Abstract
A new, simple, inexpensive, and rapid 96-well plate UV spectrophotometric method was developed and validated for the quantification of compound 48/80 (C48/80) associated with particles. C48/80 was quantified at 570 nm after reaction with acetaldehyde and sodium nitroprusside in an alkaline solution (pH 9.6). The method was validated according to the recommendations of the ICH Guidelines for specificity, linearity, range, accuracy, precision, and detection and quantification limits (DL and QL). All the validation parameters were assessed in three different solvents, i.e., deionized water, blank matrix of chitosan nanoparticles, and blank matrix of chitosan/alginate nanoparticles. The method was found to be linear in the concentration range of 5 to 160 μg/ml (R2 > 0.9994). Intraday and interday precision was adequate, with relative standard deviation lower than those given by the Horwitz equation. The mean recoveries of C48/80 from spiked samples ranged between 98.1% and 105.9% for calibration curves done with the blank matrices and between 89.3% and 103.3% for calibration curves done with water, respectively. The DL were lower than 1.01 μg/ml and the QL were lower than 3.30 μg/ml. The results showed that the developed method is sensitive, linear, precise, and accurate for its intended use, with the additional advantages of being cost-effective and time-effective, allowing the use of small-volume samples, and the simultaneous analysis of a large number of samples. The proposed method was already successfully applied to evaluate the loading efficacy of C48/80 chitosan-based nanoparticles and can be easily applied during the development of other C48/80-based formulations.
KEY WORDS: C48/80, chitosan nanoparticles, mast cell activator, method validation, p-Methoxy-N-methylphenethylamine
INTRODUCTION
Compound 48/80 (C48/80; polymer formed from p-Methoxy-N-methylphenethylamine monomers) is a mast cell activator that has been widely used in allergies-related studies due to its ability to induce the release of histamine (1). More recently, it was demonstrated that C48/80 can also act as a vaccine adjuvant by inducing dendritic cell migration to draining lymph nodes via a mast cell-dependent mechanism (2). In fact, different studies showed that the coadministration of C48/80 with an antigen improves the immunogenicity of the antigen, resulting in higher titers of specific antibodies compared to the antigen alone (2–5).
The vectorization of C48/80 to targeted cells could result in an improvement of the adjuvant effect. This can be achieved by the incorporation of the mast cell activator in nanoparticles. Besides, depending on the strategy used, the co-association of the adjuvant C48/80 and the antigen in the same nanoparticulate delivery system may prove advantageous since it will allow the delivery of both agents in the same antigen-presenting cell (6). The development of the method with the aim to encapsulate C48/80 into particles can only be possible if an efficient method for the measurement of C48/80 exists since the evaluation of the loading efficacy (LE) of the compound in the delivery system is imperative. To our better knowledge, no method has been described so far for the quantification of C48/80, neither in the supernatant of centrifuged particles nor in any other solvent or matrix. Therefore, to support pharmaceutical formulation development efforts, a method for the measurement of C48/80 needs to be established.
C48/80 is cationic polymer with secondary amine groups produced by the condensation of p-Methoxy-N-methylphenethylamine with formaldehyde (7) (Fig. 1). It is known that secondary amines in an alkaline solution react with acetaldehyde and sodium nitroprusside to form a blue–violet compound, a reaction that can be used to quantify secondary amines such as dialkylamines by spectrophotometry (8,9). This reaction is also routinely used in forensic laboratories as a preliminary test, called Simon’s test, for the qualitative detection of secondary amines used as drugs of abuse, namely, 3,4-methylenedixoymethamphetamine (MDMA) and methamphetamine (10). Therefore, based on this theoretical hypothesis, a quantitative method for the determination of C48/80 was developed in our laboratory. Optimization of the reaction conditions like concentration of reagents, finding the suitable pH for the formation of the blue–violet compound, reaction time, and so on were previously defined as a result of a several experiments in our laboratory (data not published). The necessity to evaluate simultaneously an immense number of samples using a small amount of each sample and small amounts of the reagents during the formulation phase of a new C48/80-loaded nanoparticulate delivery system was the obvious reason to adapt the method to be performed in 96-well plates. So, here, the method (optimized protocol) and the validation parameters obtained for three different solvents, i.e., deionized water, blank matrix of chitosan nanoparticles, and blank matrix of chitosan/alginate nanoparticles is described for the first time. Results of the C48/80 LE in chitosan-based nanoparticles are reported as well as a proof of the first application of this simple, reproducible, and reliable method.
