Abstract
Type 1 diabetes mellitus (T1DM) is characterized by recognition of beta cell proteins as self-antigens, called autoantigens (AAgs), by patients’ own CD4+ and CD8+ T cells and/or the products of self-reactive B cells, called autoantibodies. These AAgs are divided into two categories on the basis of beta-cell-specificity. The list of the targets associated with beta cell-specific AAgs is continuously growing. Many T1DM-associated AAgs are well characterized and have important clinical applications for disease prediction, diagnosis, and antigen-specific tolerance immunotherapy. Identification of T1DM-associated AAgs provides insight into the pathogenesis of T1DM and to understanding the clinical aspects of the disease. Since many excellent reviews have covered the previously identified T1DM-associated AAgs exhaustedly, here we only focus on several recently discovered T1DM-AAgs (PDX1, ZnT8, CHGA, and IAAP).
Keywords: Type 1 diabetes, autoantigen, autoantibodies, Pdx1, ZnT8, IAPP, CHGA, immunotherapy, immune tolerance
Type 1 diabetes mellitus (T1DM)
The autoimmune nature of T1DM
Although the etiology of T1DM is not fully understood, a well-accepted view is that T1DM is an autoimmune disease caused by genetic and environmental factors [1]. Evidence for the autoimmune nature of T1DM includes: 1) The human leukocyte antigen (HLA) has strong linkage with disease, especially HLA-DR3/4 and DQ2/8 [1]. 2) The presence of antibodies to islet autoantigens (AAgs) occurs many years before clinical onset of T1DM [2,3]. Several of these autoantibodies have already become very good predictive and diagnostic markers for the development of T1DM. 3) Lymphocytic infiltrates appear in the islets during the development of insulitis. 4) Autoreactive CD4+ and CD8+ T cells to islet antigens are often present in recently diagnosed diabetic patients and in high-risk subjects [2-5]. 5) T1DM patients have increased susceptibility to develop multiple organ specific autoimmune diseases such as thyroid disorders, celiac disease, and Addison’s disease [6,7].
The presence of autoantibodies and autoreactive T cells indicates that certain islet antigens are erroneously recognized as foreign and initiate an immune response. Previously, many islet AAgs have been implicated in relation to T1DM. Well-established AAgs include non-specific islet cell AAgs (ICA) [8], insulin [9], glutamic acid decarboxylase 65 (GAD65) [10], insulinoma antigen-2 (IA-2) [11], heat shock protein (HSP) [12], islet-specific glucose-6-phosphatase catalytic subunit related protein (IGRP) [13], and imogen-38 [14]. The newly discovered beta cell specific AAgs include zinc transporter-8 (ZnT8) [15], pancreatic duodenal homeobox factor 1 (PDX1) [16], chromogranin A (CHGA) [17], and islet amyloid polypeptide (IAPP) [18]. Understanding the nature and clinical utility of AAgs is a central focus in diabetes research and has important implications for prediction prior to disease onset, diagnosis, and intervention by restoring immune tolerance. For well-established AAgs, many excellent reviews are available that detail their nature and utilities [19-21]. In this brief review, we will only focus on several recently identified AAgs (ZnT8, PDX1, CHGA, and IAPP) and discuss their basic biology and clinical relevance. Before discussing these new AAgs, we will briefly introduce the role of AAgs in the pathogenesis of T1DM.
The role of AAgs in the pathogenesis of T1DM
The discovery of AAgs in T1DM
Growing evidence demonstrates that CD4+ helper and CD8+ cytotoxic T lymphocytes are critical in the pathogenesis of T1DM. Although the initial events triggering autoreactive responses remain unclear, specific AAg presentation by disease associated MHC class II molecules is thought to contribute to priming and expansion of pathogenic T cells. Since identification and characterization of AAgs provide insights into the pathogenic process and supports the foundation for developing diagnostic assays and potential new therapeutic strategies, there has been much effort to discover these AAgs. Several approaches have been used to identify and confirm AAgs in T1DM [21] including: 1) detection of autoantibodies from patient sera, 2) detection of islet autoreactive T cells, 3) identification of candidate proteins based on selective expression of beta cell proteins as defined by cDNA subtraction libraries or microarrays, and 4) via adoptive transfer of specific T cells or by expression knock-down in animal models of T1DM.
The characterization of T cell epitopes has potential diagnostic and therapeutic applications and may provide clues to environmental agents that could be triggered to exacerbate autoimmune disease. T cell epitopes can be identified using a molecular biology strategy. Using a T cell epitope predicting tool, potential peptide epitope sequences can be identified. Then mini-mRNAs encoding these epitope sequences can be transfected into autologous antigen presenting cells (APCs), or the synthesized peptides can be loaded into autologous APCs and used to challenge purified peripheral T cells using the ELISPOT assay. CD4+ T cell epitopes have been identified in proinsulin, IA-2, GAD65, CHGA, IAPP, and IGRP, and CD8+ T cell epitopes have been identified in proinsulin, GAD65, IAPP, IGRP, and ZnT8 [1,20]. These epitopes can be used for preparation of MHC tetramers (tetramers, which consist of four biotinylated peptide-bound MHC complexes linked to streptavidin, can be used to detect antigen epitope-specific T cells using flow cytometry or magnetic cell enrichment technology to detect extremely low frequency epitope-specific T cells) for monitoring the changes in T cell phenotype that correlate with changes in disease course. Moreover, among the steadily expanding list of AAgs, reactivity is often shared between patients and non-obese diabetic (NOD) mice, the murine model of the disease. Examples include GAD65, insulin, IA-2, PDX1, and CHGA [16,22,23].
The role of AAgs in the pathogenesis of T1DM
Pancreatic beta cell AAgs are the targets of immune responses in T1DM. Therefore, antigen specific immune reactions are believed to be involved in beta cell destruction. The steps in initiation and progression of beta cell destruction are still not clear, but it is generally believed that beta cell AAgs are processed by antigen presenting cells (APC) including macrophages, dendritic cells (DC), or B cells in the pancreatic islets, and presented to naïve T cells in draining pancreatic lymph nodes to generate autoreactive CD4+ T cells and to the lesser degree, T-regulatory cells. These autoreactive CD4+ T cells become activated and then secrete cytokines, which activate beta cell specific cytotoxic CD8+ T cells. The activated T cells are recruited to the pancreatic islets and produce cytokines, which further stimulate macrophages and other T cells, contributing to the destruction of beta cells [23,24]. Thus, beta cell AAgs, macrophages, DCs, B lymphocytes, and T lymphocytes have all been implicated in the pathogenesis of T1DM (Figure 1).
Figure 1.
