Abstract
Onychomycosis is associated with the cutaneous fungal infection of the nail and the nail folds (skin surrounding the nail). It is therefore important to target drug delivery into the nail folds along with nail plate and the nail bed. Systematic and strategic selection of the penetration enhancers specific for the skin and the nail is discussed. Twelve penetration enhancers were screened for their ability to improve solubility, in vitro nail penetration, in vitro skin permeation, and in vitro skin penetration of the antifungal drug ciclopirox olamine. In contrast to transdermal drug delivery, the main selection criteria for skin penetration enhancer in topical drug delivery were increased drug accumulation in the epidermis and minimal permeation across the skin. Thiourea improved the solubility and nail penetration of ciclopirox olamine. It also showed enhancement in the transungual diffusion of the drug. Propylene glycol showed a 12-fold increase in solubility and 3-fold increase in epidermal accumulation of ciclopirox olamine, while minimizing the transdermal movement of the drug. Thiourea was the selected nail permeation enhancer and propylene glycol was the selected skin penetration enhancer of ciclopirox olamine. A combination of the selected enhancers was also explored for its effect on drug delivery to the nail and nail folds. The enhancer combination reduced the penetration of ciclopirox in the skin and also the permeation through the nail. The proposed preformulation strategy helps to select appropriate enhancers for optimum topical delivery and paves way towards an efficient topical formulation for passive transungual drug delivery.
Key words: ciclopirox olamine, onychomychosis, penetration enhancers, preformulation, topical
INTRODUCTION
Onychomycosis is the infection of toe nails and fingernails, characterized by thickening and discoloration of the nail and involving the nail apparatus. The nail apparatus consists of the proximal nail folds, lateral nail folds, the nail matrix, nail bed, nail plate, and hyponychium. Any of these or all of these associated tissues can get infected depending upon the type and the stage of the infection (1). Apart from the cosmetic effect of onychomychosis, there is a severe impact on the quality of life of the patients suffering from the disease (2–6). Onychomycosis acts as an opportunistic disease in patients suffering from HIV/AIDS (7–10). The patients suffering from psoriatic nails are reported to show greater occurrence of toe nail onychomycosis than patients without psoriasis (11). However, it is not confirmed if psoriasis predisposes the nails to onychomycosis, but psoriatic nails show similar symptoms to onychomycosis and it is therefore important to rule out the presence of fungal infection in psoriatic nails (12). Patients suffering from diabetes and peripheral neuropathy face additional complications due to onychomychosis including the risk of terminal limb amputations (10,13). These patient subpopulations are on a number of medications to treat the primary indication. Therefore, the treatment of onychomychosis with systemic antifungal agents has to be proceeded with caution in these patients to avoid drug–drug interactions and hepatotoxicity. Currently, the topical treatment approaches are limited to: (1) mild to moderate stages of the disease, (2) in combination with other systemic agents, (3) to prevent relapse, and (4) in cases where the patients are not good candidates for oral antifungal therapy (14). The topical therapy can be improved by improving the amount of drug reaching the target tissues. This would result in shortening the duration of therapy. The primary target tissues for therapy are the nail plate and the nail bed (15). The infection of the nail folds (paronychia) is associated with proximal subungual onychomycosis, distal and lateral subungual onychomycosis, and primary total dystrophic onychomycosis (1). The skin infection and inflammation are caused by the dermatophytes associated with onychomychosis (16). The package insert of Penlac 8% topical solution also states “PENLAC® NAIL LACQUER (Ciclopirox) Topical solution, 8% should be applied evenly over the entire nail plate and 5 mm of surrounding skin.”
It is therefore important to consider drug delivery into the nail folds (skin surrounding the nail) to ensure complete topical treatment. Ciclopirox olamine (CPO) is the hydroxyl ethanolamine salt form of the free acid Ciclopirox. CPO was used as the antifungal drug in the current project because it shows better aqueous solubility than the free acid and shows a unique mechanism of antifungal activity (17). The ethanolamine salt was therefore expected to permeate the hydrophilic nail plate better than the free acid. One of the approaches used to increase passive permeation of topically applied antifungal drugs into and through the nail is the use of chemical penetration enhancers (PEs). Table I shows the PEs used in the present study and their reported mechanism of action.
Table I.
