM-based methods.(11)
Figure 1. Internal standard protein in MRM-MS assay for apoA-I in HDL.
The relative concentration of apoA-I in HDL measured in 30 samples by MRM-MS is compared with the concentration measured using a nephelometric immunoassay. The line of the equation of the Deming regression is denoted with a dashed line. The Pearson correlation coefficient (r) is shown.
To further test the analytical characteristics of the normalized MRM-protein ratio approach, we evaluated its repeatability and linearity as outlined in Supplemental Figure 1A. To test the repeatability of the LC-MRM/MS step alone we analyzed a single digestion of the same HDL 12 times over the course of 12 days interspersed between injections of other HDL samples. We also tested the overall repeatability of the assay by analyzing 5 replicate digestions of a single pooled HDL sample twice (day 1 and day 12). The normalized MRM-protein ratio approach demonstrated excellent repeatability (<2%CV) in both experiments (Table 1). To assess the linearity of the ISprot (normalized MRM-protein ratio) and ISpep (normalized MRM-peptide ratio) methods, we analyzed single digestions of a 9-point serial dilution of human HDL into mouse HDL. Both approaches demonstrated acceptable linearity over more than two-orders of magnitude with ISprot (r2 = 0.9996) and ISpep (r2 = 0.9936) (Supplemental Fig. 3).
Table 1. Performance characteristics of a multiplexed MRM-MS assay.
Isotope labeled apoA-I was used as the internal standard for all six proteins in the assay and correlation with immunoassay was determined along with linearity and imprecision.
| Imprecision |
|||||
|---|---|---|---|---|---|
| Correlation with Immunoassay (N=30) |
Linearity | LC-MS (%CV) |
Digestion (%CV) |
Overall (%CV) |
|
| A-I | 0.95 | 0.9996 | 1.15% | 0.25% | 1.18% |
| B | 0.61 | 0.9997 | 4.59% | 4.43% | 6.38% |
| C-II | 0.92 | 0.9948 | 8.38% | 7.99% | 11.58% |
| C-III | 0.88 | 0.9976 | 8.39% | 4.41% | 9.48% |
| E | 0.92 | 0.9980 | 6.03% | 4.93% | 7.79% |
| J | 0.79 | 0.9996 | 9.51% | 7.43% | 12.07% |
We then evaluated the performance of mass spectrometric methods for the measurement of five other HDL proteins. For each protein we synthesized stable isotope-labeled analogs of each peptide selected for the LC-MRM/MS assay (i.e., ISpep) (Supplemental Table 3) or used labeled apoA-I as internal standards (i.e., ISprot). Similar to apoA-I, the ISpep and ISprot approaches demonstrated good repeatability (<10%CV) and linearity for the other 5 proteins (Table 1, Supplemental Fig. 4). For each protein we also calculated the correlation of the relative concentration measured by each method with the concentration determined by an immunoassay. Remarkably, both approaches yielded very similar results for all 5 proteins (Table 1), with ISprot providing better overall correlations than ISpep. As we observed for apoA-I, the label-free shotgun proteomics quantitative measures (spectral counting and XIC) correlated poorly with the immunoassay data (Supplemental Figs. 5-9).
The overall aim of the present study was to determine whether multiplexed protein quantification using mass spectrometry can provide accurate, linear, and reproducible measurements of endogenous protein concentrations in complex human specimens. We found that the isotope dilution MRM-MS methods using either internal standard peptides or a single internal standard protein have operating characteristics and accuracy comparable to biochemical approaches. Our data strongly support the proposal that MRM-MS methods may provide accurate and reproducible quantitative data for basic and clinical studies and have great potential to be readily translated into clinical practice.
Supplementary Material
Acknowledgments
FUNDING SOURCES This work was supported in part by awards from the University of Washington (UW) Nutrition and Obesity Research Center (NIH P30DK035816; to TV and ANH), the American Heart Association (0830231N; to TV), and the UW Diabetes and Endocrinology Research Center (NIH P30DK017047; to ANH). Additional support was provided by the UW Clinical Mass Spectrometry Facility (to all), UW Proteomics Resource (UWPR95794, to all), National Institutes of Health (HL089504 to TV and HL77268 to MNO) and the New Investigator Award from Tobacco-Related Disease Research Program of California (#18KT-0021, to GC).