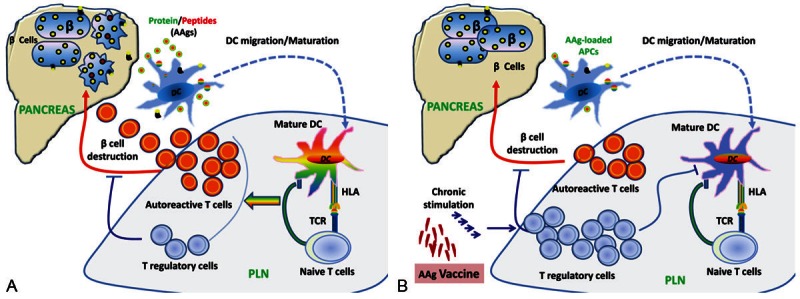
A. AAg-mediated pancreatic beta cell destruction. Unknown environmental and/or immunological insults trigger beta cell AAg shedding into the surroundings. Antigen-presenting cells (APCs, DCs, or B cells) pick up the AAgs and ferry them to the draining pancreatic lymph node (PLN), where they prime naïve autoreactive CD4+ and CD8+ T cells. CD8+ T cells become activated upon engaging MHC-I on AAg-loaded APCs in the presence of autoreactive CD4+ T helper cells and pro-inflammatory cytokines. The autoreactive CD4+ and CD8+ T cells then expand and differentiate into cytotoxic T lymphocytes (CTLs). CTLs migrate into pancreatic islets and damage the beta cells. T regulatory cells are recruited to the PLN and islets in response to antigen and IL-2 and attempt to suppress AAg presentation and T cell activation. B. Antigen-specific immunoregulation. Chronic stimulation by vaccination using soluble antigen-specific peptides from AAgs or DNA vaccine causes low-avidity autoreactive T cells to differentiate into memory-like autoregulatory T cells that suppress both autoreactive CTLs and the presentation of AAgs by DCs, protecting beta cells from further damage.
The pathogenic roles of pancreatic islet AAgs have already been demonstrated in both animal T1DM models and in human T1DM. In animal models, the pathogenic roles for GAD65, insulin, CHGA, and IAPP were confirmed via adoptive transfer of specific T cells or by expression knock-down. For example, GAD65 reactive T cells are diabetogenic in NOD mice [25] and the suppression of GAD65 expression in the beta cells results in the prevention of autoimmune diabetes in NOD mice [26]. The elimination of insulin and insulin reactive T cells prevents diabetes in NOD mice. In particular, expression of insulin B chain amino acids 9-23 (insulin B: 9-23) in pancreatic islets is required for NOD mice to develop anti-islet autoimmunity [27]. In addition, CD4+ T cell clones, which were used for discovery of the new AAgs, CHGA and IAPP, were highly diabetogenic in NOD mice [17,18]. All of the aforementioned evidence supports the pathogenic role of islet AAgs in T1DM mouse models.
Because the above experimental strategies (adoptive transfer of specific T cells or expression knock-down) cannot be applied to humans, there is no convincing evidence to show the pathogenic role of islet AAgs in human T1DM. However, some evidence indirectly indicates that islet AAgs are also involved in disease progression in patients. For example, T cell responses, including proliferation and cytokine secretion to multiple AAgs, are detected in peripheral blood of T1DM patients [20]. This T cell response involves both CD4+ T cells and CD8+ T cells. For example, GAD65 and insulin specific CD4+ T cells have been observed in recently diagnosed diabetic patients and in high-risk subjects, but not in healthy control subjects expressing the same HLA type [28,29]. Also, AAg specific memory CD4+ T cells were found early in progression to autoimmune diabetes [30], and the autoantibodies against insulin and GAD65 correlate with the disease progression in most T1DM patients [30]. Moreover, a clinical trial of heat shock protein 60 peptide vaccine (Diapep277) shows that Diapep277 injection inhibits the inflammatory T cell response to islet antigen and preserves beta cell function [31]. These data indirectly support the idea that islet AAgs may play key roles in human T1DM pathogenesis.
Although many AAgs such as GAD65, insulin, ZnT8, CHGA, and IGRP are already confirmed as T cell targets in T1DM, it is currently unclear whether single or multiple AAgs are required for triggering and exaggerating islet inflammation, and which antigen is most important. Identification of the target AAgs for T cells that play the key role in different stages is very important because manipulation of immune responses to these antigens offers the hope of specifically removing the cells responsible for beta cell destruction. For example, if early AAgs can be identified, this would provide a target for prevention at an earlier stage in the disease process before the majority of beta cells are damaged. Insulin is exclusively expressed in pancreatic beta cells, whereas other AAgs are also expressed in multiple endocrine organs and many studies are focused on exploring the role of insulin in the development of T1DM. Reviews summarize the results from these studies and indicate that insulin is a primary AAg in the development of T1DM [19,27]. In NOD knockout and transgenic models, elimination of several islet AAgs and AAg-specific T cells provides convincing data supporting the primary role of insulin in T1DM. In human T1DM, it is much more difficult to identify autoantigenic targets, especially primary AAgs for initiating early autoimmune attack. Currently, research data only suggest that insulin plays a central role in inducing anti-islet autoimmunity [32], although it is possible that multiple primary AAgs are present and remain to be discovered. One potential candidate as a primary AAg is PDX1 due to its early and exclusive expression in pancreatic progenitors and islet beta cells, but further evidence is required to confirm this. Therefore, it is still unknown which AAg is responsible for initiation of T1DM. Continuing to explore new T cell AAgs that initiate and drive disease progression has the potential to lead to effective methods for preventing or curing T1DM.
The clinical applications for AAgs in T1DM
AAgs can be used in two settings for immunotherapy of T1DM. First, specific assays used to detect autoantibodies against multiple islet AAgs are both diagnostic and predictive markers for T1DM. Second, islet AAgs can be used as therapeutic agents to induce immune tolerance, called antigen-specific immunotherapy.
Currently, major autoantibody markers for T1DM include ICA (islet cell autoantibodies), GADA (GAD65 autoantibodies), IA-2A (insulinoma antigen-2 autoantibodies), IAA (insulin autoantibodies), and ZnT8A (ZnT8 autoantibodies) [7,33]. Islet autoantibody testing can be useful in the following situations: 1) to discriminate T1DM and T2DM, 2) to diagnose acute-onset, ketoacidotic diabetes in obese individuals, 3) to diagnose nonketotic diabetes onset in lean individuals, and 4) to predict and prevent the development of T1DM [7,33]. A combination of GADA and IA-2A for initial screening, followed by ICA and IAA testing has been proposed for prediction of T1DM [33]. Because ZnT8A are present in most patients with T1DM and assay specificity and sensitivity are similar to those for GADA [15], ZnT8A could help to further confirm and predict T1DM. The importance of predicting T1DM is that early treatment could preserve beta cell function, prolong the honeymoon period, prevent the development of microvascular and neuropathic complications, and provide an opportunity for entrance into clinical trials of antigen-specific immunotherapy to prevent T1DM [7].
