Abstract
The effect of ternary solid dispersions of poor water-soluble NSAID meloxicam with moringa coagulant (obtained by salt extraction of moringa seeds) and polyvinylpyrrolidone on the in vitro dissolution properties has been investigated. Binary (meloxicam–moringa and meloxicam–polyvinylpyrrolidone (PVP)) and ternary (meloxicam–moringa–PVP) systems were prepared by physical kneading and ball milling and characterized by Fourier transform infrared spectroscopy, differential scanning calorimetry, and X-ray diffractometry. The in vitro dissolution behavior of meloxicam from the different products was evaluated by means of United States Pharmacopeia type II dissolution apparatus. The results of solid-state studies indicated the presence of strong interactions between meloxicam, moringa, and PVP which were of totally amorphous nature. All ternary combinations were significantly more effective than the corresponding binary systems in improving the dissolution rate of meloxicam. The best performance in this respect was given by the ternary combination employing meloxicam–moringa–PVP ratio of [1:(3:1)] prepared by ball milling, with about six times increase in percent dissolution rate, whereas meloxicam–moringa (1:3) and meloxicam–PVP (1:4) prepared by ball milling improved dissolution of meloxicam by almost 3- and 2.5-folds, respectively. The achieved excellent dissolution enhancement of meloxicam in the ternary systems was attributed to the combined effects of impartation of hydrophilic characteristic by PVP, as well as to the synergistic interaction between moringa and PVP.
KEY WORDS: amorphization, moringa coagulant, solubility enhancement, synergism, ternary solid dispersions
INTRODUCTION
Solubility is a critical determinant of oral bioavailability of poorly water-soluble drugs such as meloxicam (1,2). Various techniques such as size reduction, use of co-solvent and surfactants, inclusion complex formation, microparticles, salt formation, pro-drug, and solid dispersion are employed for increasing the solubility of the drugs of which the technique of solid dispersions seems to be the most appealing. Amorphization of drugs transforms them to a higher energy state, which is of more solubility compared to crystalline counterparts, but needs to be stabilized (3). For the purpose of stabilization, polymers such as polyvinylpyrrolidone (PVP), hydroxypropyl cellulose, Gelucire, etc. are used (4). Recently, the domain of natural polymers particularly proteins and amino acids is also getting increasing attention, and in a study, it was reported that arginine increased the solubility of gluten, octyl-gallate, and coumarin (5). However, spray drying remains the most used methodology for amorphous state transition of drugs. In our previous work, we attempted dissolution enhancement and amorphous state stabilization of meloxicam with moringa coagulant (MC) as a polymer and using spray drying as the methodology (6). MC is a heterogeneous, cationic biopolymer of molecular weight 6–12 kDa and isoelectric point between 10 and 11, obtained by aqueous extraction of Moringa seeds belonging to family Moringaceae, which has been used for the purification of water since time immemorial (7,23). Structural and amino acid analysis of MC has revealed a total of 60 residues having high contents of glutamine, arginine, and proline in the sequence; “QGPGRQPDFQRCGQQLRNISPPQRCPSLRQAVQLTHQQQGQVGPQQVRQMYRVASNIPST” (8). Spray drying being expensive and non-green limits the complete exploitation of solid dispersion technology. Hence, the present work was planned with the objective of using ball milling and physical kneading for the amorphization of meloxicam using MC alone and in combination with PVP K30. The use of MC and PVP K30 in combination may also further enhances the solubility and stability of the solid dispersions. Recently, arginine has been reported to possess synergistic action with hydroxypropyl-ß-cyclodextrin with respect to enhancing the aqueous solubility of naproxen (9,10). Numerous studies have reported arginine to enhance the dissolution of various drugs such as meloxicam (11), indomethacin (12), and lornoxicam (13). Exhaustive research has been carried out on dissolution enhancement of drugs using water-insoluble polymers such as crospovidone (14,15) and enteric polymers such as hydroxypropyl methylcellulose phthalate, cellulose acetate phthalate, Eudragit® L100 and S100 (16), and Eudragit® E (17,18). As a rule, inclusion of water-soluble carriers results in a fast release of the drug from the matrix, and a poorly soluble or insoluble carrier leads to a slower release of the drug from the matrix (19,20). On a similar line, the use of MC along with PVP K30 may nullify the drawback associated with PVP. PVP K30 being highly water soluble dissolves rapidly than the drug, forming drug-rich layers over the surfaces of dissolving plugs, which prevents further dissolution of drug from the solid dispersions (21). In the present study, solid dispersions of meloxicam and MC and PVP K30 alone and in combination were prepared by using green methodologies of ball milling and physical kneading and investigated for any synergism between the two polymers with respect to stability and dissolution enhancement using infrared spectroscopy, differential scanning calorimetry (DSC), powder X-ray diffractometry (PXRD), and United States Pharmacopeia (USP) type II dissolution apparatus.