Fig. 1.
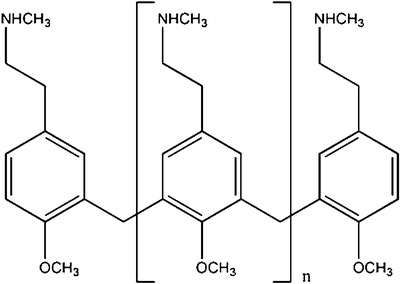
Chemical structure of C48/80
MATERIAL AND METHODS
Materials
C48/80 (mixture of low-molecular-weight [LMW] polymers having a degree of polymerization between 3 and 6; MW = 153 g/mol (monomer)), sodium nitroprusside dehydrate, and acetaldehyde were purchased from Sigma-Aldrich (Sintra, Portugal). LMW chitosan (ChitoClear™) was purchased from Primex Bio-Chemicals AS (Avaldsnes, Norway) and purified as previously described with some modifications (11). Pharmaceutical-grade alginate (Manucol LB®) was kindly donated by ISP Technologies Inc. (Surrey, UK). All other reagents were of analytical grade.
Instruments
All reactions and blue–violet compound measurements were performed using flat-bottom 96-well plates and a Multiskan EX 96-well plate reader (Thermo Scientific, Waltham, Massachusetts, USA).
Preparation of Nanoparticles
Chitosan/alginate particles were prepared using a method described elsewhere (12) with slight modifications introduced. Briefly, a CaCl2 solution 2 mg/ml was added to a 0.063% (w/v) sodium alginate solution while stirring in order to prepare a pre-gel. The particles were formed upon mixing the pre-gel and 0.05% (w/v) chitosan solution by high-speed vortexing.
The second delivery system, chitosan particles, was prepared by adding a solution 2.04 mg/ml of Na2SO4 dropwise to a 0.1% chitosan solution. C48/80-loaded chitosan/alginate and chitosan particles were obtained by addition of the compound to chitosan and Na2SO4 solutions, respectively, in each preparation method. Subsequently, particles were isolated by centrifugation for 20 min at 12,450×g and the supernatants collected. The supernatants of the C48/80-unloaded chitosan and chitosan/alginate particles were used as solvents for the establishment of the calibration curve, here named blank matrices. The method for C48/80 quantification was validated using these blank matrices.
Size and zeta potential of the particles were analyzed by dynamic light scattering using a Delsa TM Nano C (Beckman Coulter, Brea, California, USA). Size was measured by diluting the nanoparticles suspension in Milli-Q water, and for zeta potential measurements, the particles were dispersed in a solution of NaCl 1 mM.
Quantification of the C48/80 by UV Spectrophotometry
The method was primarily developed in our laboratory to quantify C48/80 in diluted aqueous solutions. Subsequently, the method was applied and validated to quantify C48/80 in samples obtained by the centrifugation of C48/80-loaded particles. In a 96-well plate, 25 μl of 0.85 M carbonate buffer pH 9.6 was added to 175 μl of sample. Then, 50 μl of a 15% acetaldehyde solution containing 1.5% of sodium nitroprusside was added and mixed by means of a plate shaker for 30 s. The absorbance was measured after 10 min at 570 nm in a 96-well plate reader.
Calibration Curve
One stock solution of 2 mg/ml of C48/80 was prepared in distilled water or in the supernatants of unloaded particles. The standards for the calibration curve were prepared using the stock solution as described in the succeeding sections (see the “Linearity and Range” section).
