Abstract
As microvascular techniques continue to improve, perforator flap free tissue transfer is now the gold standard for autologous breast reconstruction. Various options are available for breast reconstruction with autologous tissue. These include the free transverse rectus abdominis myocutaneous (TRAM) flap, deep inferior epigastric perforator flap, superficial inferior epigastric artery flap, superior gluteal artery perforator flap, and transverse/vertical upper gracilis flap. In addition, pedicled flaps can be very successful in the right hands and the right patient, such as the pedicled TRAM flap, latissimus dorsi flap, and thoracodorsal artery perforator. Each flap comes with its own advantages and disadvantages related to tissue properties and donor-site morbidity. Currently, the problem is how to determine the most appropriate flap for a particular patient among those potential candidates. Based on a thorough review of the literature and accumulated experiences in the author's institution, this article provides a logical approach to autologous breast reconstruction. The algorithms presented here can be helpful to customize breast reconstruction to individual patient needs.
Keywords: Breast cancer, Breast reconstruction, Free tissue flaps
INTRODUCTION
Since Hartrampf introduced the pedicled transverse rectus abdominis myocutaneous (TRAM) flap for breast reconstruction [1], lower abdominal tissue has become the first-line treatment for autologous breast reconstruction among most surgeons [2]. With technical advancements in microsurgery and extended applications of perforator flaps, the original lower abdominal flap evolved into the free TRAM flap, the muscle sparing free TRAM (MS-TRAM) flap, the deep inferior epigastric perforator (DIEP) flap, and the superficial inferior epigastric artery (SIEA) flap. This progression was made possible because of the increased understanding of the blood supply to the lower abdominal tissue, advances in free tissue transfer, and perforator dissection, along with a paradigm shift toward minimizing unnecessary donor site morbidity [3-13]. Medial thigh-based flaps such as the transverse upper gracilis (TUG) and vertical upper gracilis (VUG), as well as gluteal-based flaps such as the superior gluteal artery perforator (SGAP) and inferior gluteal artery perforator (IGAP), are also very useful flaps for autologous tissue breast reconstruction [14-20]. The pedicled TRAM flap, latissimus dorsi (LD) flap, thoracodorsal artery perforator (TAP) flap, anterolateral thigh free flap, and deep inferior circumflex artery flap (Rubens flap) are other options [21-24]. With so many options available for autologous breast reconstruction and each having its own unique benefits and drawbacks, an algorithm for total breast reconstruction with autologous tissue would be vital to the reconstructive breast surgeon.
NAVIGATION BY ALGORITHM
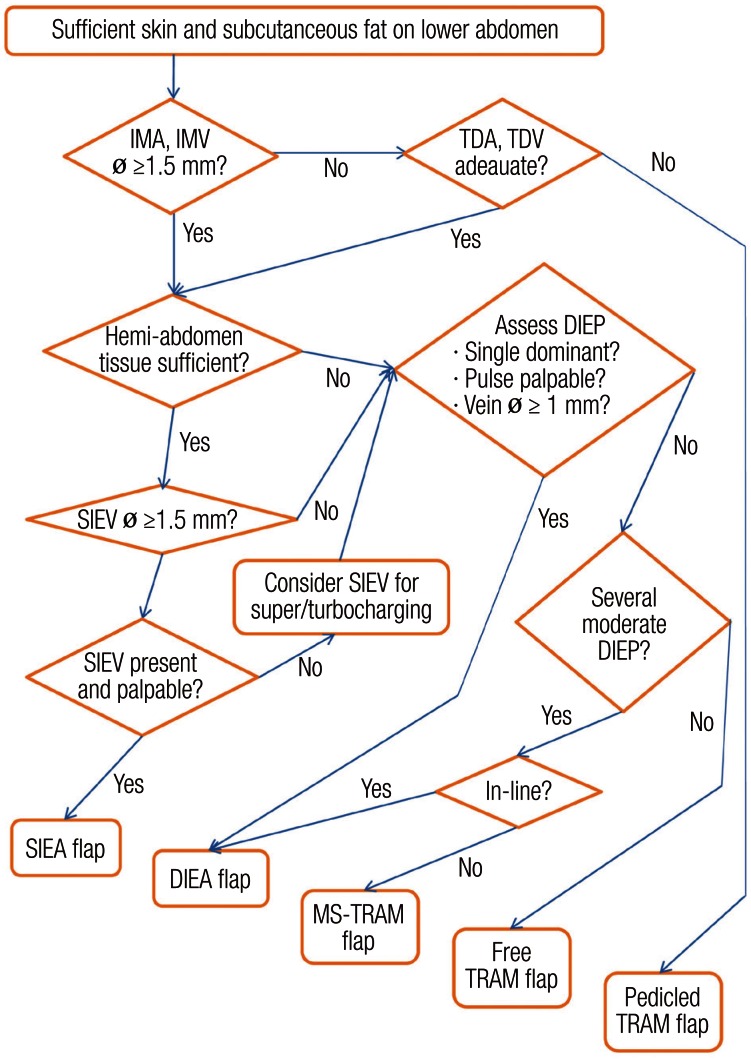
Navigation starts with preoperative selection of a suitable donor site. First, the lower abdomen of the patient should be evaluated. If it has proper skin and subcutaneous fat tissue then the primary choice is limited to one of the lower abdominal flaps (SIEA, DIEP, MS-TRAM, pedicled TRAM). The specific type of flap is decided under the guidance of the lower abdominal algorithm.