Antigen-specific immunotherapy has the advantage of focusing treatment on self-reactive T cells without impairing immune responses to unrelated antigens, especially tumor and infectious antigens. Antigen specific immunotherapies have already been applied for preventing and treating T1DM in preclinical models and in patients. Although some immune-modulatory agents showed very promising effects in animal models, none have resulted in stable, long-term insulin independence in human diabetic patients [34,35]. The reasons for this disparity may include: 1) Subgroups of the patients may respond differently. Ludvigsson, et al. found a significant effect of GAD-alum therapy in four subgroups such as male patients, the patients with a baseline tanner pubertal stage of 2 or 3, the patients with a baseline daily insulin dose of 0.398 to 0.605 IU per kilogram of body weight, and the patients from non-Nordic countries (France, Germany, Italy, The Netherlands, Slovenia, Spain, and The United Kingdom) [36]. Thus, AAg therapy benefits some subgroups of patients with T1DM. For prevention, the type of patient is equally important. Discrimination of subgroups depends on screening islet autoantibodies, genetic markers in the HLA region, and some risk factors. Its accuracy is very important for designing immunotherapy; 2) Administration protocols for AAgs require optimization including the type of AAg used, dose, frequency, and administration route [34]; 3) There is a lack of knowledge concerning the role that beta cell AAgs play in islet inflammation and restoration of immunological tolerance; 4) Multi-component vaccines, including several beta cell AAgs and adjuvants, may increase vaccine efficiency [37]; and 5) Combination therapies may be the best approach. The combination may include antigen specific immunotherapy, anti-CD3 antibody, and beta cell regeneration [38,39]. Thus, in order to improve diagnostic and predictive accuracy of islet autoantibodies and preventive and therapeutic efficiency of antigen specific immunotherapy, it is extremely necessary to explore and study the new AAgs that may initiate and drive disease progression.
Overview of the newly discovered T1DM-associated AAgs
Zinc transporter 8 protein (ZnT8)
Zinc ions are essential for the proper storage, secretion, and action of insulin. The zinc content in the pancreatic beta cell is among the highest of the body. Zinc ions are transported from the cytoplasm to insulin secretory vesicles in pancreatic beta cells by the SLC30A zinc transporter 8 protein (ZnT8). ZnT8 [40] is specifically expressed in pancreatic beta cells and has been identified as a novel T1DM autoimmune target [15].
The molecular aspects of ZnT8
ZnT8 is localized to insulin-containing secretary granule membranes and is a pancreatic beta cell specific zinc transporter [40]. ZnT8 is highly conserved evolutionarily, suggesting a central role for zinc transport within pancreatic beta cells. Like other zinc transporters, ZnT8 has six transmembrane domains, as well as cytoplasmic NH2- and COOH-terminal tails. The major B cell autoepitopes are in the cytoplasmic portions of the protein [15]. This is quite interesting because the cytoplasmic portions of the protein are unlikely to be exposed on the cell surface during granule exocytosis. It was speculated that this antigen might encounter immune surveillance after apoptosis of beta cells early in postnatal life or after immune mediated destruction of islets targeted at other cell surface or secreted AAgs. Moreover, it was found that using the entire protein for detection of ZnT8A actually inhibited autoantibody detection compared to NH2-terminal and COOH-terminal constructs and this was likely due to the improper folding of the transmembrane domain in aqueous solution.
The function of ZnT8 and its role in diabetes
The major role of ZnT8 is to provide zinc for insulin maturation and storage processes in insulin-secreting beta cells. Zinc also protects pancreatic beta cells from cytokine induced destruction, which is mostly observed in patients with T1DM, but also in T2DM. In addition, ZnT8 is reported to be associated with beta cell survival [41].
ZnT8 autoantibodies in diabetic patients
ZnT8 was originally identified as an AAg candidate for T1DM from multi-dimensional analysis of microarray mRNA expression profiling experiments and screening using radioimmunoprecipitation assays (RIPA) with recent-onset T1DM and prediabetic sera [15]. ZnT8A was shown to be associated with T1DM and demonstrated to be a good predictive and diagnostic marker in T1DM. 60-80% of recent-onset T1DM patients are positive for ZnT8A, with 4% of cases positive for ZnT8A only [42]. Individuals followed from birth to T1DM onset developed ZnT8A as early as 2 years of age with increasing levels and prevalence persisting to disease onset, which then decline after diagnosis of T1DM [43,44]. The prevalence of ZnT8A is inversely related to the age at T1DM onset with the highest prevalence of 70% in the patients less than 10 years of age [42]. The same correlations also have been found with IA-2A and GADA. However, sera from fulminant T1DM patients was not reactive to ZnT8, indicating that ZnT8A is not a diagnostic marker for fulminant T1DM [41]. It is now widely accepted that ZnT8A testing in combination with other autoantibodies improves the accuracy of disease prediction [33]. Moreover, several studies reported that there is genetic association of specific ZnT8 types in T1DM cases. There are three variants of the AAg at amino acid position 325, ZnT8-R (Arginine), ZnT8-W (Tryptophan), and ZnT8-Q (Glutamine), respectively, and ZnT8A are presented as three types, ZnT8-RAb, ZnT8-WAb, and ZnT8-QAb. Among these three variants, ZnT8-Q is observed rarely. The CC genotype was highly associated with the presence of ZnT8RAb, the TT genotype associated with the ZnT8WAb subtype, and the CT genotype associated with both ZnT8RAb and ZnT8WAb subtypes [41,45,46]. It is interesting that carriers of the CC and CT genotype groups had higher stimulated C–peptide levels the first year after onset compared with those of the TT genotype group [46]. This indicates that the C allele of the SLC30A8 gene is associated with preserved beta cell function in T1DM patients. In addition, the major C allele of the SLC30A8 gene was identified to confer greater risk for T2DM [47]. Thus, investigating and analyzing reactivity of sera with ZnT8A variants will help to improve diagnosis and prognosis of T1DM.
ZnT8 as target of islet reactive T cells in human T1DM
In addition to being a target of autoantibodies, ZnT8 was also identified as a major AAg of disease associated autoreactive CD4+ T cells in human T1DM [48]. Dang et al. found that new-onset T1DM patients who expressed at least one copy of the risk-conferring HLA-DR4/DQ8 and/or DR3/DQ2 haplotypes exhibit a significantly higher frequency of proinflammatory ZnT8 specific T cells in their peripheral blood than do age and HLA matched control subjects, indicating that ZnT8-directed T cell autoimmunity is associated with both the DR4/DQ8 and DR3/DQ2 high risk haplotypes.
Moreover, several studies indicate that ZnT8 is also a major CD8+ T cell recognized AAg [49-51]. Since the autoimmune cascade leading to beta cell destruction in T1DM is propagated by T cell recognition of specific epitopes, epitope mapping may be critical for staging of autoimmunity during the disease, as well as for developing and monitoring immunomodulatory therapies. Thus, exploring T cell epitopes in ZnT8 revealed that ZnT8 (186-194) is an immunodominant CD8+ T cell epitope (60-85% of patients) and ZnT8 (153-161) is a second rank epitope (20-25% of patients) in HLA-A2+ T1DM patients [50]. Another study further confirmed that ZnT8 (153-161) is a CD8+ T cell epitope in human T1DM [51]. Currently, the role of ZnT8 in the pathogenesis of T1DM remains unknown. Because CD8+ T effector cells take center stage in the destruction of pancreatic beta cells and contribute to sustaining islet inflammation, ZnT8 as a major AAg recognized by CD8+ T cells may play a key role in disease progression. Thus, developing and testing novel preventative or therapeutic reagents targeting ZnT8 specific T cells has the potential to arrest the progression of the disease. There is currently no information about ZnT8 as an autoimmune target in T1DM mouse models.
Pancreatic and duodenal homeobox 1 (PDX1)
PDX1, also known as insulin promoter factor 1 (IPF-1), is a transcription factor necessary for pancreatic development, beta cell maturation, and maintenance of beta cell function [52]. In adults, PDX1 is mostly restricted to the beta cells within islets. Recently, we have identified that PDX1 is a novel AAg in both NOD mice and human T1DM patients [16].