Analytical Method Validation
The method was validated according to the recommendations of ICH Guideline Q2(R1) (13) in order to evaluate the specificity, linearity, range, accuracy, precision, and finally, the detection and quantification limits (DL and QL) of the method.
Specificity
The supernatants obtained after centrifugation of C48/80-loaded particles will have some unreacted compounds that result from particle production that may possibly interfere with the quantification method. The use of blank matrices, the supernatant of unloaded nanoparticles prepared under the same conditions, will more likely simulate the solvent of our sample of interest. Therefore, C48/80 at a concentration of 80 μg/ml was prepared in deionized water and in supernatant of both unloaded chitosan and chitosan/alginate particles and analyzed at a wavelength of 570 nm (n = 9) according to the described method. The means of the resultant absorbance values were compared by Student’s t test at 95% confidence level.
Linearity and Range
For the determination of linearity, seven different concentrations of C48/80 in distilled water were prepared from the stock solution and analyzed. The stock solution of C48/80 was prepared in distilled water at the concentration of 2 mg/ml and the calibration standards were prepared by diluting the stock solutions to 5, 10, 20, 40, 80, 120, and 160 μg/ml. In a similar way, the calibration curves were prepared using the supernatants of chitosan-unloaded and chitosan/alginate-unloaded particles. The linearity of the method in the proposed range was evaluated by least square regression analysis. The linearity and range of the proposed methods were evaluated in three independent experiments.
Accuracy
The accuracy of the proposed method was investigated by spiking the supernatant of unloaded particles with known concentrations of C48/80 at three different levels (lower, intermediate, and higher concentrations) corresponding to C48/80 final concentrations of 10, 80, and 160 μg/ml (n = 6). The percent recovery of the added compound was calculated using Eq. 1:
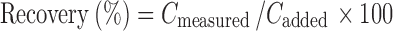 |
1 |
Precision
The intraday precision was evaluated by measuring different levels of C48/80 concentrations (10, 80, and 160 μg/ml) in triplicates at the same day under the same experimental conditions. The interday precision was evaluated following the same procedure for the three different days (n = 9). The precision of the measurements was reported as the relative standard deviation (%RSD).
Detection and Quantification Limits
The DL and QL were determined based on the standard deviation of the response and on the slope of the calibration curve, according to Eqs. 2 and 3, respectively:
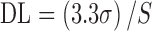 |
2 |
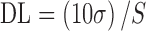 |
3 |
where S is the slope of the calibration curve and σ is the standard deviation of the y-intercept of the regression equation (n = 9).
Application of the Method
The developed method was applied for the determination of C48/80 LE in chitosan and chitosan/alginate particles. C48/80-loaded chitosan and chitosan/alginate nanoparticles had a mean size of 501 ± 65 nm (n = 19) and 564 ± 201 nm (n = 20), respectively. Both delivery systems were positively charged. Chitosan/alginate had a mean zeta potential of +25.5 ± 4.0 mV (n = 15) and chitosan particles of +23.8 ± 3.7 mV (n = 12). The C48/80-loaded particles were prepared as described above and the supernatant collected by centrifugation. C48/80 LE was determined indirectly by quantifying the C48/80 not associated with particles (supernatant) using Eq. 4:
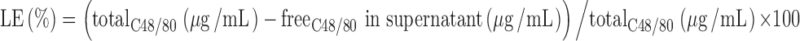 |
4 |
The supernatants of unloaded particles were used as solvents for the calibration curve. Blank matrix of chitosan/alginate particles was used for the determination of C48/80 LE in chitosan/alginate particles and the blank matrix of chitosan particles was used for the C48/80 LE assessment in chitosan particles.
Statistical Treatment
All data analyses described above were done using GraphPad Prism (GraphPad Software, Inc., La Jolla, California, USA).