To select the optimal flap among lower abdominal flaps, the availability of recipient vessels, the requirements of tissue volume, and the vasculature of the donor-site are assessed, for the most part, intraoperatively (Fig. 1).
Fig. 1.
Algorithm for use of abdominal based free flaps
Lower abdominal algorithm for determining the optimal flap among the lower abdominal flaps. IMA, internal mammary artery perforator; IMV, internal mammary vein; ø, external diameter; TDA, thoracodorsal artery; TDV, thoracodorsal vein; DIEP, deep inferior epigastric perforator; SIEV, superficial inferior epigastric vein; SIEA, superficial inferior epigastric artery; DIEA, deep inferior epigastric artery; MS-TRAM, muscle sparing free transverse rectus abdominis myocutaneous (From Park and Song [28], with permission from Lippincott Williams & Wilkins).
For the recipient vessels, the internal mammary artery and vein are our preferred choice. When compared to the thoracodorsal vessels, they are routinely preserved from the mastectomy, less affected by post-radiation scarring, and they allow the flap to be inset more medially. In this case, a contralateral low abdominal flap is usually adopted because it provides optimal pedicle position for the flap based on the superficial or deep inferior epigastric vessels. If, however, the internal mammary vessels are damaged or not usable, then the thoracodorsal vessels are assessed as a second option. In cases of thoracodorsal vessels as recipients, an ipsilateral low abdominal flap is commonly employed for a similar reason to that above. A vessel diameter of 1.5 mm or more is sufficient for any case. If both internal mammary and thoracodorsal systems are inadequate, then a pedicled TRAM flap is a remaining choice. On a rare occasion, the case may be aborted and an alternative reconstruction may be planned for a later date with consideration of vascular loops and vein grafts.
Next, to select the type of flap, the tissue requirements for the new breast such as the skin envelope and overall volume should be determined. A SIEA flap can support an entire hemiabdominal flap, but it does not reliably perfuse across the midline [25]. If the SIEA hemiabdominal flap has been determined to be an adequate size for the tissue needs, then the algorithm for choosing the flap is as follows.
The superficial inferior epigastric system is first examined. The superficial inferior epigastric vein (SIEV) should have a diameter of at least 1.5 mm. If the SIEV is not present or is too small, then the deep inferior epigastric system is explored. If an adequate vein is present, the SIEA is then assessed. This artery is absent or insufficient more often than the vein. It is present in approximately 30% of our cases and does not necessarily travel with the vein. It may be lateral to the vein up to 1 to 1.5 cm, and it typically lies just deep to the Scarpa's fascia (Fig. 2). While some centers have advocated that the vessel diameter be 1.5 mm as a criterion for a usable SIEA [13,26], we have found that a palpable pulse is a more reliable criterion for an acceptable artery irrespective of vascular diameter. If both the artery and vein are adequate, then a SIEA flap is the best choice. If the vein is adequate but the artery is not, then the vein may be dissected as a long pedicle to supercharge the venous drainage, if necessary.
