Abstract
This paper presents a novel one-step process for converting a liquid stabilized nano-suspension into a solid formulation via hot-melt extrusion combined with an internal devolatilization process (nano-extrusion, NANEX). A polymer (Soluplus®) was fed into the extruder and molten, after which a stable nano-suspension was added via side-feeding devices. The solvent (water) was removed by devolatilization and the polymer solidified at the outlet. The solid material can be tableted or filled in a capsule directly. The results showed that the obtained extrudates comprised nanocrystals in the de-aggregated form, confirming that a solid nano-formulation was prepared. This method is capable of overcoming many of the problems associated with other processes involving solid nano-dosage forms and poses a straightforward approach towards manufacturing such products.
KEY WORDS: hot-melt extrusion, nano-suspension, one-step process, solid nano-formulation
INTRODUCTION
Many active pharmaceutical ingredients (APIs) have poor aqueous solubility and bioavailability, posing a significant problem for drug development. With regard to formulation, several approaches have been developed to date, including morphology alteration, complexation of APIs with hydrophilic excipients, and particle size reduction (1–4). Various techniques have been used to reduce the size to the nanometer scale to produce so-called nanocrystals, which contain only API. These approaches include precipitation (5,6), (wet-media) milling (7–9), and high-pressure homogenization (10,11). However, since nanocrystals have a strong tendency to agglomerate, stabilizers must be used to cover the particle surfaces. One disadvantage of a nano-suspension is particle growth due to Ostwald ripening, which can occur during processing, storage, and handling of suspensions (11–14). Apart from solubility and stability considerations, patient compliance must be considered (12,15): whereas oral administration with intestinal absorption is the most patient-friendly method of drug delivery, nano-suspensions are typically delivered parenterally (10). Thus, in order to develop a preferred solid dosage form, the nano-suspension must be transformed into a solid product, presenting additional challenges. Five products (Rapamune®, Emend®, TriCor®, Megace®ES, and Triglide™ (13,16)) produced via nanoparticle technology are currently available in the market, of which four are based on the media milling technique (Elan NanoCrystal® technology (13,14,16)). The remaining one (Triglide™) is manufactured using high-pressure homogenization (DissoCubes®, SkyePharma PLC (17)) and requires several steps, including freeze-drying, spray-drying, spray coating, and pelletizing or granulation, to convert the nanoparticles in a solid powder (18) for compaction into tablets or filling into capsules. Since these complex processes are time-consuming and cost-intensive, new technologies are needed to transfer a nano-suspension into a solid application form while avoiding any agglomeration.
In our study a one-step nano-extrusion (NANEX) process was developed where the nano-suspension is directly fed to a hot-melt extruder. Hot-melt extrusion is an innovative technology for the production of a variety of dosage forms, offering several advantages over traditional processing techniques and is cost-effective (19). Moreover, it is a continuous process that combines feeding, mixing, and melting in one unit operation. In our novel approach, a stabilized nano-suspension is added through a side-feeding device and the solvent is removed continuously via devolatilization, which is standard procedure during extrusion of solvent-containing drug-excipient mixtures. Specifically, an aqueous model nano-suspension was stabilized, fed into an extruder, and embedded in a molten polymer matrix via the co-rotating twin screw. The water was removed during the process via devolatization as described above.
Our goal was to obtain extrudates that contain homogenously distributed nanocrystals produced via a one-step process. To visualize and verify the presence and dispersion of nanoparticles in the extrudate, crystalline nano-TiO2 was used as a model substance. Particle dispersion was investigated via electron microscopy (SEM) combined with energy-dispersive X-ray spectroscopy (EDX). In future studies, pharmaceutical nanocrystals will be used to demonstrate applicability to pharmaceutical products.
MATERIALS AND METHODS
Materials
Titanium(IV) oxide (TiO2, rutile, 80 nm, 99%, NO-0037-HP) purchased from Io-Li-Tec, Ionic Liquids Technologies, Heilbronn, Germany and citric acid monohydrate (CA) and sodium hydroxide from Carl Roth GmbH & Co. KG, Karlsruhe, Germany were used. The stabilizers were sodium dodecyl sulfate (SDS; Herba Chemosan, Graz, Austria), Tween 60 (T60, Sigma-Aldrich®, Munich, Germany), Cremophor EL, and Cremophor RH 40 (CEL, CRH; both, BASF, Ludwigshafen, Germany). Soluplus® was donated by BASF, Ludwigshafen, Germany.