RESULTS AND DISCUSSION
Microplate readers detect and process biological and chemical data using absorbance, luminescence, and fluorescence simultaneously for a great number of samples. Microplate readers using absorbance (UV–Vis) are widely used in laboratories as the reagents used in protocols are less expensive, when compared to fluorescence or luminescence detection. For that reason, the first-line detection method is the determination of absorbance, and frequently, the protocols indicate the utilization of 96-well plates as a physical support to measure simultaneously 96 small-volume samples (maximum volume of around 250 μl). What appears to be a detail constitutes an important and decisive advantage of the microplate readers over conventional spectrophotometer protocols. Examples of standard protocols, which are routinely used in laboratories using microplate readers, with absorbance as the detection method, are protocols for nucleic acids, enzyme activity, and protein quantification. In this paper, we describe the validation of a spectrophotometric method, using the 96-well plate, for the quantification of C48/80. The method was also validated for samples obtained by centrifugation of freshly prepared C48/80-loaded particles. During the development of this method, the effect of several parameters was evaluated by modifying one parameter and maintaining the others unchanged. Factors such as reaction time, concentration of acetaldehyde or sodium nitroprusside, and buffer solution characteristics were studied, and the most favorable conditions were established and rigorously followed in validation experiments. The optimal pH for the reaction was described elsewhere (9) to be between 9.6 and 10.2. We found that the pH is the most critical factor and the selected carbonate buffer should be freshly prepared (once weekly). The samples were measured 10 min after the formation of the C48/80–acetaldehyde–sodium nitroprusside complex. During this period, there is no need to determine light sensitivity. Similar to the BCA protein assay, this reaction does not reach a true end point, so color development will continue after the recommended measurement time. Linearity is still observed at 30 min (data not shown), although the reading at a later point may not be accurate. Finally, the observation of the correct storage of acetaldehyde is also a critical factor. It should be stored under an inert atmosphere since aldehyde oxidation easily occurs, which would compromise the final color development.
Specificity
The assay was performed with C48/80 solutions at 80 μg/ml in order to confirm the suitability of the method to unequivocally determine the concentration of C48/80 in the presence of other components that may be present (for example, compounds that were not incorporated during the preparation of the particles or compounds that were released after particle preparation). The statistical treatment of the results allowed us to observe that the calculated t values were higher than the tabulated t values, indicating that there are statistical differences between the mean absorbance of the C48/80 in water or in the presence of the nanoparticle constituents (Table I). These differences are possibly related to the interaction of the matrix components with C48/80 affecting the results measured. In order to minimize the interference of other constituents present in the formulations, all validation parameters were evaluated not only with distilled water but also with the supernatants of unloaded chitosan and chitosan/alginate nanoparticles so the suitability of these matrices as solvents for C48/80 quantification could be assessed.
Table I.
Statistical Data of the Regression Equations and Resume of Validation Parameters for C48/80 (n = 9); 570 nm, 10 min
| Parameter | H2O | Chi NPs supernatant | Chi/Alg NPs supernatant |
| Optical characteristics | |||
| Molar absorptivity, λ = 570 nm (L mol−1 cm−1)a | 122.10 | 99.14 | 113.78 |
| Regression analysis (n = 9) | |||
| Slope | 0.005197 ± 3.124 × 10−5 | 0.005267 ± 5.733 × 10−5 | 0.005269 ± 4.455E − 005 |
| 95% confidence interval of slope | 0.005116 to 0.005277 | 0.005119 to 0.005414 | 0.005155 to 0.005384 |
| Intercept | 0.009681 ± 0.002601 | −0.0008062 ± 0.004773 | 0.00022210 ± 0.003710 |
| 95% confidence interval of intercept | 0.005186 to 0.01214 | −0.01308 to 0.01147 | −0.009315 to 0.009759 |
| Regression coefficient (R 2) | 0.9998 | 0.9994 | 0.9996 |
| SD of the residuals (sy.x) | 0.00458 | 0.008405 | 0.006532 |
| Validation parameters | |||
| Specificity, t cal (t crit)b | 20.58 (2.12) | 5.887 (2.12) | |
| Linearity (μg/ml) | 5–160 | 5–160 | 5–160 |
| Detection limit (μg/ml) | 0.93 | 0.71 | 1.01 |
| Quantification limit (μg/ml) | 2.80 | 2.15 | 3.30 |
aMolar absorptivity for the monomer of C48/80, MW = 153 g/mol
b t test comparing absorbance in supernatant of unloaded nanoparticles to absorbance values in distilled water. t cal is the calculated t value and t crit is the tabulated t value based on unpaired t test at α = 0.05 level of significance
Linearity and Range
According to the results of the regression analysis (Table I), the method was found to be linear over the concentration range of 5 to 160 μg/ml for the three matrices used at good correlation coefficients (0.9994 to 0.9998) (Fig. 2). The goodness of fit of the regression equations was supported by the low standard deviations of the residuals.