Fig. 2.
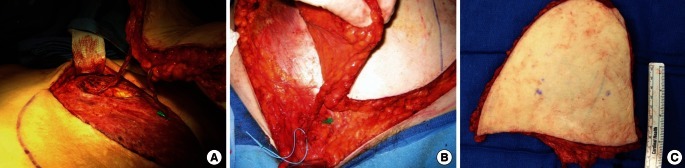
Superficial inferior epigastric artery flap
(A) A superficial inferior epigastric artery (SIEA) flap was elevated on the right lower abdomen. The SIEA was dissected to just below the inguinal ligament near its origin of the femoral artery. It typically lies just deep to the Scarpa's fascia and often travels laterally to the superficial inferior epigastric vein (SIEV) by 1 or 1.5 cm. (B) But sometimes SIEAs go with SIEVs. (C) Another SIEA flap showed its spread pedicle right after division.
If either the SIEA or the SIEV is not acceptable, the low abdominal flap is dissected in a suprafascial plane to expose the perforators of the deep inferior epigastric system. When it comes to perforators, an adequate vein should measure at least 1 mm in diameter, and an adequate artery should have a palpable pulse or visible pulsation. If a dominant perforator is found, then a DIEP flap is harvested based on that perforator.
If there is not a proper single, dominant perforator, then the location and caliber of the other perforators are assessed. If there are two or more medium-sized perforators in the same medial or lateral row and they fall within the same slit of myotomy, a DIEP flap may still be harvested based on that row of perforators (Fig. 3). However, if those perforators originated from different rows, then a MS-TRAM flap may be performed, which includes the multiple perforators and the cuff of muscle around them [5-7]. The preference is to first use the medial row over the lateral because the innervation of the rectus muscle comes laterally, and there is a theoretic advantage of minimizing the damage to the nerve supply, thus sparing the more innervated muscle. If there is no suitable collection of perforators, a free TRAM flap may be considered.
Fig. 3.
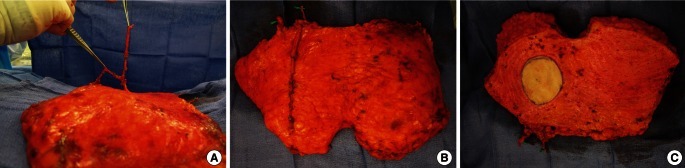
Deep inferior epigastric artery perforator flap
(A, B) A deep inferior epigastric perforator (DIEP) flap was harvested and turned over. It is based on the two medium-sized perforators in the same medial row. (C) Two-thirds of the low abdominal tissue was captured on a single pedicle. The preserved superficial inferior epigastric vein is shown lateral to the main pedicle. It could be used to supercharge or turbocharge the outflow of the DIEP flap if venous congestion occurs or is suspicious (From Park and Song [28], with permission from Lippincott Williams & Wilkins).