Preparation and Characterization of TiO2 Nano-suspensions
T60, CEL, CRH (steric stabilization), SDS (electrosteric stabilization), and CA (electrostatic stabilization) were used as stabilizers. All stabilizer concentrations (0.1–3%) used for the preparation of the nano-suspensions were chosen in accordance to the literature (13). As stated by Ramirez-Garcia et al. (20), a concentration of 0.4% CA is best suited to produce TiO2 suspensions with the smallest particle sizes, suitable zeta potentials, and ideal polydispersity indices (PdI), which indicates the particle distribution in a suspension. A high PdI (1 being the maximum) indicates a broad size distribution. Another important issue, when using CA, is the pH. Thus, the pH values of the CA solutions were adjusted to 1, 3, 4, 5, and 7. Briefly, TiO2 nanoparticles (10 mg/ml; 1% (w/w)) were mixed with each aqueous stabilizer solution using a magnetic stirrer, followed by ultrasonification (Transsonic T700, Elma®, Singen, Germany) for 20 h. Hydrodynamic size and PdI of the particles were measured via photon correlation spectroscopy (PCS; Zetasizer Nano ZS, Malvern Instruments, Malvern, UK) equipped with a 532-nm laser. The zeta potential was evaluated via laser Doppler velocimetry (scattering angle of 173°) coupled with PCS (Zetasizer Nano ZS, Malvern Instruments, Malvern, UK) and calculated according to the Helmholtz–Smoluchowski equation (n = 3) (21). Samples showing the best stability (i.e., small particle size, suitable zeta potential, and an ideal PdI) were stored in the refrigerator (2–8°C) and at room temperature (15–25°C) for 14 or 28 days. Their sizes and surface charges were evaluated via PCS and the sizes were confirmed by microscopic images (data not shown).
Hot-Melt Extrusion
Extrusion was performed with a ZSK 18 twin screw extruder (Coperion GmbH, Stuttgart, Germany) through a 1-mm die with two identical holes. The barrel had ten sections (barrel zones), which were individually heated with electric heaters. The temperatures of the individual barrels were in a range of 100–150°C. The screw configuration was adapted to the process requirements (see Fig. 1). Soluplus® was fed gravimetrically in zone 1, degassing was conducted in barrel zone 4, the nano-suspension was added in zone 5 by using a mass flow controller, and water was eliminated via devolatization (80 mbar) in barrel zone 8. The extrudates were investigated via SEM and EDX (Zeiss Ultra 55 GEMINI®).
Fig. 1.
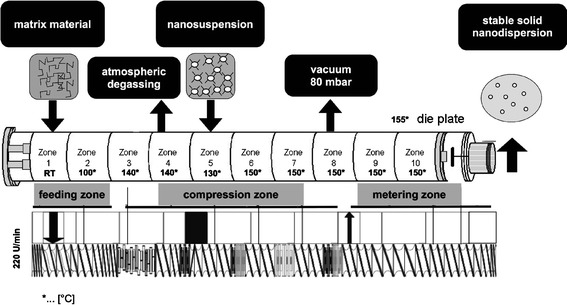
Illustration of the extruder setup, including the process temperatures of the barrel zones in degree Celsius and the screw configuration
RESULTS AND DISCUSSION
The type and amount of stabilizer have a significant impact on the stability of nanocrystals. According to the literature, systems are classified as stable with a PdI value ≤0.5 (reliable and reproducible) and a zeta potential ≥±30 mV (21). The results of the PCS measurements demonstrated that only CA and SDS were appropriate for the preparation of a stable nano-suspension. However, after 14 days of storage at 5°C, a stable suspension was obtained only with CA at pH 5. The established mean sizes decreased to 156.5 from 557.5 nm. The PdI value decreased to 0.239 from 0.443. The zeta potential increased slightly to −53.9 from −44.2 mV. The other stabilizers lead to nanoparticle aggregates and thus were not suitable for further experiments (see Table I). The stabilization process was time-dependent and was not affected by the storage conditions (Table II).