The molecular aspects of PDX1
The PDX1 gene consists of two exons encoding a 283 amino acid protein with a predicted molecular mass of 31 kDa in its unmodified form [53]. The PDX1 protein is highly conserved and the protein sequence has close homology among different species [52]. The NH2-terminus contains the transactivation domain and the middle region contains a homeodomain, which is responsible for DNA binding and protein-protein interactions via a transcriptional activation mechanism. Although the role of the COOH-terminus is largely unknown, with some evidence that it may have inhibitory function, we have shown that the COOH-terminus harbors major autoepitopes for T and B cell recognition in NOD mice [16,54]. Moreover, glucose can regulate the insulin gene promoter through activation and nuclear translocation of PDX1 [55,56].
The function of PDX1 and its role in diabetes
It is now widely accepted that PDX1 plays a crucial role in pancreas development and beta cell differentiation, and in maintaining mature beta cell function [52]. During embryonic development, only PDX1+ epithelial cells develop into pancreatic buds, and eventually, the whole pancreas including its exocrine, endocrine, and ductal populations. In adults, PDX1 expression is restricted mainly to beta cells and plays a key role in glucose-dependent insulin secretion. Genetic defects of PDX1 are the cause of maturity-onset diabetes of the young type 4 (MODY4) in humans [57,58]. In mice, PDX1 knockout resulted in pancreas agenesis [57,59,60]. The treatment of streptozotocin-induced diabetic mice with recombinant PDX1 protein promotes beta cell regeneration and transient liver cell reprogramming, leading to restoration of normoglycemia [61]. Besides the role as a transcription factor for regulating insulin gene expression, PDX1 was also found to have immunomodulatory function when given to diabetic mice. Shternhall-Ron et al. found that in NOD mice treated with PDX1 delivered by an adenovirus vector, the autoimmune T cell profile shifted from Th1 to Th2 and was accompanied by a down regulated autoimmune attack [62]. Furthermore, our own study found that treatment of prediabetic female NOD mice with recombinant PDX1 [61] or with a non-functional mutant PDX1 protein (unpublished observation) prevented the onset of diabetes. The aforementioned evidence indicates that PDX1 is involved in immunoregulation.
PDX1 autoantibodies (PAA) in NOD mice and in patients with T1DM
PAA were originally detected in NOD mice using ELISA, western blotting, and radioimmunoprecipitation of [35S]–labeled insulinoma cell line derived PDX1 protein [16]. PAA were detected as early as 5 weeks of age, generally peaked before the onset of clinically overt diabetes, and often decreased to lower positive levels or disappeared completely after the onset of diabetes. Furthermore, the immunodominant B cell autoepitopes were located to the COOH-terminus of the PDX1 protein (p200-283). PAA were also detected in the serum of human T1DM patients and two majors B cell epitopes were identified within amino acids 159-200 and 200-283. These two epitopes contain numerous trypsin sensitive (arginine or lysine) sites.
Trypsin sensitive arginine or lysine residues are critical for the antigenicity of epitopes recognized by anti-histone antibodies [63] and basic amino acids are believed to be important for autoantibody recognition of the U1-70K component of the U1 small nuclear ribonucleoprotein (U1snRNP) [64]. In this regard, it is interesting to note that NOD mice also produce autoantibodies against U1snRNP [65]. The relationship between these autoantibodies and PAA remains to be determined. However, it has been proposed that long runs of charged amino acids may render certain self-antigens immunogenic [66]. In addition, autoantibodies frequently recognize epitopes located on the NH2- and COOH-termini of proteins [67], as was the case here.
We recently developed a liquid-phase luciferase immunoprecipitation systems (LIPS) assay for detecting PAA, GADA, IA-2A, and IA-2βA autoantibodies and demonstrated a similar sensitivity and specificity to the clinical RIPA using the clinical results from GADA and IA-2A detection. Using the LIPS assay, PAA were detected in human serum samples (Donelan et al., IJCEP, in press). For future studies, the focus will be to survey the prevalence of PAA in the normal population, T1DM patients, high-risk or prediabetic populations, and in other diseases. This will help to evaluate the clinical value of PAA for prediction, diagnosis, or monitoring of T1DM.
T cell reactivity to PDX1 in NOD mice
PDX1 was also found to be a target of cellular immunity in NOD mice [16]. The location of the T cell epitope was explored and may lie within the same COOH-terminal region as the B cell epitope. Moreover, overlap between autoreactive B cell and T cell epitopes has also been found for GAD65 [68,69] and IA-2 AAgs [70]. The proximity of B and T cell epitopes within the antigen structure suggests that antigen/antibody complexes may influence antigen processing by accessory cells, thereby affecting T cell reactivity [68]. Furthermore, we have successfully isolated PDX1-specific CD4+ T cell clones and characterized one clone (2E3, see Figure 2) from prediabetic NOD mice after PDX1 immunization. Although these T cell clones’ pathogenic roles are not yet characterized, it suggests that PDX1 may be a CD4+ T cell target in the NOD mouse model. Whether PDX1 is targeted by T cells in human T1DM patients remains to be investigated in future studies.
Figure 2.
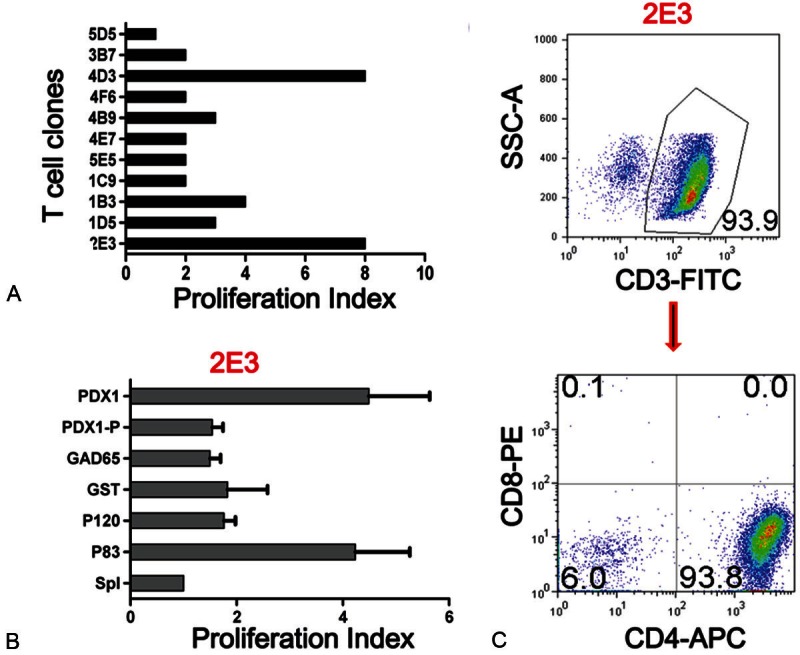
PDX1 is a CD4+ T cell target in NOD mice. Female NOD mice, 6-8 weeks of age, were injected subcutaneously at the base of tail with PDX1 (50 μg/mouse) mixed with CFA. 7 days later, the mice were sacrificed. The draining lymph nodes and spleen cells were harvested for assay of PDX1 specificity, and then PDX1-specific T cell lines and clones were prepared through repeated stimulation with PDX1 loaded irradiated syngeneic splenic cells. Using this protocol, several clones were obtained from NOD mice. A. Proliferative response of 10 representative T cell clones to PDX1 using the H3TdR incorporation assay. These T cell clones (5x104/well) were separately co-cultured with PDX1 protein loaded or non-loaded irradiated syngeneic spleen cells (5x105/well) in a 96-well U bottom plate for 72 hours, and H3TdR was added in the last 6 hours. B. Specific proliferation response of clone 2E3. T cell clone 2E3 was separately stimulated by PDX1 protein, PDX1 peptide, GAD65, GST, P120, P83, or SpI loaded irradiated syngeneic spleen cells and T cell proliferation was measure using the H3TdR incorporation assay. C. The phenotype of T cell clone 2E3 with flow cytometry staining for CD3, CD4, and CD8.
