Abstract
Abstract: Having previously shown that levels of the citrullinated vimentin peptide VICM are raised in liver fibrosis in rats, we aimed to investigate whether inhibition of citrullination as measured by VICM levels could affect fibrogenesis. Methods: Fibrogenesis was evaluated by quantitative histology and circulating levels of collagen type III in a carbon tetrachloride (CCl4) rat model of liver fibrosis for 6 weeks (n=40+10 untreated controls). The first treatment group (n=20) was treated exclusively with CCl4 for the duration of the study.The second treatment group (n=20) was additionally treated, for the same period, with N-a-benzoyl-N5-(2 Chloro-1-iminoethyl)-L-Ornithine amide, a known PAD inhibitor. Results: All 40 CCl4 treated animals showed a statistically significant increase in total collagen (p<0.0001) and C3M levels (p<0.001) compared with controls assessed by quantitative histology. Animals additionally treated with the PAD inhibitor showed a statistically significant increase when compared with controls for both total collagen (p<0.001) and C3M levels (p<0.0001) but no statistically difference when compared with animals treated only with CCl4. The mean systemic level of VICM in control animals was 115 ng/ml at 6 weeks. In CCl4-treated animals, mean systemic VICM levels increased 324% at week 6 (p<0.001). The mean level of the marker in CCl4-treated rats was not statistically significant from that in controls (P>0.05). In PAD-treated animals VICM levels were 51% (P<0.05) lower than in non-PAD CCl4-treated animals. Conclusion: The PAD inhibitor did not reduce fibrogenesis in this preclinical model. However circulating VICM marker levels were decreased in the presence of the PAD inhibitor.
Keywords: Biomarker, citrulline, PAD inhibitor, VICM, vimentin, liver fibrosis, CCl4
Introduction
Post-translational modifications (PTMs) of proteins are non-DNA coded modifications that, as their name implies, occur after translation takes place and can amplify both the structural and functional diversity of the proteome [1]. PTMs of collagens are of great importance for the formation of a stable extracellular matrix (ECM) structure [2].
Citrullination or deimination is a PTM which enzymatically converts the amino acid arginine into citrulline in the presence of calcium [3]. The arginine substitution is irreversible and induces a decrease in the charge of the modified proteins, which shifts proteins towards a basic pH and has a direct impact on the protein’s structure and function [4]. The reaction also reduces the mass of the protein by 1 Da for each arginine substituted in the peptide chain [1]. Citrullination is catalysed by a family of enzymes called peptidylarginine deiminases (PAD). This family consist of 6 members (PAD-1, -2, -3, -4, -5, -6) all of which are calcium dependent. They are expressed in a variety of different tissues and can act on a range of different substrates including nuclear and cytoskeletal [1,5].
Citrullination influences both intra- and inter-molecular interactions and has been shown to make proteins prone to proteolytic degradation. Under physiologic conditions, calcium levels are too low to promote PAD activity. It is therefore suggested that loss of calcium homeostasis and subsequent PAD activation and citrullination may be of importance in the transition from physiology to pathology [6,7]. An alternative theory proposes that PAD enzymes may be regulated by additional, non-calcium related, factors and therefore may also be able to catalyse the citrullination reaction at physiologic concentrations of calcium [1].
We have previously demonstrated that the recently introduced matrix metalloproteinase (MMP)-degraded citrullinated vimentin marker (VICM) is related to liver fibrosis progression in a CCl4 model of liver fibrosis and is statistically significantly upregulated in hepatitis C and NAFLD clinical populations [8]. In this experiment we aimed to investigate whether VICM levels are associated with PAD enzyme levels in a preclinical liver fibrosis model. We therefore measured circulating VICM levels in animals that were treated with CCl4 and N-Alpha-benzoyl-N5-I-Ornithine, a recently described pan-PAD inhibitor [9].
Methods
ELISA assay
The VICM assay procedure previously described [8] was followed. Briefly, a 96-well streptavidin plate (Roche Diagnostics, Basel, Switzerland) was coated with 2.5 ng of the biotinylated synthetic peptide, Biotin-RLRSSVPGV-Citrulline, dissolved in assay buffer (50 mM Tris, 1% BSA, 0.1% Tween-20, 0.36% Bronidox, adjusted to pH 7.4 at 20°C) and incubated for 30 minutes at 20°C. Twenty µL of the peptide calibrator or sample was added to appropriate wells, followed by 100 µL of 4 ng/ml horse radish peroxidase (HRP) labelled monoclonal antibody and incubated for 1 hour at 20°C. Finally, 100 µL tetramethyl benzinidine (TMB) (Kem-En-Tec cat. 438OH, Taastrup, Denmark) was added, and the plate was incubated for 15 minutes at 20°C in the dark. All the above incubation steps included shaking at 300 rpm. After each incubation step the plate was washed five times in washing buffer (20 mM Tris, 50 mM NaCl, pH 7.2). The TMB reaction was stopped by adding 100 µL of stopping solution (1% HCl) and measured at 450 nm with 650 nm as the reference. Coating and assay buffers were left to equilibrate to room temperature. The plate was coated with 2.5 ng/ml biotinylated antibody and was left incubating for 30 minutes at 20°C and shaking at 300 rpm. The C3M assay procedure was followed as previously described [10].
