Abstract
Extracts of phylogenetically diverse plans were surveyed for their ability to synthesize the following homoserine esters which are potential precursors for methionine and threonine synthesis in green plants: O-acetyl-, O-oxalyl-, O-succinyl-, O-malonyl-, and O-phosphohomoserine. Synthesis of O-acylhomoserine esters was detected only in Pisum sativum L. and Lathyrus sativus L. Extracts of P. sativum, a plant known to accumulate O-acetylhomoserine, catalyzed the specific synthesis of this ester from homoserine and acetyl-CoA. Extracts of L. sativus, a plant known to accumulate O-oxalylhomoserine, catalyzed the specific synthesis of this ester from homoserine and oxalyl-CoA. None of the other plants surveyed, including representatives of the green algae, horsetails, gymnosperms, and angiosperms, catalyzed the synthesis of any of the O-acylhomoserine esters studied. In contrast, synthesis of O-phosphohomoserine by the reaction catalyzed by homoserine kinase was demonstrated in extracts of all plants examined, including the two exceptional legumes.
These results suggest that, among the five homoserine esters studied, O-phosphohomoserine is the major activated homoserine derivative in plants. Direct confirmation of the dominant physiological role of O-phosphohomoserine in the synthesis of cystathionine in the transsulfuration pathway of methionine biosynthesis in plants has recently been provided (Datko, A. H., Giovanelli, J., and Mudd, S. H. 1974. J. Biol. Chem. 249: 1139-1155).
Full text
PDF



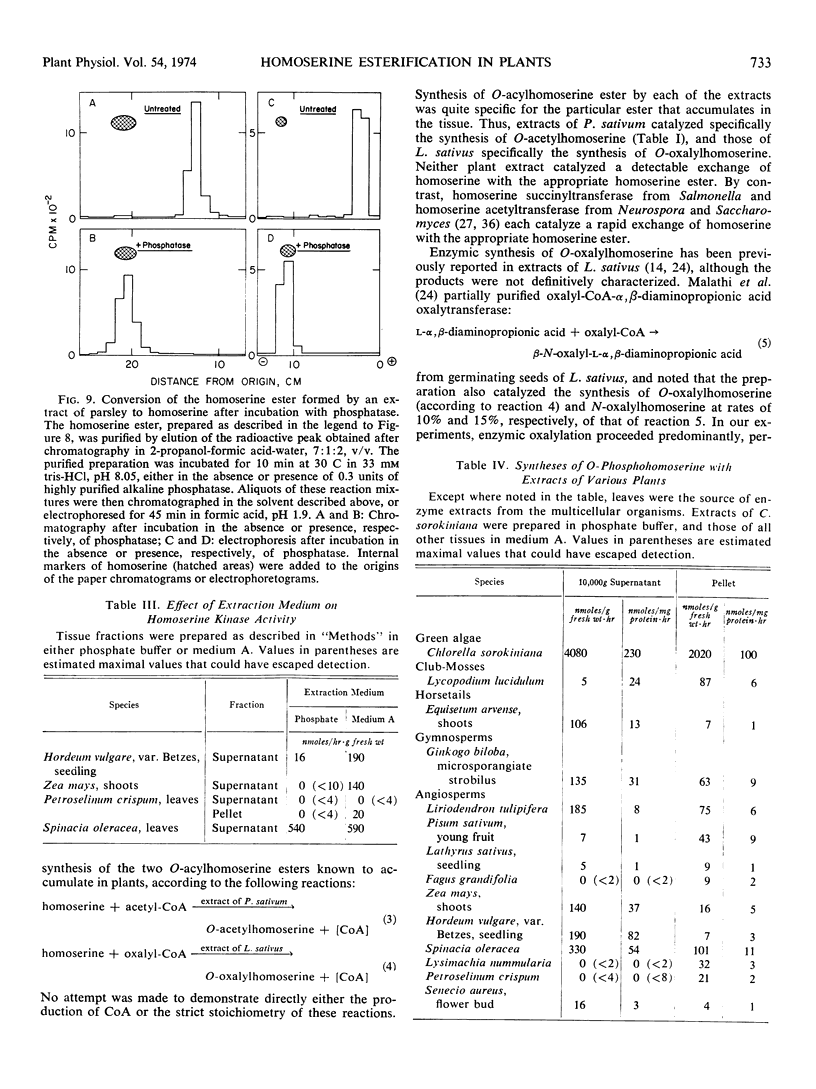
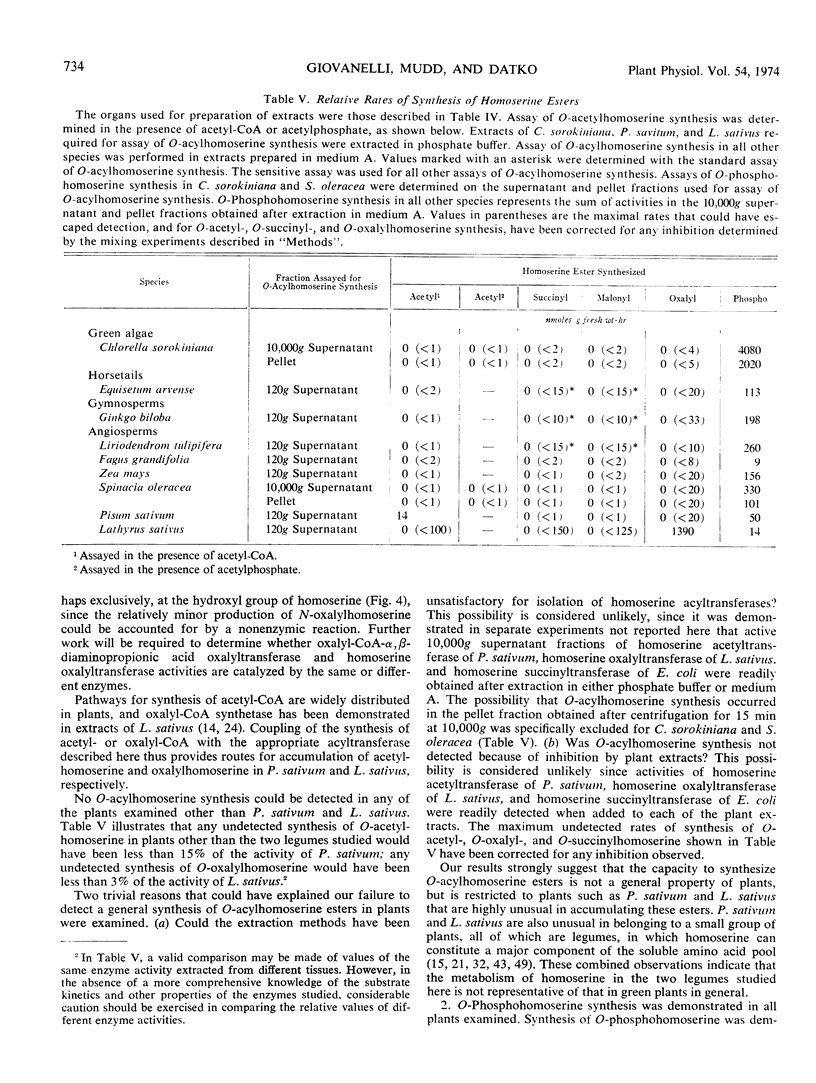


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brush A., Paulus H. The enzymic formation of O-acetylhomoserine in Bacillus subtilis and its regulation by methionine and S-adenosylmethionine. Biochem Biophys Res Commun. 1971 Nov 5;45(3):735–741. doi: 10.1016/0006-291x(71)90478-5. [DOI] [PubMed] [Google Scholar]
- Cafferata R. L., Freundlich M. Evidence for channeling of homoserine in Salmonella typhimurium. Biochim Biophys Acta. 1970 Dec 29;222(3):671–674. doi: 10.1016/0304-4165(70)90196-0. [DOI] [PubMed] [Google Scholar]
- Datko A. H., Giovanelli J., Mudd S. H. Homocysteine biosynthesis in green plants. O-Phosphorylhomoserine as the physiological substrate for cystathionine gamma-synthase. J Biol Chem. 1974 Feb 25;249(4):1139–1155. [PubMed] [Google Scholar]
- Dougall D. K., Fulton M. M. Biosynthesis of Protein Amino Acids in Plant Tissue Culture IV Isotope Competition Experiments using Glucose-U-C and Potential Intermediates. Plant Physiol. 1967 Jul;42(7):941–945. doi: 10.1104/pp.42.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Enzymatic synthesis of homocysteine or methionine directly from O-succinyl-homoserine. Biochim Biophys Acta. 1967 Mar 15;132(2):400–405. doi: 10.1016/0005-2744(67)90158-1. [DOI] [PubMed] [Google Scholar]