MATERIALS
Meloxicam (4-hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-2H-1,2-benzothiazine-3-carboxamide1,1-dioxide) was a gift sample from Cipla Pharmaceuticals (Mumbai, India). Delipidated MC seed powder and PVP K30 were purchased from Veg India Ltd (Tamil Nadu, India) and Himedia Laboratories Pvt Ltd (Mumbai), respectively. All other reagents and solvents were of analytical grade.
Isolation, Extraction, and Purification of MC
The extraction and purification methods of MC, developed by Ndabigengesere et al., were used (22,23). Delipidated MC seed powder was extracted with 3% sodium chloride solution under continuous agitation for 6 h in an orbital shaker, for the sake of leaching out the proteins. The extract was filtered by Whatman filter paper no. 44 and further heated until white precipitate forms at the bottom of the solution. The precipitate was purified by dialysis for a period of 8 h in cold water to remove any remaining traces of salt. The purified MC was homogenized with cold acetone, for further delipidation in a homogenizer, and subsequently dried in an oven, at temperature not exceeding 45°C.
Preparation of Solid Dispersions
Binary solid dispersions of meloxicam with either MC or PVP K30 in different proportions by weight (1:0.5, 1:1, 1:2, 1:3, 1:4, 1:5, and 1:6) and ternary solid dispersions of meloxicam–MC–PVP K30 in the ratios [1:(1:1), 1:(1:2), 1:(1:3), 1:(3:1), and 1:(2:1)] were prepared, according to kneading method, by triturating in a mortar and pestle for 25 min with uniform speed and force and by ball milling, employing the respective optimum parameters (100 rpm and 8 h for solid dispersions containing only MC or greater concentration of MC and 100 rpm and 12 h for solid dispersions containing only or greater concentration of PVP K30). Distilled water was used as solvent for both the methods and the solid dispersions prepared were then dried in a hot air oven at temperature not exceeding 40°C for 12 h to remove any residual moisture. The batch size of all batches of solid dispersion prepared by both methods, viz., physical kneading and ball milling was 10 g. For the purpose of ball milling, grinding jars (volume 12 cm3) and stainless steel balls (9 and 12 mm diameter) were used. The parameters of ball milling such as time and revolutions per minute were optimized by trial and error method, based on the degree of dissolution enhancement obtained.
Evaluation of Solid Dispersions
Drug Content and Yield
Drug content percent and yield percent of the optimum batches of solid dispersions prepared by using MC, PVP K30, and their mixtures were calculated. For calculating drug content %, a solution of 20 mg of optimized batch of Meloxicam-MC-PVP K30 solid dispersions (SDMMP) in dimethyl formamide was sonicated till complete extraction of drug. The solution was filtered by Whatman filter paper no. 45 and the filtrate was assayed by UV–visible spectrophotometer at 362 nm and drug content was calculated by comparing the absorbance with standard curve. For the purpose of calculating yield %, the weight of dried solid dispersions (W1) recovered from each of the batches and initial weight of drug and polymer/s (W2) was used to calculate yield using the formula:
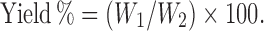 |
1 |
Micromeritics and Compressibility Evaluation
Optimized ternary batch of meloxicam–MC–PVP K30 solid dispersions at ratio of 1:3:1 (SDMMP) was evaluated for various micromeritic and compressibility properties, namely, flowability (angle of repose, Carr's compressibility index, and Hausner's ratio) and tabletability (Kawakita plot, Heckel's plot, and Leuenberger equation). SDMMP was first gently passed through a sieve with an aperture of 1.0 mm, to break up any agglomerates before carrying out any micromeritics and compressibility evaluation studies.
The angle of repose was determined using fixed funnel free-standing cone method using the formula:
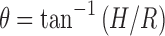 |
2 |
where H is height between lower tip of the funnel and the base of heap of solid dispersion, and R is radius of the base of heap formed (24).
The Carr's compressibility index (CCI) and Hausner's ratio (HR) were calculated using bulk density apparatus according to the formulas:
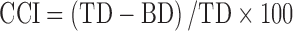 |
3 |
and
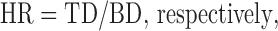 |
4 |
where TD and BD are tapped density and bulk density (25).
For the purpose of Kawakita plot, the reduction in volume of powder bed with tappings was noted using bulk density apparatus and the plot of number of tappings (n) versus degree of volume reduction (n/c) was plotted. The values of constants a and b were calculated using the following equation:
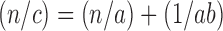 |
5 |
where n is the number of tappings, and c is the degree of volume reduction equal to c = initial volume − (volume after tapping / initial volume) (26).