Rat CCl4 liver fibrosis model
Liver fibrosis was induced in 40 male Sprague-Dawley rats (Harlan, Holland and Germany), aged 6 months, as shown in Figure 1.
Figure 1.
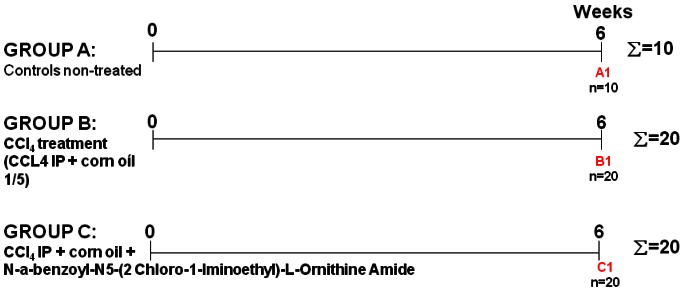
Animal study design.
Animals in group A served as controls. CCl4 (0.45 mL/kg) was injected twice a week by intraperitoneal injections (IP) and phenobarbital (0.3 g/l) was added to the drinking water of animals in group B and C for 6 weeks. Animals in group C additionally received daily injected treatment of N-Alpha-benzoyl-N5-I-Ornithine (3 mg/kg) [11]. Blood was collected at termination and was allowed to stand at room temperature for 20 minutes to clot, before centrifugation at 2500 rpm for 10 min. Samples were stored at -80°C. Liver sections 4 µm thick were stained with 0.1% Sirius red (F3B) in saturated picric acid (Sigma-Aldrich, St Louis, MO, USA). From each animal, the amount of fibrosis expressed as a percentage of total collagen in the total liver area was measured by digital quantitative histology (VisioMorph, Visiopharm, Hørsholm, Denmark) using 3 adjacent histology slides from each animal.
Statistical analyses
Comparison of groups was performed using an ANOVA test with Dunnett correction. Correlations were performed using the Spearman correlation. Differences were considered statistically significant if p<0.05. GRAPH PAD PRISM 5 (Graph Pad Software, La Jolla, CA, USA) was used for the calculations.
Results
Histology
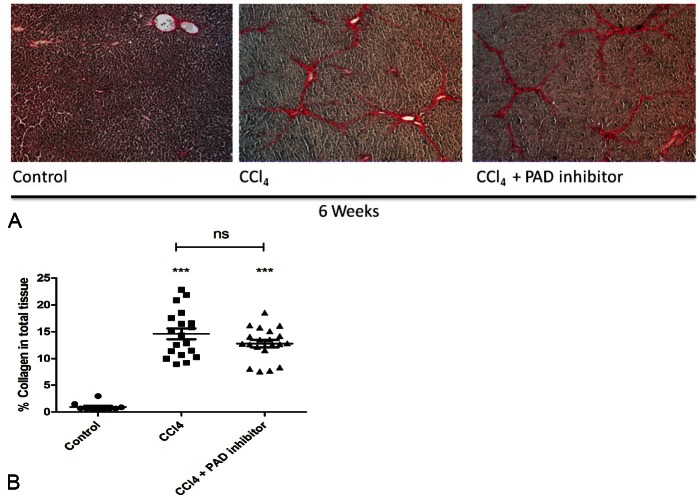
Quantitative histology measurement revealed a statistically significant difference in the total collagen levels between control animals and animals receiveing either CCl4 or CCl4+PAD. No statistically signinificant difference was observed between CCl4 and CCl4+PAD treatment groups (Figure 2A and 2B).
Figure 2.
Sirius red staining of liver tissue for all groups at 6 weeks. A. Total collagen levels as measured by quantitative histology on sirius red staining. B. The mean value for the control group was 0.9%, for the CCl4 group, 14.1% (P<0.001) and for the CCl4+PAD group, 12.7% (P<0.001). No statistically significant difference was observed between CCl4 and CCl4+PAD groups (P>0.05).
Rat CCl4 liver fibrosis model
The mean citrullinated vimentin level for control animals was 115 ng/ml. Compared with controls, the increase in marker levels for animals treated solely with CCl4 was highly statistically significant (488 ng/ml, P>0.001) representing an increase of 324%. In animals treated with both CCl4 and the PAD inhibitor, VICM levels were found to be 252 ng/ml (P>0.05) representing an increase of 119% over controls. A statistically significant difference (P>0.05) was observed in VICM levels of animals receiving solely CCl4 and those also receiving CCl4+PAD treatment (Figure 3A). Mean C3M levels for control animals was 14.1 ng/ml. Compared with controls the animals treated solely with CCl4 showed a highly statistically significant difference (20.9 ng/ml, P>0.001) in their collagen levels, representing an increase of 48%. The animals treated with the combination of CCl4 and PAD inhibitor also showed a statistically significant increase (23.3 ng/ml, P>0.0001) representing an increase of 65% over controls. No statistically significant difference was observed between the group of animals treated with CCl4 and CCl4+PAD inhibitor (Figure 3B).