Fig. 2.

Calibration curves obtained with C48/80 standard solutions in water and in supernatant of unloaded nanoparticles using the proposed spectrophotometric method (n = 9)
Accuracy
Accuracy was assessed by the recovery of the C48/80 added as a spike into the supernatant of unloaded nanoparticles. In order to evaluate the effect of the matrix, the percentage of recovery was assessed by two methods, using the calibration curve done with deionized water and the calibration curve in the corresponding supernatant of unloaded particles. The mean values of the percent recovery for each concentration level of C48/80 are shown in Table II. For chitosan particle supernatants, mean recoveries of 89.31% to 100.95% and 100.88% to 105.90% were found, calculated by applying the calibration curve prepared in water and in particle supernatant, respectively. In chitosan/alginate particle supernatant, recoveries of the C48/80 were 96.84% to 103.32% and 98.05% to 101.30% for determinations with calibration curve in water and in particle supernatants, respectively. When the calibration curve in water is used for the determination of the percentage of C48/80 recovery in chitosan particle supernatants, the mean recovery values obtained for concentrations of 80 and 160 μg/ml (89.31% and 93.04%, respectively) are out of the suggested acceptable range often considered to be between 98% and 101%. However, when using the calibration curve in the supernatant of unloaded particles, the mean recovery values (Table III) were within the acceptable range (14) and %RSD values were lower than the recommended values predicted from the Horwitz equation (Table III adapted from (15)).
Table II.
Results of Recovery (in Percent) for C48/80 from Spiked Samples (n = 6)
| Sample | Method | C48/80 added (μg/ml) | Measured concentration (μg/ml) ± SD | Percent recovery ± SD | Confidence interval 95% | %RSD |
|---|---|---|---|---|---|---|
| Chi NPs | Water | 10 | 10.09 ± 1.09 | 100.95 ± 10.86 | ±8.69 | 10.76 |
| 80 | 71.45 ± 3.52 | 89.31 ± 4.40 | ±3.52 | 4.92 | ||
| 160 | 148.86 ± 10.02 | 93.04 ± 4.40 | ±5.01 | 6.73 | ||
| NPs supernatant | 10 | 10.59 ± 0.90 | 105.9 ± 9.02 | ±7.22 | 8.52 | |
| 80 | 79.92 ± 1.25 | 101.50 ± 3.49 | ±2.80 | 3.44 | ||
| 160 | 161.42 ± 1.42 | 100.88 ± 0.89 | ±0.71 | 0.88 | ||
| Chi/Alg NPs | Water | 10 | 9.68 ± 0.59 | 96.84 ± 5.88 | ±4.71 | 6.08 |
| 80 | 82.66 ± 1.06 | 103.32 ± 1.33 | ±1.06 | 1.28 | ||
| 160 | 159.13 ± 1.29 | 99.45 ± 0.81 | ±0.65 | 0.81 | ||
| NPs supernatant | 10 | 10.12 ± 1.07 | 101.30 ± 10.76 | ±8.61 | 10.62 | |
| 80 | 80.62 ± 4.27 | 100.78 ± 4.34 | ±4.28 | 5.30 | ||
| 160 | 156.89 ± 2.44 | 98.05 ± 1.53 | ±1.22 | 1.56 |
Table III.