Chromogranin A (CHGA)
CHGA is a member of the granin family of neuroendocrine secretory proteins [71]. It is the precursor to several peptides including vasostatin-1, pancreastatin, catestatin, chromostatin, and WE-14. WE-14 and vasostatin-1 derived peptide CHGA29-42 were identified as the antigenic peptides for highly diabetogenic CD4+ T cell clones from NOD mice [17,72].
The molecular aspects of CHGA
CHGA is located in secretory vesicles of neurons and endocrine cells. Human CHGA is a 439 amino acid protein stored in the secretory granules of many normal and neoplastic cells of the diffuse neuroendocrine system [73]. Comparison of the protein sequences of human and bovine CHGA shows high conservation of NH2-terminal and COOH-terminal domains and also in the potential dibasic cleavage sites [74]. CHGA is processed differently in various cell types to yield several biologically active peptides including vasostatin-1, pancreastatin, catestatin, and serpinin, which have a variety of different functions [71].
The relationship of CHGA to T1DM
CHGA is regarded as a major, nonspecific gastroenteropancreatic neuroendocrine tumor marker [75]. Like other granins, CHGA has a granulogenic role in secretory granule biogenesis and is stored in these organelles. CHGA is exocytotically released, together with hormones, first into the extracellular environment and then into the circulation. Increased levels of circulating CHGA have been detected in patients with neuroendocrine tumors, non-small cell lung cancer, prostate, and breast cancer [73]. Moreover, elevated serum CHGA levels were also detected in patients with heart failure, renal failure, hypertension, rheumatoid arthritis, sepsis, and other inflammatory diseases. Vasostatin-1 is a fragment of CHGA spanning the NH2-terminal residues 1-78. It can inhibit a series of effects induced by VEGF on endothelial cells [73]. WE-14 is a natural 14 amino acid cleavage product of CHGA and has been identified in pancreatic islet beta cells and in other gastroentero pancreatic tissues, such as the adrenal gland [17]. Its function remains unknown.
CHGA as target of highly diabetogenic CD4+ T cell clones in mouse T1DM
Using a biochemical purification strategy combined with mass spectrometric analysis of chromatographic fractions antigenic for diabetogenic T cell clones, CHGA was found to be a source of antigenic ligands for diabetogenic CD4+ T cells [17]. These antigenic ligands include WE-14 and vasostatin-1 derived peptide CHGA29-42, and they both could bind IAg7 and stimulate BDC T cell clones which include BDC-2.5. BDC clones were isolated from the spleens and lymph nodes of NOD mice and can initiate or accelerate diabetes in vivo. Compared with WE-14, CHGA29-42 has better binding affinity to IAg7. However, antigenicity of the WE-14 peptide for BDC-2.5 is altered by posttranslational modification. The modified WE-14 found in beta cell extracts is far more potent than the unmodified one in stimulating BDC-2.5 T cells. Furthermore, studies on T cells from human T1DM patients indicate similar responsiveness to WE14, with a significant difference in responses to the modified peptide between patients and controls [22]. This indicates that post-translational modification plays a key role in the generation of AAg peptide WE-14 during disease progression of T1DM. In fact, post-translational modification is a well-established mechanism for the generation of new AAgs and is relevant to the development of autoimmune disease [76]. In T1DM, a previous study [77] on CD4+ T cell from human patients showed that oxidative modification of the cysteines is required for T cell recognition of a peptide from the insulin A chain, A1-14. Post-translational modifications can occur spontaneously or as the results of an ordered enzymatic process. Certain cellular processes such as aging, disease such as diabetes, inflammation, and trauma are known to increase the frequency of post-translational modifications [76]. Thus, post-translational modifications in self-antigens, such as insulin and WE-14, may affect the analyses of B and T cell specificity, current diagnostic techniques, and the development of immunotherapies for T1DM.
T cell responses to CHGA have already been documented in mice [17]. However, it is unclear if this occurs in human T1DM. In addition, autoantibodies to CHGA have not been reported in humans or mice with T1DM. Recently, we have developed a LIPS assay to detect CHGA and validated the assay with commercial anti-CHGA antibodies. Using the LIPS assay, we have screened human serum samples from the normal population (n=100) and patients with recent-onset (n=100) and long-standing (n=50) T1DM and failed to detect autoantibodies against CHGA (unpublished data). Since autoantibodies to CHGA might be transient during the development of T1DM and disappear at the clinical onset of diabetes, future studies may focus on detection of autoantibodies in prediabetic patients or high-risk populations.
Islet amyloid polypeptide (IAPP)
IAPP, or amylin, is a 37 amino acid peptide hormone and is one of the major secretory products of beta cells, co-secreted with insulin [78,79]. It plays a role in glycemic regulation with putative function both locally in the islets, where it inhibits insulin and glucagon secretion, and at distant targets in prevention of post-prandial spikes in blood glucose levels. Aggregated IAPP has cytotoxic properties and is known as a component of the amyloid plaques found in the pancreatic islets of patients with T2DM [79] and also in pancreatic islets transplanted into individuals with T1DM [79]. Autoantibodies to IAPP have been previously identified in human T1DM patients, but without correlation to disease [80]. Recently, IAPP was demonstrated to be a target antigen for diabetogenic CD4+ T cell clone BDC-5.2.9, from NOD mice [18].
The molecular aspects of IAPP
IAPP is processed from proislet amyloid polypeptide (ProIAPP) [79]. ProIAPP is produced as a 67 amino acid propeptide and undergoes post translational modifications, including protease cleavage, to produce IAPP. IAPP is strongly conserved but with notable variation in the 20-29 region [81]. IAPP and insulin genes contain similar promoter elements and the transcription factor PDX1 regulates both genes in response to glucose [79]. In addition to islet beta cells, IAPP was also found in delta cells in the rat and mouse, and in the gastrointestinal tract of the rat, mouse, cat, and human [79].
The relationship of IAPP to T1DM
IAPP is colocalized to beta cell granules and co-secreted into the blood stream with insulin in response to glucose and amino acid stimulated insulin secretion [79]. Likewise, IAPP hyposecretion has been observed in T1DM. Moreover, it was found that synthetic IAPP injection together with insulin improved glycemic control by suppressing glucagon secretion [82].