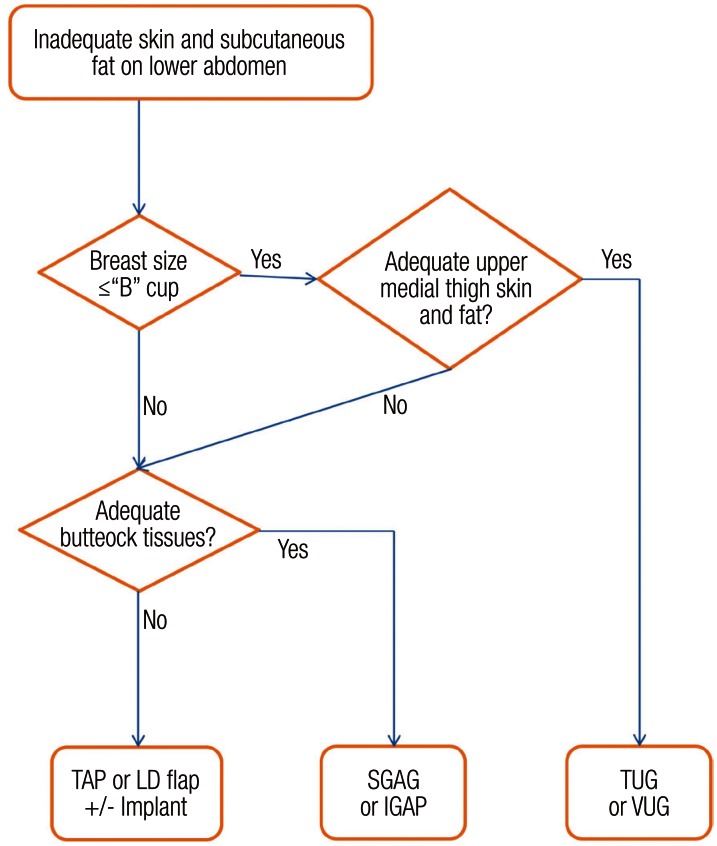
If the lower abdomen is not available as a donor, then the medial thighs are evaluated (Fig. 4). If the required breast volume is approximately a B cup or less, and there are sufficient tissues on the upper medial thigh, then a TUG flap or a VUG flap is chosen (Fig. 5) [14-17]. If the required volume exceeds the thigh donor site, then the buttocks are evaluated. If there are adequate tissues, then a SGAP flap or an IGAP flap is chosen (Fig. 6) [18-20]. If the buttock tissue is insufficent or if the patient refuses to sacrifice the buttock then a TAP flap or a LD myocutaneous flap is considered [21,22]; both are pedicled flaps and commonly accompanied by an expander or an implant to adjust the volume of the reconstructed breast. There are also alternative flaps such as the deep circumflex iliac artery flap (Rubens flap), the anterolateral thigh free flap, and omental flap, which have already been reported on for breast reconstruction, but are generally not accepted as the starting line-up [23,24,27].
Fig. 4.
Algorithm for use of non-abdominal based free flaps
Extra-abdominal algorithm for selecting a donor site other than the lower abdomen. IGAP, inferior gluteal artery perforator; LD, latissimus dorsi; SGAP, superior gluteal artery perforator; TAP, thoracodorsal artery perforator; TUG, transverse upper gracilis; VUG, vertical upper gracilis.
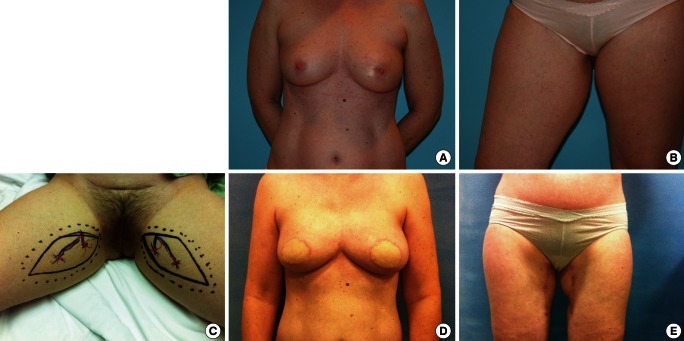
Fig. 5.
Bilateral vertical upper gracilis flaps
(A, B) Preoperative view of the 48-year-old woman who underwent immediate bilateral breast reconstruction using bilateral vertical upper gracilis (VUG) flaps. (C) Bilateral VUG flaps were designed on the two upper medial thighs. Cutaneous perforators around the gracilis muscle were traced with the help of a hand-held Doppler. (D, E) Postoperative view at 5 months is shown. Depressed scars on the upper medial thighs are easily concealed.
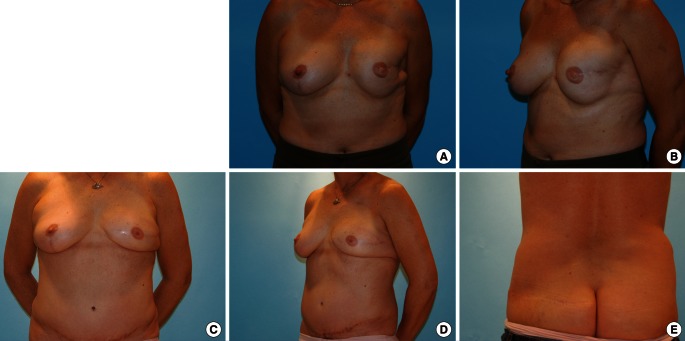
Fig. 6.