For the sake of Heckel's plot, 300 mg of SDMMP was compressed using a hydraulic press (Techno Search Instruments, Mumbai, Maharashtra, India) having a 13-mm flat-faced punch and die set at pressures of 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 tons for 1 min of dwell time in triplicate for each ton. Lubrication of the die and punches was performed by a 1% w/v dispersion of magnesium stearate in acetone. Compacts were allowed to relax for 24 h at ambient conditions and pressure (P) − relative density (ρr) data were analyzed using the Heckel equation:
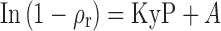 |
6 |
where ρr is packing fraction of the tablet; P is the applied pressure in tons; and K is Heckel constant; K = 1/3σ0, where σ0 is yield strength; and mean yield pressure (MyP) is equal to 3σ0. The constant A expresses densification at low pressure (27).
The compacts used for Heckel studies were also used for tensile strength determination for Leuenberger equation. After determination of diameter (D) and thickness (t), the compacts used for compression study were subjected to hardness determination using Monsanto-type hardness tester and the data were subjected to tensile strength (σt) determination by using following equation:
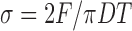 |
7 |
where D is diameter, t is thickness of compacts, and F is the force required to break the compacts (28).
Pressure–tensile strength data were subject to Leuenberger equation to calculate compression susceptibility (γ) and compactibility (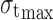 ) of agglomerates. The plot of tensile strength versus relative density × pressure in tons was obtained for the same in accordance with the formula:
) of agglomerates. The plot of tensile strength versus relative density × pressure in tons was obtained for the same in accordance with the formula:
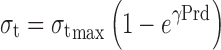 |
8 |
where σt is tensile strength; 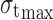 is compactibility; γ compression susceptibility; P pressure; and ρr the relative density calculated from tablet dimensions (29).
is compactibility; γ compression susceptibility; P pressure; and ρr the relative density calculated from tablet dimensions (29).
Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) spectra of all ball milled components (meloxicam, MC, and PVP K30) and ternary batch of meloxicam–MC–PVP K30 solid dispersion at ratio of 1:3:1 (SDMMP) were recorded using an infrared spectrophotometer (Jasco-V-530 model). About 2 mg of sample was ground thoroughly with KBr, uniformly mixed, and the spectra were recorded over the wave number of 400–4,000 cm−1.
Differential Scanning Calorimetry
DSC thermograms of all fresh ball milled components (meloxicam, MC, and PVP K30) and prepared optimized batch solid dispersions (SDMMP) were obtained by using a DSC 821e (Mettler Toledo, Switzerland) instrument, operating with STARe software, version 9.2, and equipped with an intracooler. The samples (3–5 mg) were heated 10°C to 350°C at rate of 10°C/min under dry nitrogen purge (80 ml/min) in crimped and pin-holed aluminum pans.
Powder X-ray Diffraction
PXRD patterns of all fresh ball milled components (meloxicam, MC, and PVP K30) and SDMMP were recorded at room temperature on an X-ray diffractometer (Philips Analytical XRD, PW 3710) with CuKα radiation (1.54 Å), at 40 kV and 40 mA. Samples were subjected to X-ray powder diffraction analysis in continuous mode with a step size of 0.01° and step time of 1 s.
In Vitro Dissolution Studies
In vitro dissolution kinetics of the different batches of solid dispersions was carried out using the USP type II dissolution test apparatus. The dissolution medium used was 900 ml of phosphate buffer 7.4 maintained at a temperature of 37.5 ± 0.5°C. The paddle speed was kept constant at 100 rpm. Ten-milligram samples of the solid dispersions were introduced and aliquots of 5 ml were withdrawn at specific time interval and same amount of fresh phosphate buffer was used to replace the amount of dissolution media withdrawn. The withdrawn samples were analyzed by UV spectroscopy at the wavelength maxima of 362 nm. Analysis of data was done using PCP Disso V3 software (Poona College of Pharmacy, Pune, India). To ascertain that the dissolution enhancement was not due to pH change by the basic amino acids in MC, a solubility study of optimum batches of meloxicam–MC (1:3), meloxicam–PVP K30 (1:4), and meloxicam–MC–PVP K30 (1:3:1) solid dispersions was also performed in an unbuffered distilled water. Excess amount of meloxicam–MC (1:3), meloxicam–PVP K30 (1:4), and meloxicam–MC–PVP K30 (1:3:1) solid dispersions was added to 10 ml distilled water in sealed glass containers, magnetically stirred (500 rpm) at constant temperature of (25 ± 0.5°C) till equilibrium was achieved. The concentration of meloxicam was then calculated using ultraviolet spectroscopy (Jasco 630).