- GROBBELAAR N., STEWARD F. C. O-acetylhomoserine in Pisum. Nature. 1958 Nov 15;182(4646):1358–1359. doi: 10.1038/1821358a0. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Synthesis of homocysteine and cysteine by enzyme extracts of spinach. Biochem Biophys Res Commun. 1967 Apr 20;27(2):150–156. doi: 10.1016/s0006-291x(67)80054-8. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Owens L. D., Mudd S. H. beta-Cystathionase In Vivo Inactivation by Rhizobitoxine and Role of the Enzyme in Methionine Biosynthesis in Corn Seedlings. Plant Physiol. 1973 Mar;51(3):492–503. doi: 10.1104/pp.51.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T., Seto K., Murooka Y. O-alkylhomoserine and methionine biosynthesis in Corynebacterium. J Biochem. 1969 Mar;65(3):493–496. doi: 10.1093/oxfordjournals.jbchem.a129041. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Lloyd H. J. Oxalyl-coenzyme A synthetase and the neurotoxin beta-n-oxalyl-l-alpha, beta-diaminopropionate. Aust J Biol Sci. 1967 Dec;20(6):1241–1244. doi: 10.1071/bi9671241. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Flavin M. Threonine biosynthesis. On the pathway in fungi and bacteria and the mechanism of the isomerization reaction. J Biol Chem. 1965 Oct;240(10):3928–3933. [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. Synthesis of cystathionine from O-acetylhomoserine in Neurospora: a step in methionine biosynthesis. Biochem Biophys Res Commun. 1968 Apr 5;31(1):124–130. doi: 10.1016/0006-291x(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. The regulation of methionine synthesis and the nature of cystathionine gamma-synthase in Neurospora. J Biol Chem. 1970 Apr 10;245(7):1842–1855. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee L. W., Ravel J. M., Shive W. Multimetabolite control of a biosynthetic pathway by sequential metabolites. J Biol Chem. 1966 Nov 25;241(22):5479–5480. [PubMed] [Google Scholar]
- Miyajima R., Shiio I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. VII. Properities of homoserine O-transacetylase. J Biochem. 1973 May;73(5):1061–1068. doi: 10.1093/oxfordjournals.jbchem.a130160. [DOI] [PubMed] [Google Scholar]
- Murooka Y., Seto K., Harada T. O-Alkylhomoserine synthesis from O-acetylhomoserine and alcohol. Biochem Biophys Res Commun. 1970 Oct 23;41(2):407–414. doi: 10.1016/0006-291x(70)90519-x. [DOI] [PubMed] [Google Scholar]
- Nagai S., Flavin M. Acetylhomoserine. An intermediate in the fungal biosynthesis of methionine. J Biol Chem. 1967 Sep 10;242(17):3884–3895. [PubMed] [Google Scholar]