IAPP as target of highly diabetogenic CD4+ T cell clones in mouse T1DM
The activation of autoreactive CD4+ T cells is a major event in autoimmune disease such as T1DM. IAPP is a protein located within secretory granules of beta cells, as is insulin, CHGA, and ZnT8, that is found to be targeted by pathogenic CD4+ T cells [18]. Similar to insulin and CHGA, IAPP is produced from a large precursor (ProIAPP), which is post-translationally processed within the secretory granules [79]. There is currently no study examining whether human CD4+ T cells react to IAPP peptide. Because the sequence of IAPP is quite similar between mouse and human, with only one amino acid difference, it is possible that IAPP peptide is also the target of human CD4+ T cells, and this should be investigated in future studies.
In addition, IAPP5-13 and IAPP9-17 were reported to be recognized in the context of HLA-A2 by peripheral blood mononuclear cells form T1DM patients and at risk individuals [83,84]. This demonstrated that IAPP is a CD8+ T cell recognized antigen in human T1DM. Future studies will be required to determine if there are autoantibodies to IAPP in human T1DM patients.
Concluding remarks
Because of their important theoretical and practical relevance to T1DM, there has been considerable effort to identify and characterize the AAgs involved in T1DM. Here, we mainly discuss four newly discovered AAgs: ZnT8, PDX1, CHGA, and IAPP (see Table 1). Many other AAgs related to T1DM have previously been identified, supporting the idea that immune responses to multiple antigens contribute to the pathogenesis of T1DM. CD4+ and CD8+ T cell recognition of islet beta cell primary AAgs may be the key initiation step in the autoimmune cascade. After the disease process is initiated, inter-molecular and intra-molecular epitope spreading occurs, involving more AAgs as the islet cell damage is perpetuated. It is very interesting that two new AAgs, PDX1 and IAPP, have a close relationship to insulin. PDX1, the master pancreatic transcription factor, controls the expression of insulin and IAPP, suggesting that intra-molecular epitope spreading has its own selectivity and may not be a random process. Discovery of beta-cell-specific CD4+ and CD8+ T cell epitopes is an important area of T1DM research. This should aid in our understanding of the disease pathogenesis and in the development of new strategies for monitoring disease development and progression, as well as for developing therapeutic interventions. It is expected that more T1DM related AAgs and T cell epitopes will be discovered in the future.
Table 1.
Summary of Novel AAgs targeted by the adaptive immune system in T1DM
| AAg | Tissue Distribution | Mouse | Human | References | ||
|---|---|---|---|---|---|---|
|
| ||||||
| AAbs | T cells | AAbs | T cells | |||
| ZnT8 | Beta cells | Unknown | Unknown | Yes | CD4, CD8 | 15, 48, 49, 50, 51 |
| PDX1 | Beta cells | Yes | CD4 | Yes | Unknown | 16, Figure 2 |
| CHGA | Endocrine cells | Unknown | CD4 | Unknown | Unknown | 17, 72 |
| IAPP | Islets | Unknown | CD4 | Yes | CD8 | 18, 80, 84 |
Acknowledgements
This work was supported in part by grants from the National Institutes of Health, NIDDK DK064054 and DK071831 (to LJ Yang) and NIH-T32 (W.R.).
References
- 1.Van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Gianani R, Nakayama M, Liu E, Kobayashi M, Baschal E, Yu L, Babu S, Dawson A, Johnson K, Jahromi M, Aly T, Fain P, Barker J, Rewers M, Eisenbarth GS. Type 1 diabetes: chronic progressive autoimmune disease. Novartis Found Symp. 2008;292:85–94. doi: 10.1002/9780470697405.ch7. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Eisenbarth GS. Prediction and prevention of Type 1 diabetes mellitus. J Diabetes. 2011;3:48–57. doi: 10.1111/j.1753-0407.2010.00102.x. [DOI] [PubMed] [Google Scholar]
- 4.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, Roep BO, von Herrath MG. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012 Jan 16;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reijonen H, Novak EJ, Kochik S, Heninger A, Liu AW, Kwok WW, Nepom GT. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 2002;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 6.Lazzarotto F, Basso D, Plebani M, Moscon A, Zanchetta R, Betterle C. Celiac disease and type 1 diabetes. Diabetes Care. 2003;26:248–249. doi: 10.2337/diacare.26.1.248. [DOI] [PubMed] [Google Scholar]
- 7.Winter WE, Harris N, Schatz D. Type 1 diabetes islet autoantibody markers. Diabetes Technol Ther. 2002;4:817–839. doi: 10.1089/152091502321118838. [DOI] [PubMed] [Google Scholar]
- 8.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974 Nov 30;2:1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 9.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983 Dec 23;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 10.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347:151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 11.Bonifacio E, Lampasona V, Bingley PJ. IA-2 (islet cell antigen 512) is the primary target of humoral autoimmunity against type 1 diabetes-associated tyrosine phosphatase autoantigens. J Immunol. 1998 Sep 1;161:2648–2654. [PubMed] [Google Scholar]
- 12.Birk OS, Elias D, Weiss AS, Rosen A, van der Zee R, Walker MD, Cohen IR. NOD mouse diabetes: the ubiquitous mouse hsp60 is a beta-cell target antigen of autoimmune T cells. J Autoimmun. 1996;9:159–166. doi: 10.1006/jaut.1996.0019. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Danke NA, Berger D, Reichstetter S, Reijonen H, Greenbaum C, Pihoker C, James EA, Kwok WW. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J Immunol. 2006 Mar 1;176:2781–2789. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- 14.Arden SD, Roep BO, Neophytou PI, Usac EF, Duinkerken G, de Vries RR, Hutton JC. Imogen 38: a novel 38-kD islet mitochondrial autoantigen recognized by T cells from a newly diagnosed type 1 diabetic patient. J Clin Invest. 1996 Jan 15;97:551–561. doi: 10.1172/JCI118448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007 Oct 23;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li SW, Koya V, Li Y, Donelan W, Lin P, Reeves WH, Yang LJ. Pancreatic duodenal homeobox 1 protein is a novel beta-cell-specific autoantigen for type I diabetes. Lab Invest. 2010;90:31–39. doi: 10.1038/labinvest.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler J, Haskins K. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delong T, Baker RL, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Barbour G, Bradley B, Haskins K. Islet amyloid polypeptide is a target antigen for diabetogenic CD4+ T cells. Diabetes. 2011;60:2325–2330. doi: 10.2337/db11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasinski JM, Eisenbarth GS. Insulin as a primary autoantigen for type 1A diabetes. Clin Dev Immunol. 2005;12:181–186. doi: 10.1080/17402520500078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallone R, Brezar V, Boitard C. T cell recognition of autoantigens in human type 1 diabetes: clinical perspectives. Clin Dev Immunol. 