Screened Penetration Enhancers for Transungual Formulation
| No. | Penetration enhancers (PEs) | Abbreviation | Concentration of PEs (% w/w) | Mechanism of action (18,19) |
|---|---|---|---|---|
| 1 | Diethylene glycol monoethyl ether or (transcutol) | TCL | 100 | Increase drug solubility and formation of drug depot in skin |
| 2 | N-methyl pyrrolidone | NMP | 100 | Altering membrane solvent nature, increase drug partition into skin |
| 3 | Poly(oxyethylene)(4) lauryl ether or BRIJ 30 | B30 | 2.5 | Potential to solubilize lipids in stratum corneum of skin, increase solubility of drug, emulsify sebum |
| 4 | Polyoxyethylene (20) oleyl ether or BRIJ 98 | B98 | 2.5 | |
| 5 | Sorbitan laurate or SPAN 20 | S20 | 2.5 | |
| 6 | Propylene glycol | PG | 100 | In the skin shows “solvent drag,” solubilizes α keratin in SC |
| 7 | Polyethylene glycol 400 | PEG | 100 | |
| 8 | Isopropyl myristate | IPM | 100 | Penetrates into lipid bilayers and increases lipid fluidity |
| 9 | Oleic acid | OA | 100 | Modify intercellular lipid domains and fluidizes the membrane |
| 10 | Thiourea | TU | 5 | Reduces disulphide linkages in keratin of nail |
| 11 | Thioglycolic acid | TA | 5 | |
| 12 | Urea-hydrogen peroxide | UH | 8.75 | Oxidizes disulphide linkages in nail |
It was hypothesized that drug delivery into the two target tissues—human hail plate and the nail folds (skin)—with the use of specific PEs will improve the drug levels in these target tissues and subsequently increase the efficiency of the resulting topical formulation. The main objective of the current work was to screen the PEs and select suitable enhancers for delivery of the antifungal drug CPO into the nail and nail folds. Figure 1 shows the proposed preformulation screening strategy for selection of PEs for transungual and epidermal drug delivery. The enhancers selected from the above strategy will be incorporated into a topical formulation.
Fig. 1.
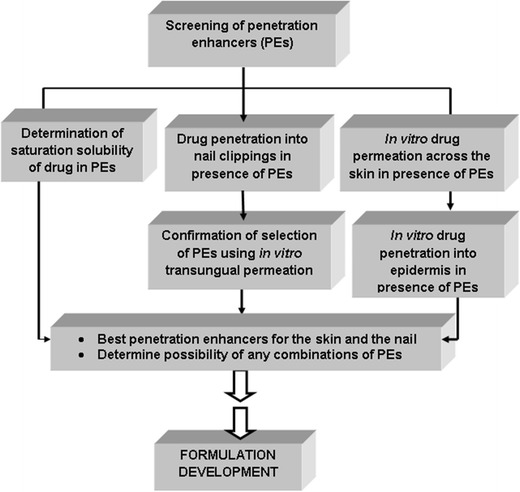
Proposed preformulation strategy for selection of PEs for transungual drug delivery
MATERIALS AND METHODS
CPO was purchased from Haorui Pharma-Chem Inc., Edison, New Jersey, USA. N-methyl-pyrrolidone (NMP) and propylene glycol (PG) (MW 400) were obtained from JT Baker. Transcutol (TCL) and BRIJ 98 (B98) were purchased from Acros Organics. B30, SPAN 20 (S20), sodium hydroxide, gentamicin sulfate, dimethyl sulfate, and triethylamine were purchased from Sigma-Aldrich. PG was obtained from MP Biochemicals. Oleic acid (OA) was bought from EMD. Isopropyl myristate (IPM) was purchased from Alfa Aesar. Franz diffusion cells and neoprene nail adapters were purchased from PermeGear. Cadaver skin and cadaver toenails were purchased from AlloSource, Cincinnati, and Anatomy Gifts Registry, Maryland, respectively. Nanopure water was used for the preparation of 10 mM pH 7.4 phosphate-buffered saline (pH 7.4 PBS). HPLC grade acetonitrile from Fischer Scientific was used for the preparation of mobile phase. All the chemicals were used as received without any further purification.
Sample Preparation for Analysis
The assay of CPO in the nail was performed by first dissolving the nails in 0.5 mL of 1 M sodium hydroxide (NaOH) solution. The nail solutions were filtered through Restek® 0.45 μm syringe filters (cellulose acetate membrane). These solutions were diluted sufficiently to reduce the strength of 1 M NaOH to 0.1 M before derivatization. For the derivatization of CPO, 25 μL of dimethyl sulfate was added to 0.2 mL of the dilute nail solution and vortexed for 5 min. These solutions were maintained at 37°C for 20 min. Twenty-five microliters of triethylamine was added and vortexed for 1 min to terminate the reaction. The solutions were then filtered through Restek® 0.22 μm syringe filters (cellulose acetate membrane) and analyzed using the HPLC method. In order to quantify the CPO in the buffer, a similar pre-column derivatization process was used. To 0.2 mL of the samples, 50 μL of 0.1 M NaOH and 25 μL of dimethyl sulfate were added and vortexed for 5 min. The solutions were maintained at 37°C for 20 min prior to the addition of 25 μL of triethylamine. The solutions were vortexed, filtered through Restek® 0.22 μm syringe filters (cellulose acetate membrane) and analyzed using HPLC.