Figure 3.
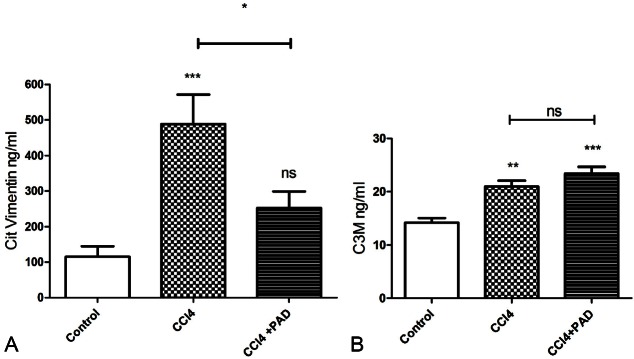
VICM levels in control, CCl4 and CCl4+PAD treated animals. A statistically significant difference was found between animals receiving the PAD inhibitor+CCl4 and those that received only CCl4 (P>0.05) (A). C3M levels in CCl4 and CCl4+PAD treated animals were not statistically significantly different (P<0.05) (B).
Discussion
We have previously demonstrated that levels of the citrullinated peptide, VICM, are increased in a CCl4 preclinical model of liver fibrosis [12]. This finding raised the question of whether citrullination is an active player in liver fibrogenesis. The aim of this study was to investigate whether PAD inhibitor treatment, against citrullination, could reduce liver fibrogenesis. The results suggest that even though VICM levels were decreased, PAD inhibitor treatment had no effect on total collagen accumulation as shown by total collagen measured by quantitative histology. An interesting finding of the study is that animals that were treated with CCl4+PAD inhibitor showed slightly increased levels of C3M marker. Even though the difference is not statistically significant between animals that were treated solely with CCl4, we believe that this finding may indicate that PAD is implicated in matrix remodelling during pathology. The exact mechanisms of this interaction warrants further study. Results from such investigations would significantly add to our understanding of citrullination modifications in ECM remodelling and their role in the transition from physiology to pathology. An important biomarker-related finding of this study is that PAD treatment can decrease VICM levels in animals developing liver fibrosis due to CCl4 intoxication. We believe that this finding implies a cause and effect relationship between PAD levels and VICM formation.
Study limitations
The study lacks immunohistochemistry and western blot data due to the fact that the utilised VICM and C3M antibodies were found to be highly specific for ELISA application.
Acknowledgement
We acknowledge the Danish Ministry of Science, Technology and Innovation and the Den Danske Forskningsfond, Centre for Clinical and Basic Research.
References
- 1.Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arita K, Shimizu T, Hashimoto H, Hidaka Y, Yamada M, Sato M. Structural basis for histone N-terminal recognition by human peptidylarginine deiminase 4. Proc Natl Acad Sci U S A. 2006;103:5291–5296. doi: 10.1073/pnas.0509639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.György B, Tóth E, Tarcsa E, Falus A, Buzás EI. Citrullination: A posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–77. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 6.Harauz G, Ishiyama N, Hill CM, Bates IR, Libich DS, Fares C. Myelin basic protein-diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron. 2004;35:503–542. doi: 10.1016/j.micron.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 8.Vassiliadis E, Oliveira CP, vares-da-Silva MR, Zhang C, Carrilho FJ, Stefano JT, Rabelo F, Pereira L, Kappel CR, Henriksen K, Veidal SS, Vainer B, Duffin KL, Christiansen C, Leeming DJ, Karsdal M. Circulating levels of citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis related pathology. Am J Transl Res. 2012;4:403–414. [PMC free article] [PubMed] [Google Scholar]
- 9.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH, Arend WP, Thompson PR, Holers VM. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barascuk N, Veidal SS, Larsen L, Larsen DV, Larsen MR, Wang J, Zheng Q, Xing R, Cao Y, Rasmussen LM, Karsdal MA. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010;43:899–904. doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH, Arend WP, Thompson PR, Holers VM. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassiliadis E, Oliveira CP, vares-da-Silva MR, Zhang C, Carrilho FJ, Stefano JT, Rabelo F, Pereira L, Kappel CR, Henriksen K, Veidal SS, Vainer B, Duffin KL, Christiansen C, Leeming DJ, Karsdal M. Circulating levels of citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis related pathology. Am J Transl Res. 2012;4:403–414. [PMC free article] [PubMed] [Google Scholar]