Abstract
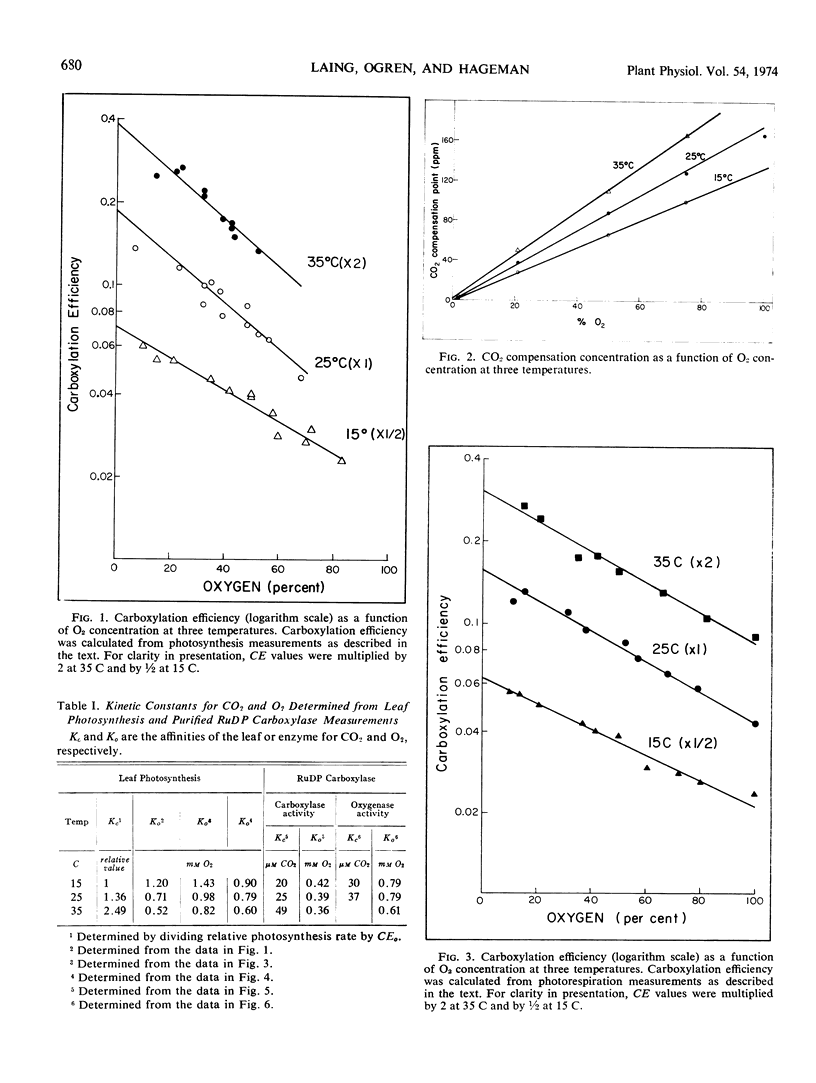
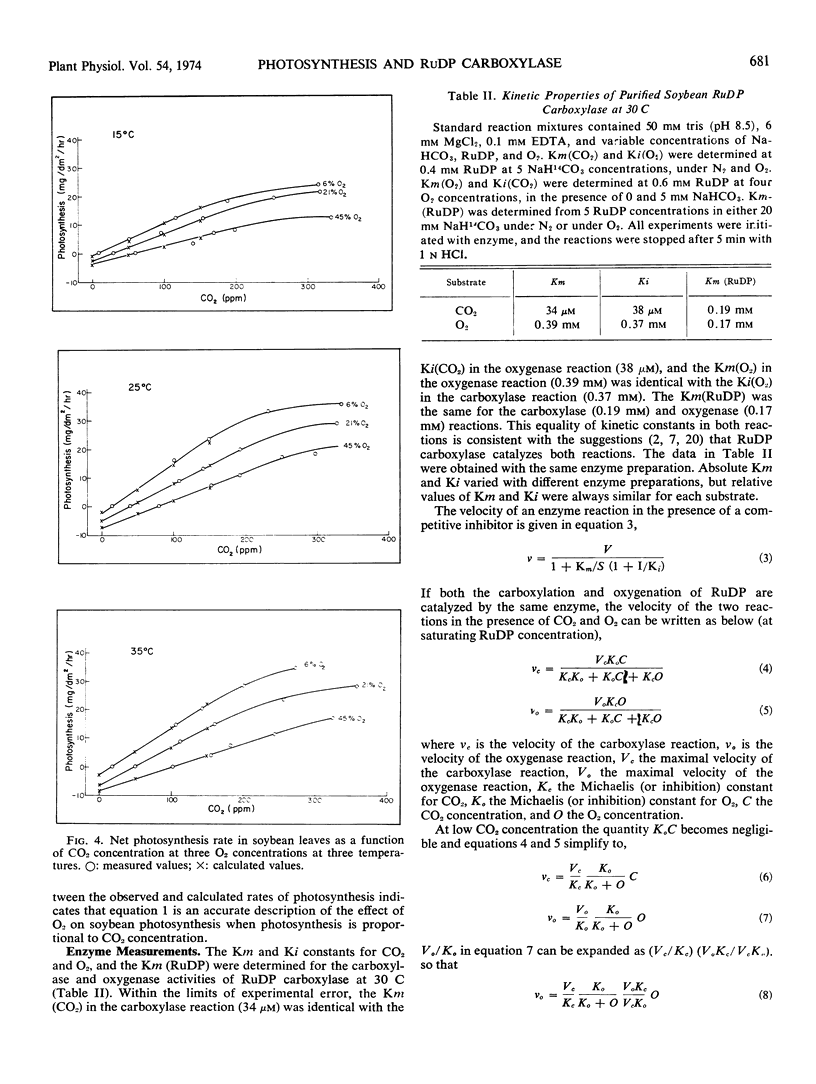
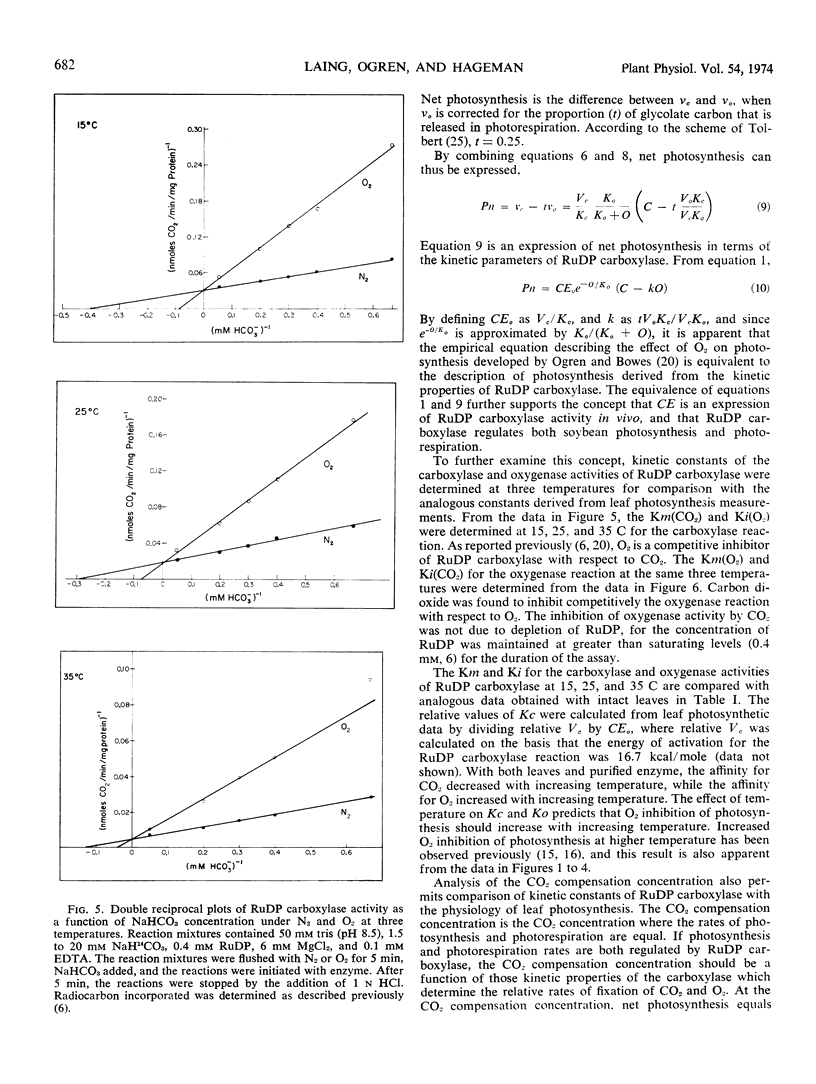
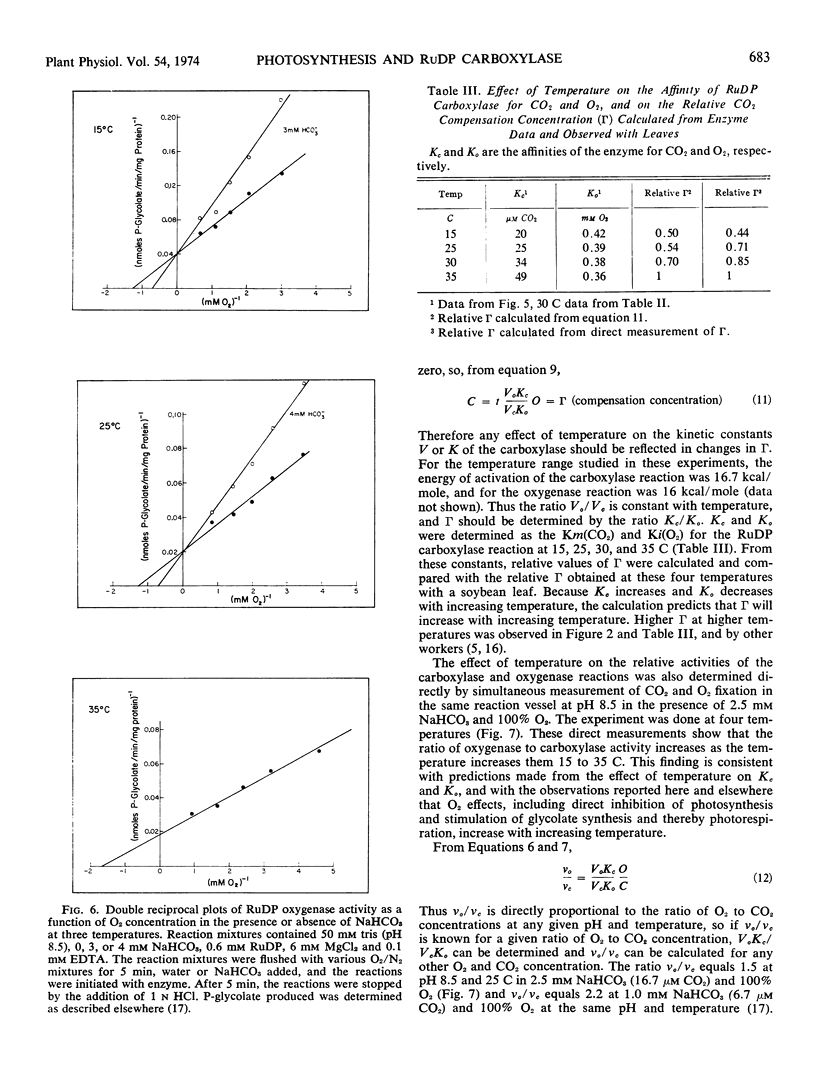
Kinetic properties of soybean net photosynthetic CO2 fixation and of the carboxylase and oxygenase activities of purified soybean (Glycine max [L.] Merr.) ribulose 1, 5-diphosphate carboxylase (EC 4.1.1.39) were examined as functions of temperature, CO2 concentration, and O2 concentration. With leaves, O2 inhibition of net photosynthetic CO2 fixation increased when the ambient leaf temperature was increased. The increased inhibition of CO2 fixation at higher temperatures was caused by a reduced affinity of the leaf for CO2 and an increased affinity of the leaf for O2. With purified ribulose 1,5-diphosphate carboxylase, O2 inhibition of CO2 incorporation and the ratio of oxygenase activity to carboxylase activity increased with increased temperature. The increased O2 sensitivity of the enzyme at higher temperature was caused by a reduced affinity of the enzyme for CO2 and a slightly increased affinity of the enzyme for O2. The similarity of the effect of temperature on the affinity of intact leaves and of ribulose 1,5-diphosphate carboxylase for CO2 and O2 provides further evidence that the carboxylase regulates the O2 response of photosynthetic CO2 fixation in soybean leaves. Based on results reported here and in the literature, a scheme outlining the stoichiometry between CO2 and O2 fixation in vivo is proposed.