Accelerated Stability Studies
For the present study, fresh ball milled meloxicam and SDMMP were packed in polyethylene-laminated aluminum foils of thickness 0.05 mm and were stored under international conference on harmonization-specified accelerated stability conditions of storage for zone IV at a temperature and relative humidity (40°C/75% RH), in an environmental test chamber (Remi). Samples were periodically removed at 1, 2, and 3 months and characterized using DSC, PXRD, and in vitro dissolution studies.
RESULTS AND DISCUSSION
Isolation, Extraction, and Purification of MC
We found salt extraction to be less time consuming and gave considerable higher yield of MC than aqueous extraction which was in complete agreement with Okuda et al. (30). The reason seems to be the salting out effect, wherein a salt increases protein dissociations leading to increased protein solubility and consequently its extraction. The percent yield of MC obtained by salt extraction was found to be 15%.
Drug Content Percent and Yield Percent
Drug content percent and yield percent of meloxicam in the optimum batches of binary and ternary solid dispersions (meloxicam–MC, meloxicam–PVP K30, and meloxicam–MC–PVP K30) have been shown in Table I. It has been observed that there was an insignificant difference between drug content of binary solid dispersions prepared by physical kneading and ball milling, whereas it was significant between ternary solid dispersions prepared by both methods (P < 0.05, paired t test). In the case of yield percent of solid dispersions, a significant difference was found between binary and ternary systems prepared by physical kneading and ball milling. However, solid dispersion obtained by ball milling gave comparatively less yield because of loss in processing.
Table I.
Percent Drug Content and Percent Yield for Different Batches of Solid Dispersions
| Preparation method | Batch | % Drug content | % Yield |
|---|---|---|---|
| Physical kneading | MEL–PVP K30 (1:4) | 84.555 ± 1.465 | 93.880 ± 2.345 |
| MEL–MC (1:3) | 87.000 ± 1.479 | 92.153 ± 3.456 | |
| MEL–MC–PVP K30 (1:(3:1)) | 90.125 ± 2.465 | 95.236 ± 1.455 | |
| Ball milling | MEL–PVP K30 (1:4) | 88.115 ± 3.458 | 83.350 ± 3.456 |
| MEL–MC (1:3) | 92.334 ± 1.456 | 78.385 ± 2.487 | |
| MEL–MC–PVP K30 (1:(3:1)) | 95.113 ± 2.012 | 84.463 ± 1.478 |
MEL meloxicam, MC moringa coagulant, PVP K30 polyvinylpyrrolidone
Micromeritics and Compressibility Evaluation
Angle of repose, Carr's compressibility index, and Hausner's ratio of the optimized batch was found to be 26.77°, 14.622, and 1.1712, respectively, indicating good flowability (28). The values of a and b in the Kawakita plot of SDMMP were 0.5789 and 0.05906, respectively, indicating large reduction in volume of powder bed with increase in number of tapings and hence good compressibility (26). The mean yield pressure, compactibility, and compression susceptibility were found to be 1.806, 17.72, and 1.473 (27).
Fourier Transform Infrared Spectroscopy
The infrared (IR) spectra of MC revealed characteristic absorption bands attributed to different groups such as at 3,301.54 cm−1 for NH stretching vibrations, 2,927.41 cm−1 for C–H stretching, 1,662.34 cm−1 for C=O stretching, 1,450.21 cm−1 for C–N stretching, and 1,535.06 cm−1 for C=C aromatic stretching, which strongly indicate the presence of amino acids like tyrosine, phenylalanine, tryptophan, arginine, cystine, etc. as shown in Fig. 1.
Fig. 1.

FTIR spectra of fresh ball milled MC, PVP, meloxicam, and SDMMP
The IR spectra of meloxicam showed their characteristic absorption bands at 3,269.96 cm−1 for –NH or O–H stretching vibrations, 1,619.91 cm−1 for C=N stretching vibrations, 1,535.06 cm−1 for C–O stretching, 2,915.84 cm−1 for –CONH group, and 1,176.36 cm−1 for S=O stretching vibration confirming their identity. Similarly, PVP K30 was identified by its FTIR spectra showing its characteristic peaks at 1,593.64 cm−1 for C=O stretching, 2,942.99 cm−1 for C–H stretching, and 1,406.11 cm−1 for the N–H band. The broad band visible at 3,898.24 cm−1 was attributed to the presence of water, confirming the very broad endotherm at approximately 100°C in the DSC of PVP K30.