Table I.
Mean Particle Sizes [in Nanometer], PdI, and Zeta Potential Values [in Millivolt] of TiO2-Nano-suspensions Stabilized with T60, CEL, CRH, SDS, and CA
| Stabilizer [w/w, %] | Mean particle size ± SD [nm] | PdI ± SD | Zeta potential ± SD [mV] | Evaluation before storage | Evaluation after 2 weeks storage |
|---|---|---|---|---|---|
| T60 (0.1%; 0.5%; 1%, 2%, 3%) | 501.5 ± 36.9–796.4 ± 78.0 | 0.374 ± 0.007–0.626 ± 0.162 | 10.1 ± 3.0 24.0 ± 1.7 | Not appropriate: zeta potential ≤30 mV | |
| CEL (0.1%; 0.5%; 1%, 2%, 3%) | 277.9 ± 39.3–706.4 ± 68.1 | 0.254 ± 0.009–0.514 ± 0.098 | 6.7 ± 7.3–22.1 ± 6.0 | Not appropriate: zeta potential ≤30 mV | |
| CRH (0.1%; 0.5%; 1%, 2%, 3%) | 217.6 ± 12.2–578.5 ± 70.8 | 0.305 ± 0.057–0.439 ± 0.100 | 5.4 ± 2.5–18.4 ± 1.2 | Not appropriate: zeta potential ≤30 mV | |
| SDS (0.1%; 0.5%; 1%, 2%, 3%) | 380.5 ± 27.3–754.5 ± 49.3 | 0.335 ± 0.085–0.571 ± 0.105 | 15.9 ± 2.2–40.2 ± 0.7 | 1% SDS appropriate | Increase mean particle size: from 380.5 ± 27.3 to 535.3 ± 10.4 |
| Increase PdI: from 0.335 ± 0.085 to 0.381 ± 0.049 | |||||
| Decrease zeta potential: from 36.4 ± 1.1 to 31.7 ± 1.7 | |||||
| CAa (0.4%, pH 1, 3, 4, 5, 7) | 519.3 ± 77.8–2,236.3 ± 519.5 | 0.395 ± 0.034–0.924 ± 0.127 | 5.8 ± 1.9–45.1 ± 0.8 | 0.4% CA, pH 5 appropriate | Decrease mean particle size: from 557.5 ± 52.5 to 156.5 ± 7.1 |
| Decrease PdI: from 0.443 ± 0.075 to 0.239 ± 0.013 | |||||
| Increase zeta potential: from 44.2 ± 0.8 to 53.9 ± 0.4 |
Lowest and highest values are given dependent on the stabilizer concentrations. Systems with zeta potentials ≥30 mV, PdI ≤0.5, and constant or smaller particle sizes after storage were classified as suitable for further investigations
PdI polydispersity indices, T60 Tween 60, CEL Cremophor EL, CRH Cremophor RH 40, CA citric acid monohydrate, SDS sodium dodecyl sulfate, SD standard deviation
aAppropriate stabilizer concentration resulting in a de-aggregated nano-suspension
Table II.