Abbreviations
- MRM-MS
multiple-reaction monitoring mass spectrometry
- ISpep
stable isotope labeled peptide internal standard
- IS
stable isotope labeled protein internal standard
- apoA-I
apolipoprotein A-I
- XIC
extracted ion chromatogram
REFERENCES
- 1.Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. J Immunol Methods. 2009;350:125–32. doi: 10.1016/j.jim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle AN, Wener MH. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods. 2009;347:3–11. doi: 10.1016/j.jim.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agger SA, Marney LC, Hoofnagle AN. Simultaneous quantification of apolipoprotein A-I and apolipoprotein B by liquid-chromatography-multiple-reaction-monitoring mass spectrometry. Clin Chem. 2010;56:1804–13. doi: 10.1373/clinchem.2010.152264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson NL, Anderson NG, Pearson TW, Borchers CH, Paulovich AG, Patterson SD, et al. A human proteome detection and quantitation project. Mol Cell Proteomics. 2009;8:883–6. doi: 10.1074/mcp.R800015-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5:573–88. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A. 2003;100:6940–5. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong SE, Mann M. Mass spectrometry-based proteomics turns quantitative. Nat Chem Biol. 2005;1:252–62. doi: 10.1038/nchembio736. [DOI] [PubMed] [Google Scholar]
- 8.Whiteaker JR, Zhao L, Anderson L, Paulovich AG. An automated and multiplexed method for high throughput peptide immunoaffinity enrichment and multiple reaction monitoring mass spectrometry-based quantification of protein biomarkers. Mol Cell Proteomics. 2010;9:184–96. doi: 10.1074/mcp.M900254-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27:633–41. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoofnagle AN. Quantitative clinical proteomics by liquid chromatography-tandem mass spectrometry: assessing the platform. Clin Chem. 2010;56:161–4. doi: 10.1373/clinchem.2009.134049. [DOI] [PubMed] [Google Scholar]
- 11.Brun V, Dupuis A, Adrait A, Marcellin M, Thomas D, Court M, et al. Isotope-labeled protein standards: toward absolute quantitative proteomics. Mol Cell Proteomics. 2007;6:2139–49. doi: 10.1074/mcp.M700163-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Picotti P, Bodenmiller B, Mueller LN, Domon B, Aebersold R. Full dynamic range proteome analysis of S. cerevisiae by targeted proteomics. Cell. 2009;138:795–806. doi: 10.1016/j.cell.2009.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoofnagle AN, Heinecke JW. Lipoproteomics: using mass spectrometry-based proteomics to explore the assembly, structure, and function of lipoproteins. J Lipid Res. 2009;50:1967–75. doi: 10.1194/jlr.R900015-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogeser M, Seger C. Pitfalls associated with the use of liquid chromatography-tandem mass spectrometry in the clinical laboratory. Clin Chem. 2010;56:1234–44. doi: 10.1373/clinchem.2009.138602. [DOI] [PubMed] [Google Scholar]
- 16.Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, et al. Combined Statin and Niacin Therapy Remodels the High-Density Lipoprotein Proteome. Circulation. 2008;118:1259–67. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–56. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knopp RH, Retzlaff B, Fish B, Walden C, Wallick S, Anderson M, et al. Effects of insulin resistance and obesity on lipoproteins and sensitivity to egg feeding. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:1437–43. doi: 10.1161/01.ATV.0000082461.77557.C7. [DOI] [PubMed] [Google Scholar]
- 19.Deutsch EW, Eng JK, Zhang H, King NL, Nesvizhskii AI, Lin B, et al. Human Plasma PeptideAtlas. Proteomics. 2005;5:3497–500. doi: 10.1002/pmic.200500160. [DOI] [PubMed] [Google Scholar]
- 20.Bondarenko PV, Chelius D, Shaler TA. Identification and relative quantitation of protein mixtures by enzymatic digestion followed by capillary reversed-phase liquid chromatography-tandem mass spectrometry. Anal Chem. 2002;74:4741–9. doi: 10.1021/ac0256991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.