Infrared spectrum of SDMMP reveals marked spectral changes such as shifting and broadening of the characteristic peaks of all the components, viz., meloxicam, MC, and PVP K30, indicating a good degree of interaction between them. A shift in the peaks corresponding to O–H and –NH stretching of MC and meloxicam, respectively, indicates a strong possibility of hydrogen bonding via overlapping of –OH band of MC with N–H of meloxicam. A decrease in the intensity of S=O and C=N peaks of meloxicam at 3,289.52 and 1,152.64 cm−1 was also noted. Numerous peaks corresponding to PVP K30 and meloxicam in the low-frequency region (1,000–400 cm−1) of the spectra exhibited only a slight change with respect to shifting and broadening. This indicated that although meloxicam is hydrogen bonded with MC, the overall symmetry of the molecule was not affected significantly. Thus, the marked spectral change of component peaks in the FTIR spectrum of SDMMP confirms that the components are in a state of interaction and not merely mixed. The broadening of peaks can also be attributed to the amorphous form of the solid dispersion.
Differential Scanning Calorimetry
The DSC thermogram (Fig. 2) of MC showed endothermic peaks at 80°C and 239.42°C corresponding to transition point and melting point, respectively, while an exothermic peak at 278.90°C was attributed to the decomposition point corresponding to other components like carbohydrate, etc. present in MC. The DSC thermogram of PVP K30 showed endothermic peaks at 138.23°C and 102°C corresponding to melting point and transition point, respectively. The DSC curve of meloxicam indicated crystalline anhydrous state (Tonset = 257.64°C, Tpeak = 267.83°C, Hfus = 940.54 J g−1), while the exothermic peak at 280.1°C was attributed to the decomposition peak of meloxicam. The DSC profile of SDMMP shows thermal effects characteristic of its components. The broad endotherm between 60 and 100°C was attributed to water loss from MC. Distinct broadening and reduction in intensity of meloxicam endotherm at 250.19°C were observed which was indicative of complete drug amorphization. Conversely, no thermal effects attributable to free meloxicam can be observed, where mechanical treatment has led to a possible molecular inclusion and/or to the total amorphization of meloxicam. The DSC spectra of SDMMP also indicated no chemical reactivity between any of the components and no formation of any new chemical moiety.
Fig. 2.

DSC spectra of fresh ball milled MC, PVP, meloxicam, and SDMMP
Powder X-ray Diffraction
X-ray powder diffraction patterns of fresh ball milled pure components and SDMMP are shown in Fig. 3. Spectra of meloxicam reveal several intense diffraction peaks, characteristic of crystalline nature, whereas a flat pattern with many peaks of very low intensity, typical of amorphous substances, was exhibited by MC. The PXRD spectra of PVP K30 revealed greater crystalline nature than that of MC. As for SDMMP, a strong reduction in crystallinity was observed, as can be seen from the fewer number and low intensity of peaks in the PXRD spectra. Finally, the spectrum of SDMMP was actually the weighted superposition of the spectra of its respective components with the difference of reduced number and intensity of the respective peaks.
Fig. 3.

PXRD spectra of fresh ball milled MC, PVP, meloxicam, and SDMMP
In Vitro Dissolution Studies
The results of the dissolution experiments are summarized in Table II, in terms of percent drug dissolved after 1, 2, 3, 4, 5, and 6 h. Solid dispersions prepared by ball milling exhibited better dissolution profile than those prepared by physical kneading. The dissolution data of all binary and ternary batches of solid dispersions revealed an increase in solubility with an increase in the concentration of polymer/s up to a certain concentration limit, above which there was no significant dissolution enhancement and even a slight fall in dissolution (complete dissolution data not presented). In case of MC, enhancement of dissolution was observed up to meloxicam–MC concentration ratio of 1:3 above which there was a slight fall in the dissolution. Similarly, in the case of PVP K30, enhancement of dissolution was observed up to the meloxicam–PVP K30 concentration ratio of 1:4. All ternary systems showed significantly better drug dissolution parameters than the corresponding binary systems. It has been observed that the amount of drug dissolved at 6 h was significantly different amongst binary and ternary solid dispersions prepared by physical kneading and ball milling (P < 0.05, unpaired t test). Similarly significant difference was noted in drug dissolution of solid dispersions prepared by both methods (P < 0.05, paired t test).
Table II.