Summary of Acceptance Criteria for RSD According to the Horwitz Equation (%RSD = 2 (1–0.5 logC)) and for Mean Recovery (in Percent) for Each One of Concentration Levels Assayed
| Analyte (μg/ml) | Analyte (%) | Analyte ratio | Horwitz %RSD | Mean recovery (%) |
|---|---|---|---|---|
| 10 | 0.001 | 1.00E − 05 | <11.3 | 80–110 |
| 80 | 0.008 | 8.00E − 05 | <8.3 | 90–107 |
| 160 | 0.016 | 1.60E − 04 | <7.5 | 95–105 |
Precision
The precision of the proposed method was evaluated by the assessment of repeatability (intraday) and intermediate precision (interday). Precision was evaluated in three different matrices: deionized water, unloaded chitosan particle supernatant, and unloaded chitosan/alginate particle supernatant. The results are shown in Tables IV, V, and VI, respectively. Repeatability refers to the precision of the method carried out under the same operating conditions over a short interval of time. For the three analytical methods, repeatability (RSD) ranged from 1.62% to 7.48% at 10 μg/ml, from 0.98% to 2.09% at 80 μg/ml, and from 0.28% to 1.10% at 160 μg/ml concentration levels of C48/80.
Table IV.
Intraday and Interday Precision Results for the Method Using Water as the Solvent
| Standard solution (μg/ml) | Day | Measured (μg/ml) | SD | %RSD | Confidence interval 95% |
|---|---|---|---|---|---|
| Intraday variation (n = 3) | |||||
| 10 | 1 | 9.52 | 0.34 | 3.57 | ±0.38 |
| 2 | 9.60 | 0.69 | 7.23 | ±0.78 | |
| 3 | 10.36 | 0.68 | 6.56 | ±0.77 | |
| 80 | 1 | 80.89 | 1.22 | 1.51 | ±1.39 |
| 2 | 79.34 | 1.61 | 2.03 | ±1.83 | |
| 3 | 81.62 | 1.28 | 1.57 | ±1.45 | |
| 160 | 1 | 160.82 | 1.18 | 0.73 | ±1.34 |
| 2 | 156.26 | 0.44 | 0.28 | ±0.50 | |
| 3 | 159.04 | 0.68 | 0.43 | ±0.77 | |
| Interday (n = 9) | |||||
| 10 | 9.83 | 0.66 | 6.67 | ±0.43 | |
| 80 | 80.62 | 1.57 | 1.94 | ±1.02 | |
| 160 | 158.71 | 2.12 | 1.33 | ±1.38 | |
Table V.
Intraday and Interday Precision Results for the Method Using the Supernatant of Unloaded Chitosan Nanoparticles as the Solvent
| Standard solution (μg/ml) | Day | Measured (μg/ml) | SD | RSD | Confidence interval 95% |
|---|---|---|---|---|---|
| Intraday variation (n = 3) | |||||
| 10 | 1 | 11.35 | 0.85 | 7.48 | ±0.96 |
| 2 | 11.23 | 0.18 | 1.62 | ±0.21 | |
| 3 | 11.25 | 0.65 | 5.78 | ±0.74 | |
| 80 | 1 | 75.98 | 0.89 | 1.17 | ±1.01 |
| 2 | 75.17 | 0.73 | 0.98 | ±0.83 | |
| 3 | 80.88 | 1.39 | 1.72 | ±1.57 | |
| 160 | 1 | 161.56 | 0.51 | 0.32 | ±0.58 |
| 2 | 159.72 | 1.00 | 0.63 | ±1.13 | |
| 3 | 160.32 | 1.18 | 0.73 | ±1.33 | |
| Interday (n = 9) | |||||
| 10 | 11.88 | 0.53 | 4.43 | ±0.34 | |
| 80 | 77.53 | 2.78 | 3.59 | ±1.82 | |
| 160 | 160.40 | 0.93 | 0.58 | ±0.61 | |
Table VI.