Superior gluteal artery perforator flap
(A, B) Preoperative photos of 44-year-old woman who had previously taken abdominoplasty and breast reconstruction with an implant on her left breast. A severely depressed deformity associated with capsular contracture after postoperative radiation therapy is shown on the left lateral breast. Balancing mastopexy had been performed on the contralateral breast at the time of implantation on the left side. (C, D) Secondary delayed breast reconstruction using a superior gluteal artery perforator (SGAP) flap was applied. The implant was removed with anterior capsulectomy at the same time the SGAP flap was removed. The 2-year postoperative view is shown. (E) Donor site scar on the left upper buttock is inconspicuous.
DISCUSSION
These two flap chains of the lower abdominal and extra-abdominal algorithm are established based on our preference for pursuing more reliable free flaps, reducing donor site morbidity, and decreasing operative time. The two are based on our experiences in optimizing outcomes and effectively meeting the patients' expectations [28].
Structurally, the SIEA flap has a robust blood supply because it is based on a direct axial artery as its pedicle. It maximally preserves the abdominal wall function and is free from the risk of abdominal hernia [9-13,29]. The loss rate of the SIEA flap is low enough and tolerable as that of MS-TRAM [30]. Additionally, in our experience it is associated with shorter operative times, shorter hospital stays, and decreased postoperative pain compared to DIEP and other low abdominal flaps. Therefore, the SIEA flap is always pursued first if low abdominal tissue is chosen for breast reconstruction. If the SIEA flap is unavailable, the next considerations are DIEP, MS-TRAM, free TRAM, and pedicled TRAM flap in that order for same reasons discussed earlier.
To boost blood supply, the SIEA itself can be combined with a DIEP flap or a muscle-sparing free TRAM flap in a daisy-chain method for unilateral breast reconstruction that requires the entire low abdominal tissue. In that case, the SIEA is connected to the proximal deep inferior epigastric artery (DIEA) on the same side of the flap and the distal DIEA is anastomosed to the recipient pedicle, which corresponds to the turbocharged flap [31,32]. Additionally, bilateral breast reconstruction incorporating the SIEA flap has a significantly lower chance of abdominal donor-site morbidity compared to bilateral DIEP flaps and other various combinations of low abdominal flaps (Fig. 7). Preserving SIEV as long as possible is routinely attempted because it may be used to supercharge or turbocharge the outflow of the DIEP flap to help relieve venous congestion (Fig. 3). The SIEV may be anastomosed to the distal internal mammary vein, an internal mammary perforator, or the proximal outflow of the pedicle [31,32]. Examining the contribution of the superficial venous drainage system before completion of harvesting the flap is helpful. This may be performed simply by temporally occluding the SIEV with microvascular clamps and assessing the perfusion of the flap. If the flap shows significant congestive change, venous supercharge using SIEV is highly recommended.
Fig. 7.
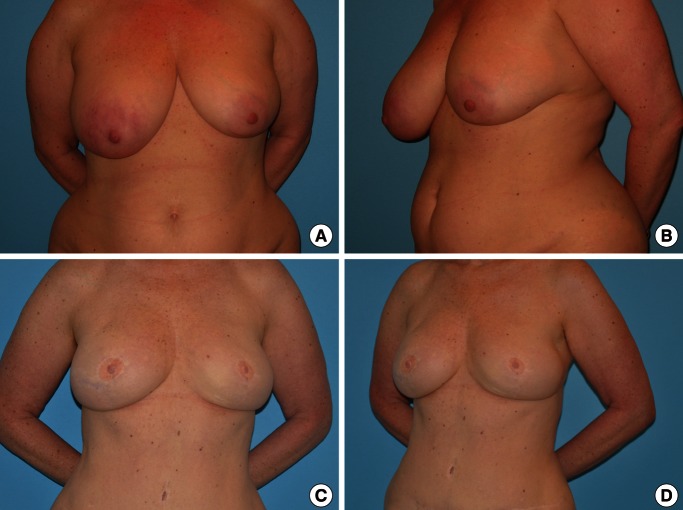
Long term results from superficial inferior epigastric artery flap
(A, B) A preoperative photo of 43-year-old woman who had immediate bilateral breast reconstruction using bilateral superficial inferior epigastric vein flaps is shown. (C, D) Postoperative appearance at 3 years is presented. Overall, good body shape is well maintained without any weakness or bulging in the lower abdomen.