In Vitro Dissolution of Meloxicam from Different Batches of Solid Dispersions
| Method | Sample | Ratio | PD 1 | PD 2 | PD 3 | PD 4 | PD 5 | PD 6 |
|---|---|---|---|---|---|---|---|---|
| Pure MEL | – | 5.675 ± 1.236 | 7.865 ± 0.879 | 11.679 ± 2.137 | 13.579 ± 1.587 | 13.003 ± 2.879 | 13.34 ± 1.879 | |
| Physical kneading | MEL–MC | 1:3 | 22.721 ± 1.046 | 28.349 ± 3.628 | 36.821 ± 5.584 | 40.492 ± 4.356 | 39.834 ± 4.012 | 39.578 ± 3.678 |
| MEL–PVP K30 | 1:4 | 19.513 ± 2.324 | 23.338 ± 2.460 | 28.478 ± 3.515 | 32.728 ± 3.510 | 31.607 ± 3.717 | 31.574 ± 2.416 | |
| MEL–MC–PVP K30 | 1:3:1 | 40.525 ± 3.285 | 46.217 ± 0.889 | 57.427 ± 1.704 | 62.698 ± 1.637 | 61.608 ± 0.567 | 61.395 ± 0.561 | |
| Ball milling | MEL–MC | 1:3 | 24.591 ± 3.608 | 29.820 ± 3.599 | 38.864 ± 2.819 | 43.962 ± 2.443 | 42.498 ± 2.122 | 42.105 ± 1.938 |
| MEL–PVP K30 | 1:4 | 21.557 ± 2.418 | 27.463 ± 4.260 | 31.843 ± 3.504 | 36.093 ± 2.978 | 35.745 ± 3.078 | 35.427 ± 3.451 | |
| MEL–MC–PVP K30 | 1:3:1 | 71.154 ± 1.971 | 76.814 ± 0.039 | 81.234 ± 0.735 | 85.928 ± 0.978 | 85.428 ± 0.764 | 85.177 ± 0.992 |
PD percent dissolved, MEL meloxicam, MC moringa coagulant, PVP K30 polyvinylpyrrolidone
There seems to be a synergistic effect between MC and PVP K30 with respect to dissolution enhancement of meloxicam as can be seen from the dissolution data. This synergistic effect cannot be simply attributed to a favorable pH variation of the dissolution medium due to the presence of basic amino acids in MC as the dissolution studies were carried out using phosphate buffer at pH 7. Further solubility study of optimum batches of meloxicam–MC (1:3), meloxicam–PVP K30 (1:4), and meloxicam–MC–PVP K30 (1:3:1) solid dispersions in unbuffered distilled water showed that the equilibrium solubility of meloxicam in meloxicam–MC–PVP K30 system was about two times higher than that of meloxicam–MC and about 2.5 times higher than that of meloxicam–PVP K30 system.
Accelerated Stability Studies
In Vitro Dissolution
The dissolution data of SDMMP at 1, 2, and 3 months revealed negligible worsening of dissolution as can be seen from the data in the Table III.
Table III.
In Vitro Dissolution of Meloxicam from the Ternary Optimized Batch of Solid Dispersions (SDMMP) for Accelerated Stability Studies
| Time (min) | Initial | 30 days | 60 days | 90 days |
|---|---|---|---|---|
| 60 | 71.154 ± 1.971 | 70.458 ± 1.244 | 69.458 ± 0.274 | 67.995 ± 0.248 |
| 120 | 76.814 ± 0.039 | 75.123 ± 0.348 | 74.458 ± 0.296 | 72.896 ± 0.124 |
| 180 | 81.234 ± 0.735 | 79.454 ± 0.248 | 77.846 ± 0.178 | 77.546 ± 0.315 |
| 240 | 85.928 ± 0.978 | 85.125 ± 0.178 | 83.478 ± 0.300 | 82.897 ± 0.222 |
| 300 | 85.428 ± 0.764 | 84.985 ± 0.087 | 83.120 ± 0.248 | 81.789 ± 0.175 |
| 360 | 85.177 ± 0.992 | 84.678 ± 0.319 | 82.759 ± 0.169 | 81.908 ± 0.076 |
SDMMP–meloxicam–moringa coagulant–PVP K30 solid dispersions in ratio 1:3:1
Differential Scanning Calorimetry
Consecutive DSC thermograms of SDMMP at 1, 2, and 3 months reveal negligible worsening of amorphous nature as denoted by the small enthalpy change at the end of 3 months, thereby clearly indicating the existence of amorphous nature as shown in Fig. 4. On the other hand, 1-month DSC spectrum of meloxicam (MEL 1) reveals an additional peak at 244.89°C and large changes in the heat capacity indicating complete reversion to highly crystalline form as compared with crystalline freshly ball milled meloxicam (MEL), which is comparatively less crystalline. This observation asserts the utility and necessity of the polymers (MC and PVP K30) as amorphous state stabilizers of meloxicam. Whereas SDMMP shows no significant increase in crystallinity, which is due to the diluting effect of the polymers and to their ability to lower the chain mobility of meloxicam, the percent crystallinity of SDMMP at 1, 2, and 3 months was found to be 17.443, 21.202, and 24.286, respectively.
Fig. 4.