- Nigam S. N., Ressler C. Biosynthesis of 2,4-diaminobutyric acid from L-[3H]homoserine and DL-[1-14C]aspartic acid in Lathyrus sylvestris W. Biochemistry. 1966 Nov;5(11):3426–3431. doi: 10.1021/bi00875a006. [DOI] [PubMed] [Google Scholar]
- Przybylska J., Pawelkiewicz J. O-Oxalylhomoserine, a new homoserine derivative in young pods of Lathyrus sativus. Bull Acad Pol Sci Biol. 1965;13(6):327–329. [PubMed] [Google Scholar]
- QUAYLE J. R. Chemical synthesis of oxalyl-coenzyme A and its enzymic reduction to glyxylate. Biochim Biophys Acta. 1962 Feb 26;57:398–400. doi: 10.1016/0006-3002(62)91142-3. [DOI] [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. O-SUCCINYLHOMOSERINE AS AN INTERMEDIATE IN THE SYNTHESIS OF CYSTATHIONINE BY ESCHERICHIA COLI. J Gen Microbiol. 1964 Sep;36:341–358. doi: 10.1099/00221287-36-3-341. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H. Diversité des types de régulation impliqués dans la biosynthèse de la thréonine et de la méthionine chez Saccharomyces cerevisiae. Biochimie. 1971;53(2):131–134. doi: 10.1016/s0300-9084(71)80043-3. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Surdin Y., Mortimer R. K. Genetic and biochemical studies of genes controlling the synthesis of threonine and methionine in Saccharomyces. Genetics. 1966 Mar;53(3):609–619. doi: 10.1093/genetics/53.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbury R. J., Woods D. D. The regulation of cystathionine formation in Escherichia coli. J Gen Microbiol. 1966 Jan;42(1):155–163. doi: 10.1099/00221287-42-1-155. [DOI] [PubMed] [Google Scholar]
- Savin M. A., Flavin M. Cystationine synthesis in yeast: an alternative pathway for homocysteine biosynthesis. J Bacteriol. 1972 Oct;112(1):299–303. doi: 10.1128/jb.112.1.299-303.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart C. E., Mann J. D., Mudd S. H. Alkaloids and Plant Metabolism. VII. The Kinetin-Produced Elevation in Tyramine Methylpherase Levels. Plant Physiol. 1964 Nov;39(6):1030–1038. doi: 10.1104/pp.39.6.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEAS H. J. Mutants of Bacillus subtilis that require threonine or threonine plus methionine. J Bacteriol. 1950 Jan;59(1):93–104. doi: 10.1128/jb.59.1.93-104.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Greer S. Minor threonine dehydratase encoded within the threonine synthetic region of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):983–993. doi: 10.1128/jb.106.3.983-993.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORMSER E. H., PARDEE A. B. Regulation of threonine biosynthesis in Escherichia coli. Arch Biochem Biophys. 1958 Dec;78(2):416–432. doi: 10.1016/0003-9861(58)90367-9. [DOI] [PubMed] [Google Scholar]