2011;2011:513210. doi: 10.1155/2011/513210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med. 2012;2:a007781. doi: 10.1101/cshperspect.a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haskins K, Cooke A. CD4 T cells and their antigens in the pathogenesis of autoimmune diabetes. Curr Opin Immunol. 2011;23:739–745. doi: 10.1016/j.coi.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadinski B, Kappler J, Eisenbarth GS. Molecular targeting of islet autoantigens. Immunity. 2010 Apr 23;32:446–456. doi: 10.1016/j.immuni.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580–591. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 25.Zekzer D, Wong FS, Ayalon O, Millet I, Altieri M, Shintani S, Solimena M, Sherwin RS. GAD-reactive CD4+ Th1 cells induce diabetes in NOD/SCID mice. J Clin Invest. 1998 Jan 1;101:68–73. doi: 10.1172/JCI119878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon JW, Yoon CS, Lim HW, Huang QQ, Kang Y, Pyun KH, Hirasawa K, Sherwin RS, Jun HS. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in beta cells. Science. 1999 May 14;284:1183–1187. doi: 10.1126/science.284.5417.1183. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama M. Insulin as a key autoantigen in the development of type 1 diabetes. Diabetes Metab Res Rev. 2011;27:773–777. doi: 10.1002/dmrr.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honeyman MC, Harrison LC, Drummond B, Colman PG, Tait BD. Analysis of families at risk for insulin-dependent diabetes mellitus reveals that HLA antigens influence progression to clinical disease. Mol Med. 1995;1:576–582. [PMC free article] [PubMed] [Google Scholar]
- 29.Panina-Bordignon P, Lang R, van Endert PM, Benazzi E, Felix AM, Pastore RM, Spinas GA, Sinigaglia F. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med. 1995 May 1;181:1923–1927. doi: 10.1084/jem.181.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oling V, Reijonen H, Simell O, Knip M, Ilonen J. Autoantigen-specific memory CD4+ T cells are prevalent early in progression to Type 1 diabetes. Cell Immunol. 2012;273:133–139. doi: 10.1016/j.cellimm.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001 Nov 24;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 32.Wong FS. Insulin--a primary autoantigen in type 1 diabetes? Trends Mol Med. 2005;11:445–448. doi: 10.1016/j.molmed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem. 2011;57:168–175. doi: 10.1373/clinchem.2010.148205. [DOI] [PubMed] [Google Scholar]
- 34.Ludvigsson J. In light of recent clinical trial results, what lies next for Type 1 diabetes vaccine research? Expert Rev Vaccines. 2012;11:263–265. doi: 10.1586/erv.11.194. [DOI] [PubMed] [Google Scholar]
- 35.Phillips B, Trucco M, Giannoukakis N. Current state of type 1 diabetes immunotherapy: incremental advances, huge leaps, or more of the same? Clin Dev Immunol. 2011;2011:432016. doi: 10.1155/2011/432016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ludvigsson J, Krisky D, Casas R, Battelino T, Castano L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, Veeze HJ, Palmer J. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012 Feb 2;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 37.Nicholas D, Odumosu O, Langridge WH. Autoantigen based vaccines for type 1 diabetes. Discov Med. 2011;11:293–301. [PMC free article] [PubMed] [Google Scholar]
- 38.Boettler T, von Herrath M. Immunotherapy of type 1 diabetes--how to rationally prioritize combination therapies in T1D. Int Immunopharmacol. 2010;10:1491–1495. doi: 10.1016/j.intimp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Todd JA, Knip M, Mathieu C. Strategies for the prevention of autoimmune type 1 diabetes. Diabet Med. 2011;28:1141–1143. doi: 10.1111/j.1464-5491.2011.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chimienti F, Favier A, Seve M. ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals. 2005;18:313–317. doi: 10.1007/s10534-005-3687-9. [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki E. ZnT8 and type 1 diabetes [Review] . Endocr J. 2012 Jul 31;59:531–7. doi: 10.1507/endocrj.ej12-0069. [DOI] [PubMed] [Google Scholar]
- 42.Howson JM, Krause S, Stevens H, Smyth DJ, Wenzlau JM, Bonifacio E, Hutton J, Ziegler AG, Todd JA, Achenbach P. Genetic association of zinc transporter 8 (ZnT8) autoantibodies in type 1 diabetes cases. Diabetologia. 2012;55:1978–1984. doi: 10.1007/s00125-012-2540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaziri-Sani F, Oak S, Radtke J, Lernmark K, Lynch K, Agardh CD, Cilio CM, Lethagen AL, Ortqvist E, Landin-Olsson M, Torn C, Hampe CS. ZnT8 autoantibody titers in type 1 diabetes patients decline rapidly after clinical onset. Autoimmunity. 2010;43:598–606. doi: 10.3109/08916930903555927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenzlau JM, Walter M, Gardner TJ, Frisch LM, Yu L, Eisenbarth GS, Ziegler AG, Davidson HW, Hutton JC. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J Clin Endocrinol Metab. 2010;95:4712–4719. doi: 10.1210/jc.2010-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen ML, Vaziri-Sani F, Delli A, Porksen S, Jacobssen E, Thomsen J, Svensson J, Steen Petersen J, Hansen L, Lernmark A, Mortensen HB, Nielsen LB. Association between autoantibodies to the Arginine variant of the Zinc transporter 8 (ZnT8) and stimulated C-peptide levels in Danish children and adolescents with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2012 Sep;13:454–62. doi: 10.1111/j.1399-5448.2012.00857.x. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen LB, Vaziri-Sani F, Porksen S, Andersen ML, Svensson J, Bergholdt R, Pociot F, Hougaard P, de Beaufort C, Castano L, Mortensen HB, Lernmark A, Hansen L. Relationship between ZnT8Ab, the SLC30A8 gene and disease progression in children with newly diagnosed type 1 diabetes. Autoimmunity. 2011;44:616–623. doi: 10.3109/08916934.2011.576724. [DOI] [PubMed] [Google Scholar]
- 47.Tabara Y, Osawa H, Kawamoto R, Onuma H, Shimizu I, Makino H, Kohara K, Miki T. Genotype risk score of common susceptible variants for prediction of type 2 diabetes mellitus in Japanese: the Shimanami Health Promoting Program (J-SHIPP study). Development of type 2 diabetes mellitus and genotype risk score. Metabolism. 2011;60:1634–1640. doi: 10.1016/j.metabol.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Dang M, Rockell J, Wagner R, Wenzlau JM, Yu L, Hutton JC, Gottlieb PA, Davidson HW. Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol. 2011 May 15;186:6056–6063. doi: 10.4049/jimmunol.1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enee E, Kratzer R, Arnoux JB, Barilleau E, Hamel Y, Marchi C, Beltrand J, Michaud B, Chatenoud L, Robert JJ, van Endert P. ZnT8 Is a Major CD8+ T Cell-Recognized Autoantigen in Pediatric Type 1 Diabetes. Diabetes. 2012;61:1779–1784. doi: 10.2337/db12-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scotto M, Afonso G, Larger E, Raverdy C, Lemonnier FA, Carel JC, Dubois-Laforgue D, Baz B, Levy D, Gautier JF, Launay O, Bruno G, Boitard C, Sechi LA, Hutton JC, Davidson HW, Mallone R. Zinc transporter (ZnT)8(186-194) is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia. 2012;55:2026–2031. doi: 10.1007/s00125-012-2543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Xu X, Gu R, Wang Z, Chen H, Xu K, Zhang M, Hutton J, Yang T. Prediction of HLA class I-restricted T-cell epitopes of islet autoantigen combined with binding and dissociation assays. Autoimmunity. 2012;45:176–185. doi: 10.3109/08916934.2011.622014. [DOI] [PubMed] [Google Scholar]