Preoperative mapping of abdominal wall perforators may be done with radiologic studies. The most effective among several imaging modalities at present appears to be computerized tomography (CT) angiography [33-35]. The CT angiogram gives the most useful information when the periumbilical perforators are mapped in a three-dimensional reconstruction image. Some studies have reported a reduction in operative time, as well as better preoperative planning based on the findings of CT angiograms [36-38].
When there is not a single obvious dominant DIEA perforator, a couple of considerations can be made to select which perforators to use. Temporary occlusion of each perforator using microvascular clamps helps identify the isolated angiosome of each perforator. This will reveal which perforator or combination of perforators is adequate for whole flap perfusion. It is also prudent to evaluate the need for supercharging or turbocharging flaps to augment venous drainage. Although augmented venous drainage is generally unnecessary, one should make this determination if needed, and take action immediately rather than risk discovery of congestion after complete harvest of the flap and anastomoses.
In the event that over two-thirds of the low abdominal tissue is needed on a single pedicle, choosing the perforators originating from the medial row of DIEA leads to less disruption of the innervated rectus muscle and theoretically preserves its function better than basing the flap off the lateral row where the nerve supply to the remaining muscle can be suspect. There can be unfavorable patient factors that may make using multiple perforators wise, such as if the patient is a smoker or a diabetic. In that case, proximally located additional DIEA perforators tend to shorten the effective pedicle length; therefore, using multiple perforators should be performed carefully.
If the lower abdominal tissue as a donor site is excluded for any reason, the remaining reasonable options for total autologous breast reconstruction include TUG, VUG, SGAP, IGAP, TAP, or LD flap. Those flaps comprise the extra-abdominal algorithm. There are no significant differences between a TUG or VUG flap other than skin paddle orientation. Of course, a patient's preferences, body habitus, and activity level, as well as past history of any failed reconstruction that may limit other options, have to be carefully considered preoperatively.
There are several contraindications for using the lower abdominal free flaps that are mostly similar to others. Contraindications include previous abdominoplasty; although there have been reports of perforator flaps after full abdominoplasty, it would be wise to perform a preoperative image of these patients if a perforator abdominal flap is considered. The presence of multiple abdominal scars can also preclude the harvest of adequate volume and can cause a severe mismatch of size and volume for the new breast, and thus may be unfavorable. Other unfavorable conditions requiring proceeding with caution include a previous history of liposuction, collagen vascular disease, chronic steroid use, uncontrolled diabetes, and morbid obesity. Smoking, while not an absolute contraindication, needs to be carefully considered, especially when proceeding with perforator flap based breast reconstruction. The complication rate is raised slightly higher for smokers, but is particularly significant in mastectomy skin flap necrosis, donor site healing problems, and partial flap fat necrosis, rather than anastomotic complications such as total flap loss [39].
CONCLUSIONS
When autologous free tissue reconstruction of the breast is required, available donor sites of a patient should first be systemically checked, and reconstructive needs should also be considered to choose the optimal flap. When the lower abdominal donor tissue is selected, the most suitable flap is decided according to the proven lower abdominal algorithm that is designed to optimize blood supply to the flap and minimize donor-site morbidity. However, if the lower abdominal tissue is ruled out, the next best flap is determined by following the extra-abdominal algorithm that incorporates other available donor sites. The two algorithms can be useful guides for patients and surgeons to organize an individualized plan and make a rational decision to navigate through the labyrinth of various donor sites and flaps for total autologous breast reconstruction.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69:216–225. doi: 10.1097/00006534-198202000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Shestak KC. Breast reconstruction with a pedicled TRAM flap. Clin Plast Surg. 1998;25:167–182. [PubMed] [Google Scholar]