Intraday and Interday Precision Result for the Method Using the Supernatant of Unloaded Chitosan/Alginate Nanoparticles as the Solvent
| Standard solution (μg/ml) | Day | Measured (μg/ml) | SD | RSD | Confidence interval 95% |
|---|---|---|---|---|---|
| Intraday variation (n = 3) | |||||
| 10 | 1 | 9.84 | 0.38 | 3.85 | ±0.43 |
| 2 | 11.01 | 0.48 | 4.40 | ±0.55 | |
| 3 | 11.05 | 0.69 | 6.23 | ±0.78 | |
| 80 | 1 | 82.16 | 1.03 | 1.26 | ±1.17 |
| 2 | 76.84 | 1.50 | 1.95 | ±1.70 | |
| 3 | 78.10 | 1.63 | 2.09 | ±1.85 | |
| 160 | 1 | 158.64 | 0.73 | 0.46 | ±0.83 |
| 2 | 162.40 | 1.79 | 1.10 | ±2.02 | |
| 3 | 160.00 | 1.68 | 1.05 | ±1.90 | |
| Interday (n = 9) | |||||
| 10 | 10.63 | 0.75 | 7.08 | ±0.49 | |
| 80 | 79.04 | 2.70 | 3.42 | ±1.77 | |
| 160 | 160.42 | 2.12 | 1.32 | ±1.39 | |
Intermediate precision hints at within-laboratory variation and was evaluated using the same method on identical test samples in the same laboratory and equipment but on different days. Intermediate precision (RSD) ranged from 4.43% to 7.08%, from 1.94% to 3.59%, and from 0.58% to 1.33% at lower, intermediate, and higher concentration levels, respectively. RSD values (in percent) found for the three analytical methods were within the acceptable range, indicating that these methods have good repeatability and intermediate precision.
Detection and Quantification Limits
The DL and QL for C48/80 in water were 0.93 and 2.80 μg/ml, respectively. In the supernatant of unloaded chitosan particles, the DL and QL were found to be 0.71 and 2.15 μg/ml, and in the supernatant of unloaded chitosan/alginate particles, the DL and QL were found to be 1.01 and 3.30 μg/ml, respectively. These values indicate that the method is sufficiently sensitive to evaluate the concentration of C48/80 in the supernatants of particles and so, indirectly, the extent of incorporation of the mast cell activator in the delivery systems.
Application of the Method
The proposed method was applied to determine the C48/80 LE of two chitosan-based delivery systems—chitosan particles and chitosan/alginate particles. Recovery studies revealed that the method was more accurate when utilizing the blank matrices of the particles to establish the calibration curve to evaluate the amounts of C48/80 present in the particle supernatants. Since the quantification method in supernatants of unloaded nanoparticles was found to be linear, precise, sensitive, and accurate for the determination of C48/80, these matrices were used instead of deionized water for the quantification of C48/80 in the supernatant of loaded particles. C48/80 LE was found to be 18.65 ± 2.99% for chitosan particles and 29.56 ± 1.59% for chitosan/alginate particles (mean ± SD; n = 12).
CONCLUSIONS
An inexpensive, rapid, sensitive, precise, and accurate small-volume UV spectrophotometric method for the determination of C48/80 was developed and validated. The simplicity of the method and the small amounts of sample and solvents required make this method attractive for C48/80 quantification in pharmaceutical dosage forms. When applied to the quantification of C48/80 in chitosan-based particles, a small effect of the other particle components was observed but was compensated by using the supernatants of unloaded formulations to establish the calibration curve. We could demonstrate that the developed method is accurate for the quantification of C48/80 in the samples of interest and so sufficient specificity of the method can be concluded (16). The observed matrix effect supports the possible need for partial method revalidation when samples in different matrices are used, as specified in different validation guidelines (13,17,18). The proposed method was already successfully applied for the determination of C48/80 incorporated into two chitosan-based delivery systems and would be useful during the development and characterization of other C48/80 formulations.