Oxygen competitively inhibited carboxylase activity with respect to CO2, and CO2 competitively inhibited oxygenase activity with respect to O2. Within the limits of experimental error, the Michaelis constant (CO2) in the carboxylase reaction was identical with the inhibition constant (CO2) in the oxygenase reaction, and the Michaelis constant (O2) in the oxygenase reaction was identical with the inhibition constant (O2) in the carboxylase reaction. The Michaelis constant, (ribulose 1,5-diphosphate) was the same in both the carboxylase and oxygenase reactions. This equality of kinetic constants is consistent with the notion that the same enzyme catalyzes both reactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973 Jan 2;12(1):11–18. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- Bassham J. A., Kirk M. Sequence of Formation of Phosphoglycolate and Glycolate in Photosynthesizing Chlorella pyrenoidosa. Plant Physiol. 1973 Nov;52(5):407–411. doi: 10.1104/pp.52.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L. Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. J Biol Chem. 1972 Apr 10;247(7):2171–2176. [PubMed] [Google Scholar]
- Ellyard P. W., Gibbs M. Inhibition of photosynthesis by oxygen in isolated spinach chloroplasts. Plant Physiol. 1969 Aug;44(8):1115–1121. doi: 10.1104/pp.44.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe P. A., Tregunna E. B. Effect of Temperature, CO(2) Concentration, and Light Intensity on Oxygen Inhibition of Photosynthesis in Wheat Leaves. Plant Physiol. 1968 Jun;43(6):902–906. doi: 10.1104/pp.43.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Andrews T. J., Tolbert N. E. Ribulose diphosphate oxygenase. II. Further proof of reaction products and mechanism of action. Biochemistry. 1973 Jan 2;12(1):18–23. doi: 10.1021/bi00725a004. [DOI] [PubMed] [Google Scholar]
- Ludwig L. J., Canvin D. T. The Rate of Photorespiration during Photosynthesis and the Relationship of the Substrate of Light Respiration to the Products of Photosynthesis in Sunflower Leaves. Plant Physiol. 1971 Dec;48(6):712–719. doi: 10.1104/pp.48.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren W. L., Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971 Mar 31;230(13):159–160. doi: 10.1038/newbio230159a0. [DOI] [PubMed] [Google Scholar]
- Plaut Z., Gibbs M. Glycolate formation in intact spinach chloroplasts. Plant Physiol. 1970 Apr;45(4):470–474. doi: 10.1104/pp.45.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. E., TOLBERT N. E. Phosphoglycolic acid phosphatase. J Biol Chem. 1961 May;236:1285–1290. [PubMed] [Google Scholar]
- Shain Y., Gibbs M. Formation of glycolate by a reconstituted spinach chloroplast preparation. Plant Physiol. 1971 Sep;48(3):325–330. doi: 10.1104/pp.48.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe T., Akazawa T. Oxidative formation of phosphoglycolate from ribulose-1,5-diphosphate catalysed by Chromatium ribulose-1,5-diphosphate carboxylase. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1173–1179. doi: 10.1016/0006-291x(73)90588-3. [DOI] [PubMed] [Google Scholar]
- Wareing P. F., Khalifa M. M., Treharne K. J. Rate-limiting processes in photosynthesis at saturating light intensities. Nature. 1968 Nov 2;220(5166):453–457. doi: 10.1038/220453a0. [DOI] [PubMed] [Google Scholar]
- ZELITCH I. The relationship of glycolic acid to respiration and photosynthesis in tobacco leaves. J Biol Chem. 1959 Dec;234:3077–3081. [PubMed] [Google Scholar]