Mean Particle Sizes [in Nanometer], PdI, and Zeta Potential Values [in Millivolt] of a TiO2-Nano-suspension Stabilized with 0.4% CA Adjusted to pH 5
| Storage condition | Duration of storage [days] | Size ± SD [nm] | PdI ± SD | Zeta potential ± SD [mV] |
|---|---|---|---|---|
| Refrigerator | 1 | 683.7 ± 39.5 | 0.550 ± 0.088 | −48.4 ± 0.8 |
| 7 | 233.5 ± 13.5 | 0.286 ± 0.043 | −45.8 ± 0.9 | |
| 14 | 257.0 ± 89.5 | 0.337 ± 0.037 | −48.0 ± 0.8 | |
| 21 | 208.9 ± 13.6 | 0.242 ± 0.041 | −48.1 ± 1.3 | |
| 28 | 240.0 ± 61.2 | 0,281 ± 0.074 | −48.5 ± 1.2 | |
| Room temperature | 1 | 517.4 ± 96.7 | 0.461 ± 0.043 | −50.2 ± 1.2 |
| 7 | 183.0 ± 17.2 | 0.291 ± 0.041 | −47.2 ± 1.8 | |
| 14 | 181.5 ± 15.8 | 0.283 ± 0.043 | −50.6 ± 1.0 | |
| 21 | 267.4 ± 69.3 | 0.289 ± 0.047 | −50.9 ± 0.2 | |
| 28 | 155.2 ± 3.2 | 0.232 ± 0.001 | −49.8 ± 1.5 |
Two different storage conditions were tested, i.e., refrigerator (2–8°C) and room temperature (15–25°C)
PdI polydispersity indices, SD standard deviation
Next, the stabilized CA nano-suspension (mean particle size 181.5 ± 15.8 nm, see Table II) was used for the extrusion experiments. Before running the extruder, the screw was optimized based on the results of previous experiments (data not shown). Soluplus®, a graft polymer composed of polyethylene glycol, polyvinyl caprolactam, and polyvinyl acetate, was used as matrix carrier, since it is miscible with water. Additionally, it has a low glass transition temperature of 70°C (22). Since the polymer contained residual moisture, steam was formed. Thus, an atmospheric degassing was required in barrel zone 4, after the kneading elements, but before introducing the nano-suspension into the molten polymer.
Evaluation of the water amount that can be added to the polymer is an important aspect during extrusion. The results showed that 30% (w/w) water could be added to the molten polymer in the melting zone without impacting the extrudability of the material. The liquid side feed was added at barrel zone 5 and water was removed from the polymer via devolatilization in barrel 8 (vacuum of 80 mbar).
The extrudate solidified at the outlet and was investigated using SEM and EDX to verify that the nanoparticles were embedded in the matrix system. The data confirmed that the nanoparticles were embedded in the matrix in a de-aggregated form. The measured particle sizes were in the range of approximately 200–400 nm, which was slightly higher than the PCS measurements, since PCS measures mean particle sizes. The visualized particles were verified by EDX, indicating that TiO2 de-aggregated nanoparticles were embedded in the Soluplus® matrix (see Fig. 2).
Fig. 2.
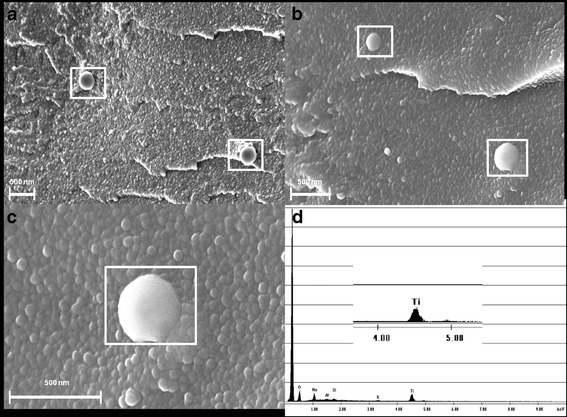
SEM images of the cross sections (a–c) of extrudates: a, b 210–340 nm TiO2 particles embedded in Soluplus® in de-aggregated form and c, d verification of a TiO2 nanoparticle (340 nm) via elemental analysis (EDX)
CONCLUSIONS
The current study demonstrates that our novel process based on hot-melt extrusion of a nano-suspension is an appropriate continuous technology for producing solid nano-formulation. It helps to eliminate problems associated with stabilization of nano-suspensions, parenteral delivery, and the conversion of nanoparticles into a (dry) solid dosage form. Further studies will focus on tailoring the process to pharmaceutical nano-materials, involving stability, process, and formulation aspects.