- 3.Schusterman MA, Kroll SS, Weldon ME. Immediate breast reconstruction: why the free TRAM over the conventional TRAM flap? Plast Reconstr Surg. 1992;90:255–261. [PubMed] [Google Scholar]
- 4.Kroll SS, Schusterman MA, Reece GP, et al. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96:616–619. doi: 10.1097/00006534-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Nahabedian MY, Momen B, Galdino G, et al. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466–475. doi: 10.1097/00006534-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Nahabedian MY, Manson PN. Contour abnormalities of the abdomen after transverse rectus abdominis muscle flap breast reconstruction: a multifactorial analysis. Plast Reconstr Surg. 2002;109:81–87. doi: 10.1097/00006534-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. 2006;117:737–746. doi: 10.1097/01.prs.0000200062.97265.fb. [DOI] [PubMed] [Google Scholar]
- 8.Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645–648. doi: 10.1016/0007-1226(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 9.Grotting JC. The free abdominoplasty flap for immediate breast reconstruction. Ann Plast Surg. 1991;27:351–354. doi: 10.1097/00000637-199110000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. 2004;113:1153–1160. doi: 10.1097/01.prs.0000110328.47206.50. [DOI] [PubMed] [Google Scholar]
- 11.Volpe AG, Rothkopf DM, Walton RL. The versatile superficial inferior epigastric flap for breast reconstruction. Ann Plast Surg. 1994;32:113–117. doi: 10.1097/00000637-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Arnez ZM, Khan U, Pogorelec D, et al. Breast reconstruction using the free superficial inferior epigastric artery (SIEA) flap. Br J Plast Surg. 1999;52:276–279. doi: 10.1054/bjps.1999.3100. [DOI] [PubMed] [Google Scholar]
- 13.Chevray PM. Breast reconstruction with superficial inferior epigastric artery flaps: a prospective comparison with TRAM and DIEP flaps. Plast Reconstr Surg. 2004;114:1077–1083. doi: 10.1097/01.prs.0000135328.88101.53. [DOI] [PubMed] [Google Scholar]
- 14.Yousif NJ, Matloub HS, Kolachalam R, et al. The transverse gracilis musculocutaneous flap. Ann Plast Surg. 1992;29:482–490. doi: 10.1097/00000637-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Arnez ZM, Pogorelec D, Planinsek F, et al. Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg. 2004;57:20–26. doi: 10.1016/j.bjps.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Schoeller T, Huemer GM, Wechselberger G. The transverse musculocutaneous gracilis flap for breast reconstruction: guidelines for flap and patient selection. Plast Reconstr Surg. 2008;122:29–38. doi: 10.1097/PRS.0b013e318177436c. [DOI] [PubMed] [Google Scholar]
- 17.Kind GM, Foster RD. The longitudinal gracilis myocutaneous flap: broadening options in breast reconstruction. Ann Plast Surg. 2008;61:513–520. doi: 10.1097/SAP.0b013e318168db64. [DOI] [PubMed] [Google Scholar]
- 18.Allen RJ, Tucker C., Jr Superior gluteal artery perforator free flap for breast reconstruction. Plast Reconstr Surg. 1995;95:1207–1212. doi: 10.1097/00006534-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Blondeel PN. The sensate free superior gluteal artery perforator (S-GAP) flap: a valuable alternative in autologous breast reconstruction. Br J Plast Surg. 1999;52:185–193. doi: 10.1054/bjps.1998.3032. [DOI] [PubMed] [Google Scholar]
- 20.Allen RJ, Levine JL, Granzow JW. The in-the-crease inferior gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg. 2006;118:333–339. doi: 10.1097/01.prs.0000227665.56703.a8. [DOI] [PubMed] [Google Scholar]
- 21.Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg. 1995;96:1608–1614. doi: 10.1097/00006534-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Hamdi M, Van Landuyt K, Hijjawi JB, et al. Surgical technique in pedicled thoracodorsal artery perforator flaps: a clinical experience with 99 patients. Plast Reconstr Surg. 2008;121:1632–1641. doi: 10.1097/PRS.0b013e31816c3bfa. [DOI] [PubMed] [Google Scholar]