ACKNOWLEDGMENTS
This study was financially supported by the Portuguese Foundation of Science and Technology (FCT), grant SFRH/BD/65141/2009, and project FCT PTDC/SAL-FAR/115044/2009.
REFERENCES
- 1.Rothschild AM. Mechanisms of histamine release by compound 48–80. Br J Pharmacol. 1970;38(1):253–262. doi: 10.1111/j.1476-5381.1970.tb10354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14(5):536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 3.Staats HF, Fielhauer JR, Thompson AL, Tripp AA, Sobel AE, Maddaloni M, et al. Mucosal targeting of a BoNT/A subunit vaccine adjuvanted with a mast cell activator enhances induction of BoNT/A neutralizing antibodies in rabbits. PLoS One. 2011;6(1):e16532. doi: 10.1371/journal.pone.0016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009;27(27):3544–3552. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SH, Kirwan SM, Abraham SN, Staats HF, Hickey AJ. Stable dry powder formulation for nasal delivery of anthrax vaccine. J Pharm Sci. 2012;101(1):31–47. doi: 10.1002/jps.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vajdy M, Srivastava I, Polo J, Donnelly J, O’Hagan D, Singh M. Mucosal adjuvants and delivery systems for protein-, DNA- and RNA-based vaccines. Immunol Cell Biol. 2004;82(6):617–627. doi: 10.1111/j.1440-1711.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- 7.Paton WD. Compound 48/80: a potent histamine liberator. Br J Pharmacol Chemother. 1951;6(3):499–508. doi: 10.1111/j.1476-5381.1951.tb00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CM, Wagner C. Quantitative determination of secondary amines: measurement of N-methylalanine. Anal Biochem. 1974;60(1):278–284. doi: 10.1016/0003-2697(74)90155-9. [DOI] [PubMed] [Google Scholar]
- 9.Cullis CF, Waddington DJ. The colorimetric determination of secondary amines. Anal Chim Acta. 1956;15:158–163. doi: 10.1016/0003-2670(56)80030-5. [DOI] [Google Scholar]
- 10.O’Neal CL, Crouch DJ, Fatah AA. Validation of twelve chemical spot tests for the detection of drugs of abuse. Forensic Sci Int. 2000;109(3):189–201. doi: 10.1016/S0379-0738(99)00235-2. [DOI] [PubMed] [Google Scholar]
- 11.Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier—systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B Biointerfaces. 2007;59(1):24–34. doi: 10.1016/j.colsurfb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate. J Pharm Sci. 1993;82(9):912–917. doi: 10.1002/jps.2600820909. [DOI] [PubMed] [Google Scholar]
- 13.ICH Harmonized Tripartite Guideline. Validation of analytical procedures: text and methodology Q2(R1). 2005.
- 14.Huber L, editor. Validation and qualification in analytical laboratories. 2. New York: Informa Healthcare; 2007. [Google Scholar]
- 15.Taverniers I, De Loose M, Van Bockstaele E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends Anal Chem. 2004;23(8):535–552. doi: 10.1016/j.trac.2004.04.001. [DOI] [Google Scholar]
- 16.Ermer J, Burgess C, Kleinschmidt G, McB. Miller JH. Performance parameters, calculations and tests. Method validation in pharmaceutical analysis. Weinheim: Wiley-VCH; 2005. p. 21–194.
- 17.Thompson M, Ellison SLR, Wood R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC technical report). Pure Appl Chem. 2002;74:835–55.
- 18.Eurachem. The fitness for purpose of analytical methods: a laboratory guide to method validation and related topics. http://www.eurachem.org/guides/pdf/valid.pdf (1998).


