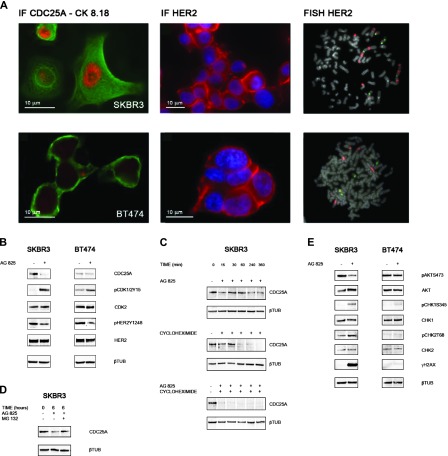
Figure 3.
(A) CDC25A expression and HER2 expression/gene amplification in breast cancer cell lines. Immunofluorescence with CDC25A and cERBB2 (Dako) antibodies revealed CDC25A overexpression (nuclear red signal) and HER2 overexpression (cytoplasmic red signal) in SKBR3 cells and basal levels of CDC25A and HER2 overexpression in BT474 cells. Green signal is the immunofluorescence staining of cytokeratin pair 8 and 18 (CK8.18) highlighting the cell cytoplasm; blue signal is DAPI counterstaining for nuclei. FISH analysis showed HER2 amplification (red signals) in SKBR3 and BT474 metaphase spreads. (B) HER2 inhibition induces CDC25A down-regulation of both protein expression and functional activity. Cell lines were treated with AG825 (45 µM) for 24 hours. Control cells were treated with DMSO. Immunoblot data showed a decrease in CDC25A protein levels in HER2/CDC25A double-positive SKBR3 cells treated with AG825. SKBR3 cells showed also a decreased in CDC25A activity after HER2 abrogation as measured by the increase of the inhibitory phosphorylation of its CDK substrates, pCDK1/2Y15. No such strong effect was seen in BT474. AG825 treatment could inhibit the kinase activity of HER2 as assessed by a decrease in HER2Y1248 phosphorylation with no effect on total HER2 levels in the HER2-positive cell lines, SKBR3 and BT474. (C) HER2 inhibition affects CDC25A protein stability in SKBR3. SKBR3 cells were treated with the protein synthesis inhibitor cycloheximide (5 µg/ml) at different time points (0, 15, 30, 60, 240, and 360 minutes) in the presence or absence of AG825: Immunoblot data showed that AG825 decreased the half-life of CDC25A protein from 60 to less than 15 minutes. β-Tubulin was used as a loading control. (D) Ubiquitin/proteasome pathway is involved in the increased turnover of CDC25A after SKBR3 AG825 treatment. To inhibit proteasome-dependent degradation of proteins, SKBR3 cells, either treated or not with AG825, were incubated with 50 µM proteasome inhibitor MG132 for 6 hours. CDC25A immunoblot analysis showed that inhibition of the proteasome by treatment with MG132 rescued CDC25A down-regulation. β-Tubulin was used as a loading control. (E) HER2 inhibition leads to CDC25A down-regulation through the PI3K/AKT pathway and DDR activation. Cell lines were treated with AG825 for 24 hours. Control cells were treated with DMSO. Immunoblot data showed, in AG825-treated SKBR3 cells, a decrease in AKT activity, as measured by the decrease in its phosphorylation on serine 473 (pAKTS473) and no reduction of total AKT observed; CHK1 and CHK2 activation, through phosphorylation of their key sites, serine 345 and threonine 68, respectively (pCHK1S345, pCHK2T68), with no effect on total CHK1 and CHK2 levels; and histone H2AX gamma phosphorylation (γH2AX). No differences were observed in BT474 cells either with or without AG825 treatment. β-Tubulin was used as a loading control.