- 23.Wei FC, Suominen S, Cheng MH, et al. Anterolateral thigh flap for postmastectomy breast reconstruction. Plast Reconstr Surg. 2002;110:82–88. doi: 10.1097/00006534-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Hartrampf CR, Jr, Noel RT, Drazan L, et al. Ruben's fat pad for breast reconstruction: a peri-iliac soft-tissue free flap. Plast Reconstr Surg. 1994;93:402–407. doi: 10.1097/00006534-199402000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Holm C, Mayr M, Hofter E, et al. The versatility of the SIEA flap: a clinical assessment of the vascular territory of the superficial epigastric inferior artery. J Plast Reconstr Aesthet Surg. 2007;60:946–951. doi: 10.1016/j.bjps.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel AJ, Khan FN. An Intraoperative algorithm for use of the SIEA flap for breast reconstruction. Plast Reconstr Surg. 2007;120:1450–1459. doi: 10.1097/01.prs.0000270282.92038.3f. [DOI] [PubMed] [Google Scholar]
- 27.Cothier-Savey I, Tamtawi B, Dohnt F, et al. Immediate breast reconstruction using a laparoscopically harvested omental flap. Plast Reconstr Surg. 2001;107:1156–1163. doi: 10.1097/00006534-200104150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Park JE, Song DH. Breast reconstruction with free tissue transfer: an algorithmic approach. In: Spear SL, Willey SC, Robb GL, et al., editors. Surgery of the breast: principles and art. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 727–735. [Google Scholar]
- 29.Wu LC, Bajaj A, Chang DW, et al. Comparison of donor-site morbidity of SIEA, DIEP, and muscle-sparing TRAM flaps for breast reconstruction. Plast Reconstr Surg. 2008;122:702–709. doi: 10.1097/PRS.0b013e3181823c15. [DOI] [PubMed] [Google Scholar]
- 30.Selber JC, Samra F, Bristol M, et al. A head-to-head comparison between the muscle-sparing free TRAM and the SIEA flaps: is the rate of flap loss worth the gain in abdominal wall function? Plast Reconstr Surg. 2008;122:348–355. doi: 10.1097/PRS.0b013e31817d60b0. [DOI] [PubMed] [Google Scholar]
- 31.Tseng CY, Lang PO, Cipriani NA, et al. Pedicle preservation technique for arterial and venous turbocharging of free DIEP and muscle-sparing TRAM flaps. Plast Reconstr Surg. 2007;120:851–854. doi: 10.1097/01.prs.0000277663.50061.83. [DOI] [PubMed] [Google Scholar]
- 32.Hamdi M, Khuthaila DK, Van Landuyt K, et al. Double-pedicle abdominal perforator free flaps for unilateral breast reconstruction: new horizons in microsurgical tissue transfer to the breast. J Plast Reconstr Aesthet Surg. 2007;60:904–912. doi: 10.1016/j.bjps.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Rosson GD, Williams CG, Fishman EK, et al. 3D CT angiography of abdominal wall vascular perforators to plan DIEAP flaps. Microsurgery. 2007;27:641–646. doi: 10.1002/micr.20423. [DOI] [PubMed] [Google Scholar]
- 34.Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the deep inferior epigastric artery: a blinded, prospective cohort study. Plast Reconstr Surg. 2008;122:1003–1009. doi: 10.1097/PRS.0b013e3181845994. [DOI] [PubMed] [Google Scholar]
- 35.Rozen WM, Stella DL, Bowden J, et al. Advances in the pre-operative planning of deep inferior epigastric artery perforator flaps: magnetic resonance angiography. Microsurgery. 2009;29:119–123. doi: 10.1002/micr.20590. [DOI] [PubMed] [Google Scholar]
- 36.Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg. 2009;62:1112–1117. doi: 10.1016/j.bjps.2007.12.090. [DOI] [PubMed] [Google Scholar]
- 37.Rozen WM, Ashton MW, Whitaker IS, et al. The financial implications of computed tomographic angiography in DIEP flap surgery: a cost analysis. Microsurgery. 2009;29:168–169. doi: 10.1002/micr.20594. [DOI] [PubMed] [Google Scholar]
- 38.Piorkowski JR, DeRosier LC, Nickerson P, et al. Preoperative computed tomography angiogram to predict patients with favorable anatomy for superficial inferior epigastric artery flap breast reconstruction. Ann Plast Surg. 2011;66:534–536. doi: 10.1097/SAP.0b013e31820b3ccc. [DOI] [PubMed] [Google Scholar]
- 39.Chang DW, Reece GP, Wang B, et al. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg. 2000;105:2374–2380. doi: 10.1097/00006534-200006000-00010. [DOI] [PubMed] [Google Scholar]