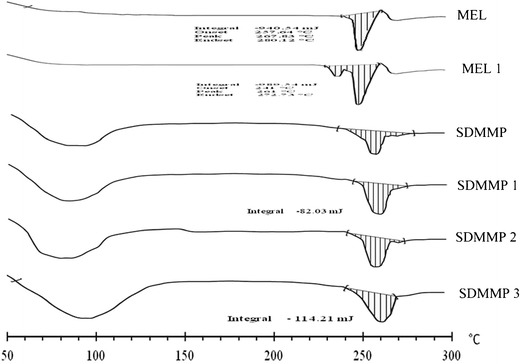
DSC spectra of fresh ball milled meloxicam and SDMMP for accelerated stability studies
Powder X-ray Diffraction
PXRD spectra of meloxicam after 1 month (MEL 1) reveal considerable increase in the number as well as intensity (counts) at respective 2θ values as compared with fresh ball milled MEL, which is comparatively less crystalline. Consecutive SDMMP spectra of 3 months gave only a slight increase in the form of low intensity peaks as seen in Fig. 5. Thus, PXRD spectra of SDMMP indicate minor worsening of the amorphous nature of meloxicam at 1-, 2-, and 3-month storage, respectively, thereby justifying the use of MC and PVP K30 as amorphous state stabilizers of meloxicam.
Fig. 5.
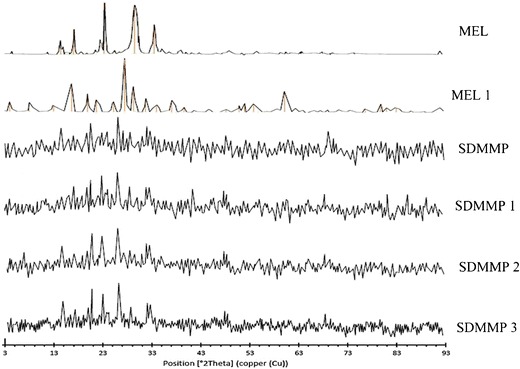
PXRD spectra of fresh ball milled meloxicam and SDMMP for accelerated stability studies
CONCLUSION
Solid dispersions of meloxicam with MC and PVP K30 were successfully prepared for improving the solubility and dissolution properties of meloxicam. The ternary systems displayed better dissolution performances than the corresponding binary systems. Excellent improvement in the dissolution profile of meloxicam was seen which was attributed to the synergistic effect between MC and PVP K30. Further solid-state studies revealed satisfactory degree of amorphization. The stability studies also showed negligible deterioration in terms of percent dissolution and amorphous nature. The micromeritic study of the above ternary solid dispersions also certified its suitability for formulation of tablets with the least possible use of excipients. Therefore, ternary system of meloxicam–MC–PVP K30 appears as to be a potential product for developing fast-release formulations of meloxicam.
ACKNOWLEDGMENTS
Noolkar Suhail B. and Bhende Santosh A. are thankful to AICTE for funding in terms of JRF.
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Yu LX, et al. A biopharmaceutics classification system: the scientific basis for bio waiver extensions. Pharm Res. 2002;19:921–925. doi: 10.1023/A:1016473601633. [DOI] [PubMed] [Google Scholar]
- 2.Chowdary KPR, Hymavathi R. Enhancement of dissolution rate of meloxicam. Indian J Pharm Sci. 2001;63(2):150–154. [Google Scholar]
- 3.Patidar K, Soni M, Sharma DK, Jain SK. Solid dispersion: approaches, technology involved, unmet need & challenges. Drug Invent Today. 2010;2(7):349–357. [Google Scholar]
- 4.Wade A, Weller PJ, editors. Handbook of pharmaceutical excipients. Washington: American Pharmaceutical Association/The Pharmaceutical Press; 1994. pp. 220–399. [Google Scholar]
- 5.Arakawa T, Kita Y, Koyamac HA. Solubility enhancement of gluten and organic compounds by arginine. Int J Pharm. 2008;355:220–223. doi: 10.1016/j.ijpharm.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Bhende S, Jadhav N. Moringa coagulant as a stabilizer for amorphous solids: part I. AAPS PharmSciTech. 2012;13:400–410. doi: 10.1208/s12249-012-9755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwaambwa HM, Maikokera R. A fluorescence spectroscopic study of a coagulating protein extracted from Moringa oleifera seeds. Colloids Surf B. 2007;60:213–220. doi: 10.1016/j.colsurfb.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Gassenschmidt U, Jany KD, Tauscher B, Niebergall H. Isolation and characterization of a flocculating protein from Moringa oleifera Lam. Biochim Biophys Acta. 1995;1243:477–481. doi: 10.1016/0304-4165(94)00176-X. [DOI] [PubMed] [Google Scholar]
- 9.Mura P, Bettinettib GP, Cirria M, Maestrellia F, Sorrentib M, Catenaccib L. Solid-state characterization and dissolution properties of naproxen–arginine–hydroxypropyl-β-cyclodextrin ternary system. Eur J Pharm Biopharm. 2005;59:99–106. doi: 10.1016/j.ejpb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Mura P, Maestrellia F, Cirri M. Ternary systems of naproxen with hydroxypropyl-β-cyclodextrin and amino acids. Int J Pharm. 2003;260:293–302. doi: 10.1016/S0378-5173(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 11.El-Maradny HA, Mortada SA, Kamel OA, Hikal AH. Characterization of ternary complexes of meloxicam-HPβCD and PVP or l-arginine prepared by the spray-drying technique. Acta Pharma. 2008;58:455–466. doi: 10.2478/v10007-008-0029-9. [DOI] [PubMed] [Google Scholar]