- 52.Hui H, Perfetti R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002;146:129–141. doi: 10.1530/eje.0.1460129. [DOI] [PubMed] [Google Scholar]
- 53.Stoffel M, Stein R, Wright CV, Espinosa R 3rd, Le Beau MM, Bell GI. Localization of human homeodomain transcription factor insulin promoter factor 1 (IPF1) to chromosome band 13q12.1. Genomics. 1995 Jul 1;28:125–126. doi: 10.1006/geno.1995.1120. [DOI] [PubMed] [Google Scholar]
- 54.Al-Quobaili F, Montenarh M. Pancreatic duodenal homeobox factor-1 and diabetes mellitus type 2 (review) Int J Mol Med. 2008;21:399–404. [PubMed] [Google Scholar]
- 55.Macfarlane WM, McKinnon CM, Felton-Edkins ZA, Cragg H, James RF, Docherty K. Glucose stimulates translocation of the homeodomain transcription factor PDX1 from the cytoplasm to the nucleus in pancreatic beta-cells. J Biol Chem. 1999 Jan 8;274:1011–1016. doi: 10.1074/jbc.274.2.1011. [DOI] [PubMed] [Google Scholar]
- 56.Marshak S, Totary H, Cerasi E, Melloul D. Purification of the beta-cell glucose-sensitive factor that transactivates the insulin gene differentially in normal and transformed islet cells. Proc Natl Acad Sci U S A. 1996 Dec 24;93:15057–15062. doi: 10.1073/pnas.93.26.15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoffers DA, Zinkin NT, Stanojevic V, Clarke W L, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 58.Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 59.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994 Oct 13;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 60.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 61.Koya V, Lu S, Sun YP, Purich DL, Atkinson MA, Li SW, Yang LJ. Reversal of streptozotocin-induced diabetes in mice by cellular transduction with recombinant pancreatic transcription factor pancreatic duodenal homeobox-1: a novel protein transduction domain-based therapy. Diabetes. 2008;57:757–769. doi: 10.2337/db07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shternhall-Ron K, Quintana FJ, Perl S, Meivar-Levy I, Barshack I, Cohen IR, Ferber S. Ectopic PDX-1 expression in liver ameliorates type 1 diabetes. J Autoimmun. 2007;28:134–142. doi: 10.1016/j.jaut.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Thomas JO, Wilson CM, Hardin JA. The major core histone antigenic determinants in systemic lupus erythematosus are in the trypsin-sensitive regions. FEBS Lett. 1984 Apr 9;169:90–96. doi: 10.1016/0014-5793(84)80295-1. [DOI] [PubMed] [Google Scholar]
- 64.James JA, Scofield RH, Harley JB. Basic amino acids predominate in the sequential autoantigenic determinants of the small nuclear 70K ribonucleoprotein. Scand J Immunol. 1994;39:557–566. doi: 10.1111/j.1365-3083.1994.tb03413.x. [DOI] [PubMed] [Google Scholar]
- 65.Baxter AG, Healey D, Cooke A. Mycobacteria precipitate autoimmune rheumatic disease in NOD mice via an adjuvant-like activity. Scand J Immunol. 1994;39:602–606. doi: 10.1111/j.1365-3083.1994.tb03419.x. [DOI] [PubMed] [Google Scholar]
- 66.Brendel V, Dohlman J, Blaisdell BE, Karlin S. Very long charge runs in systemic lupus erythematosus-associated autoantigens. Proc Natl Acad Sci U S A. 1991 Feb 15;88:1536–1540. doi: 10.1073/pnas.88.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollard KM, Cohen MG. Predicting antigenic determinants of autoantigens. Autoimmunity. 1990;5:265–275. doi: 10.3109/08916939009014711. [DOI] [PubMed] [Google Scholar]
- 68.Fenalti G, Hampe CS, Arafat Y, Law RH, Banga JP, Mackay IR, Whisstock JC, Buckle AM, Rowley MJ. COOH-terminal clustering of autoantibody and T-cell determinants on the structure of GAD65 provide insights into the molecular basis of autoreactivity. Diabetes. 2008;57:1293–1301. doi: 10.2337/db07-1461. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Purdy LE, Rabinovitch S, Jevnikar AM, Elliott JF. Major DQ8-restricted T-cell epitopes for human GAD65 mapped using human CD4, DQA1*0301, DQB1*0302 transgenic IA(null) NOD mice. Diabetes. 1999;48:469–477. doi: 10.2337/diabetes.48.3.469. [DOI] [PubMed] [Google Scholar]
- 70.Dromey JA, Weenink SM, Peters GH, Endl J, Tighe PJ, Todd I, Christie MR. Mapping of epitopes for autoantibodies to the type 1 diabetes autoantigen IA-2 by peptide phage display and molecular modeling: overlap of antibody and T cell determinants. J Immunol. 2004 Apr 1;172:4084–4090. doi: 10.4049/jimmunol.172.7.4084. [DOI] [PubMed] [Google Scholar]
- 71.Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64:2863–2886. doi: 10.1007/s00018-007-7254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nikoopour E, Sandrock C, Huszarik K, Krougly O, Lee-Chan E, Masteller EL, Bluestone JA, Singh B. Cutting edge: vasostatin-1-derived peptide ChgA29-42 is an antigenic epitope of diabetogenic BDC2.5 T cells in nonobese diabetic mice. J Immunol. 2011 Apr 1;186:3831–3835. doi: 10.4049/jimmunol.1003617. [DOI] [PubMed] [Google Scholar]
- 73.Loh YP, Cheng Y, Mahata SK, Corti A, Tota B. Chromogranin A and Derived Peptides in Health and Disease. J Mol Neurosci. 2012 Mar 3;48:347–56. doi: 10.1007/s12031-012-9728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konecki DS, Benedum UM, Gerdes HH, Huttner WB. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987 Dec 15;262:17026–17030. [PubMed] [Google Scholar]
- 75.Singh S, Law C. Chromogranin A: a sensitive biomarker for the detection and post-treatment monitoring of gastroenteropancreatic neuroendocrine tumors. Expert Rev Gastroenterol Hepatol. 2012;6:313–334. doi: 10.1586/egh.12.15. [DOI] [PubMed] [Google Scholar]
- 76.Doyle HA, Mamula MJ. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr Opin Immunol. 2012;24:112–118. doi: 10.1016/j.coi.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mannering SI, Harrison LC, Williamson NA, Morris JS, Thearle DJ, Jensen KP, Kay TW, Rossjohn J, Falk BA, Nepom GT, Purcell AW. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J Exp Med. 2005 Nov 7;202:1191–1197. doi: 10.1084/jem.20051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stridsberg M, Wilander E. Islet amyloid polypeptide (IAPP). A short review. Acta Oncol. 1991;30:451–456. doi: 10.3109/02841869109092400. [DOI] [PubMed] [Google Scholar]
- 79.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 80.Clark A, Yon SM, de Koning EJ, Holman RR. Autoantibodies to islet amyloid polypeptide in diabetes. Diabet Med. 1991;8:668–673. doi: 10.1111/j.1464-5491.1991.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 81.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomita T. Islet amyloid polypeptide in pancreatic islets from type 1 diabetic subjects. Islets. 2011;3:166–174. doi: 10.4161/isl.3.4.15875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panagiotopoulos C, Qin H, Tan R, Verchere CB. Identification of a beta-cell-specific HLA class I restricted epitope in type 1 diabetes. Diabetes. 2003;52:2647–2651. doi: 10.2337/diabetes.52.11.2647. [DOI] [PubMed] [Google Scholar]