REFERENCES
- 1.Kipp JE. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int J Pharm. 2004;284:109–122. doi: 10.1016/j.ijpharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Gardner CR, Walsh CT, Almarsson O. Drugs as materials: valuing physical form in drug discovery. Nat Rev Drug Discov. 2004;3:926–934. doi: 10.1038/nrd1550. [DOI] [PubMed] [Google Scholar]
- 3.Prentis R, Lis Y, Walker SR. Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964–1985) Brit J Clin Pharmacol. 1988;25(3):387–396. doi: 10.1111/j.1365-2125.1988.tb03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3–19. doi: 10.1016/S0169-409X(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 5.Sarkari M, Brown J, Chen X, Swinnea S, Williams RO, Johnston KP. Enhanced drug dissolution using evaporative precipitation into aqueous solution. Int J Pharm. 2002;243:17–31. doi: 10.1016/S0378-5173(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Young TJ, Sarkari M, Williams RO, Johnston KP. Preparation of cyclosporine A nanoparticles by evaporative precipitation into aqueous solution. Int J Pharm. 2002;242:3–14. doi: 10.1016/S0378-5173(02)00147-3. [DOI] [PubMed] [Google Scholar]
- 7.Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62:1569–1579. doi: 10.1111/j.2042-7158.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- 8.Liversidge GG, Conzentino P. Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats. Int J Pharm. 1995;125:309–313. doi: 10.1016/0378-5173(95)00148-C. [DOI] [Google Scholar]
- 9.Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–120. doi: 10.1016/S0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 10.Müller R, Peters K. Nanosuspensions for the formulation of poorly soluble drugs: I. Preparation by a size-reduction technique. Int J Pharm. 1998;160:229–237. doi: 10.1016/S0378-5173(97)00311-6. [DOI] [Google Scholar]
- 11.Krause KP, Müller RH. Production and characterisation of highly concentrated nanosuspensions by high pressure homogenisation. Int J Pharm. 2001;214:21–24. doi: 10.1016/S0378-5173(00)00626-8. [DOI] [PubMed] [Google Scholar]
- 12.Bhakay A, Davé R, Bilgili E. Recovery of BCS Class II drugs during aqueous redispersion of core–shell type nanocomposite particles produced via fluidized bed coating. Powder Technol. 2012. doi:10.1016/j.powtec.2011.12.066
- 13.Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364:64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Liversidge G, Cundy K. Surface modified drug nanoparticles. US Patent 5145684. 1992.
- 15.Fasano A. Innovative strategies for the oral delivery of drugs and peptides. Trends Biotechnol. 1998;16:152–157. doi: 10.1016/S0167-7799(97)01170-0. [DOI] [PubMed] [Google Scholar]
- 16.Chingunpituk J. Nanosuspension technology for drug delivery. Walailak J Sci Technol. 2011;4:139–153. [Google Scholar]
- 17.Müller R, Becker R, Kruss B, Peters K. Pharmaceutical nanosuspensions for medicament administration as systems with increased saturation solubility and rate of solution. US Patent 5,858,410. 1999
- 18.Müller RH, Böhm BHL, Grau MJ. Nanosuspensions: a formulation approach for poorly soluble drugs and poorly bioavailable drugs. In: Wise D, editor. Handbook of pharmaceutical controlled release technology. New York: Marcel Dekker; 2000. pp. 345–357. [Google Scholar]
- 19.Roblegg E, Jäger E, Hodzic A, Koscher G, Mohr S, Zimmer A, et al. Development of sustained-release lipophilic calcium stearate pellets via hot melt extrusion. Eur J Pharm Biopharm. 2011;79:635–645. doi: 10.1016/j.ejpb.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez-Garcia S, Chen L, Morris MA, Dawson KA. A new methodology for studying nanoparticle interactions in biological systems: dispersing titania in biocompatible media using chemical stabilisers. Nanoscale. 2011;3:4617–4624. doi: 10.1039/c1nr10488h. [DOI] [PubMed] [Google Scholar]
- 21.Müller RH. Zetapotential und Partikelladung in der Laborpraxis. Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, editor. 1996.
- 22.Kolter K, Karl M, Gryczke A. Hot-melt extrusion with BASF pharma polymers. BASF SE, Pharma Ingredients & Services; 67056 Ludwigshafen, Germany; 2012.