- 12.Xiaodan Q, Jing Z, Wei W, Deying C. Solubility and stability of indomethacin in arginine-assisted solubilization system. Pharm Dev Technol. 2011;13:1–4. [Google Scholar]
- 13.Bramhane D, Saindane N, Vavia P. Inclusion complexation of weakly acidic NSAID with β-cyclodextrin: selection of arginine, an amino acid, as a novel ternary component. J Incl Phenom Macrocycl Chem. 2011;69:453–460. doi: 10.1007/s10847-010-9783-7. [DOI] [Google Scholar]
- 14.Hirasawa N, Ishise S, Miyata H, Danjo K. Physicochemical characterization and drug release studies of nilvadipine solid dispersions using water-insoluble polymer as a carrier. Drug Dev Ind Pharm. 2003;29(3):339–344. doi: 10.1081/DDC-120018207. [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa N, Ishise S, Miyata H, Danjo K. Application of nilvadipine solid dispersion to tablet formulation and manufacturing using crospovidone and methylcellulose as dispersion carriers. Chem Pharm Bull. 2004;52:244–247. doi: 10.1248/cpb.52.244. [DOI] [PubMed] [Google Scholar]
- 16.Takada K, Oh-hashi M, Furuya Y, Yoshikawa H, Muranishi S. Enteric solid dispersion of ciclosporin A (CiA) having potential to deliver CiA into lymphatics. Chem Pharm Bull. 1989;37(2):471–474. doi: 10.1248/cpb.37.471. [DOI] [PubMed] [Google Scholar]
- 17.Horisawa E, Danjo K, Haruna M. Physical properties of solid dispersion of a nonsteroidal anti-inflammatory drug (M-5011) with Eudragit E. Drug Dev Ind Pharm. 2000;26:1271–1278. doi: 10.1081/DDC-100102308. [DOI] [PubMed] [Google Scholar]
- 18.Jung JY, Yoo SD, Lee SH, Kim KH, Yoon DS, Lee KH. Enhanced solubility and dissolution rate of itraconazole by a solid dispersion technique. Int J Pharm. 1999;187:209–218. doi: 10.1016/S0378-5173(99)00191-X. [DOI] [PubMed] [Google Scholar]
- 19.Sharma D, Soni M, Kumar S, Gupta GD. Solubility enhancement—eminent role in poorly soluble drugs. Res J Pharm Tech. 2009;2(2):222. [Google Scholar]
- 20.Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60:1281–1302. doi: 10.1002/jps.2600600902. [DOI] [PubMed] [Google Scholar]
- 21.Reintjes T. Solubility enhancement with BASF Pharma polymers. Solubilizer compendium. 2011;7–128. BASF chemical company. http://www.pharma-ingredients.basf.com.
- 22.Ndabigengesere A, Narasiah KS. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998;32(3):781–791. doi: 10.1016/S0043-1354(97)00295-9. [DOI] [Google Scholar]
- 23.Ndabigengesere A, Narasiah KS, Talbot BG. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995;29(2):703–710. doi: 10.1016/0043-1354(94)00161-Y. [DOI] [Google Scholar]
- 24.Train D. Some aspects of the property of angle of repose of powders. J Pharm Pharmacol. 1958;10:127T–134T. doi: 10.1111/j.2042-7158.1958.tb10391.x. [DOI] [PubMed] [Google Scholar]
- 25.Carr RL. Evaluating flow properties of solids. Chem Eng. 1965;72:163–168. [Google Scholar]
- 26.Kawakita K., Ludde K. H. Some considerations on powder compression equations. Powder Technol. 1970–1971;4:61–8.
- 27.Heckel RW. Density pressure relationship in powder compaction. Trans Metall Soc AIME. 1961;221:671–675. [Google Scholar]
- 28.Fell JT, Newton JM. Determination of tablet strength by the diametral compression test. J Pharm Sci. 1970;59:688–691. doi: 10.1002/jps.2600590523. [DOI] [PubMed] [Google Scholar]
- 29.Leuenberger H. The compressibility and compactibility of powder systems. Int J Pharm. 1982;12:41–55. doi: 10.1016/0378-5173(82)90132-6. [DOI] [Google Scholar]
- 30.Okuda T, Baes AU, Nishijima W, Okada M. Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Res. 1999;33(15):3373–3378. doi: 10.1016/S0043-1354(99)00046-9. [DOI] [Google Scholar]