High Pressure Liquid Chromatography
CPO content in the samples was assayed using an Agilent1100 series HPLC with dual wavelength detector, using a modified, pre-column derivatization method (20–22). Acetonitrile/water (50:50 v/v) was used as the mobile phase with a 150 × 4.6-mm Eclipse Plus C18 column (Agilent). The flow rate was 1 mL/min and the injection volume was 50 μL. The wavelength of detection for derivatized CPO was 298 nm.
Recovery of CPO from Human Nails
Solutions of CPO in pH 7.4 PBS with concentrations 0.0253, 0.0505, 0.202, 0.809, 3.24, and 6.47 mg/mL were prepared. Approximately 20 mg of human nail clippings was added to each concentration of CPO solution. Each concentration was studied in triplicate. The nail clippings were treated with 200 μL of each of these solutions for 3 h at 32 ± 1°C. To dissolve the nails, 200 μL of 1 M NaOH was added to each sample. The nail solutions were diluted using pH 7.4 PBS to reduce the concentration of 1 M NaOH to 0.1 M and the sample preparation method was followed to quantify the concentration of CPO.
The Recovery of CPO from Epidermis and Dermis
A stock solution containing 1.6 mg/mL of CPO in pH 7.4 PBS was prepared. Solutions with the concentrations 0.1, 0.2, 0.4, 0.8, and 1.2 mg/mL were prepared using the stock solution. Fifty microliters of each of these solutions, including the stock, contains 5.2, 10.4, 20, 40, 60, and 80 μg of CPO, respectively. Each of the above concentrations was studied in triplicate. The skin sections with 9 mm diameter were punched from a piece of human cadaver skin, using a cork borer. The epidermis and the dermis were separated for each section, using the modified heat separation method (23,24). Both the epidermis and the dermis, for each skin section, were separately treated with 50 μL of the drug solutions for 4 h in microcentrifuge tubes. After the drug treatment, 1 mL of the mobile phase was added to each of the microcentrifuge tubes and equilibrated for 24 h at 37°C for drug extraction. The extracting medium was filtered through Restek® 0.22 μm syringe filters, derivatized, and analyzed using HPLC.
Determination of the Saturation Solubility of CPO in the PEs
An excess amount of CPO was added to 0.5 mL of each PE at the concentration levels shown in Table I. The drug was allowed to equilibrate with the PEs for a period of 72 h at 32 ± 1°C. A saturated solution of CPO in pH 7.4 PBS served as the control solution. At the end of 72 h, the solutions were filtered through a 0.22-μm syringe filter. The samples were derivatized and analyzed using the HPLC method mentioned previously. The enhancement in solubility, EFsol, of CPO in presence of the PEs was calculated using the equation below.
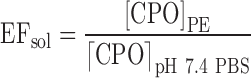 |
Where [CPO]PE is saturation solubility of CPO in presence of PEs and [CPO]pH 7.4 PBS is the saturation solubility of CPO in pH 7.4 PBS (control).
The In Vitro Nail Penetration of CPO
The PEs were screened for their ability to increase the concentration of the drug within the nail, using human nail clippings. The saturated solutions of CPO in the PEs and pH 7.4 PBS were prepared. Fourteen milligrams of previously washed and dried human nail clippings was weighed into the microcentrifuge tubes. The nail clippings were obtained from healthy volunteers (20–50 years) in the Department of Pharmaceutical Sciences, Temple University. The nail clippings were treated for 24 h with 0.5 mL of saturated solution of the drug in the enhancers at 32 ± 1°C. As a control, the nail clippings were treated with a saturated solution of CPO in pH 7.4 PBS. After 24 h of drug treatment, the nail clippings were washed three times with 5 mL of Nanopure water to remove any surface-adherent drug. The nail clippings were dried using tissue paper and were dissolved in 0.5 mL of 1 M sodium hydroxide solution at 37°C. The nail solutions were filtered using 0.45 μm syringe filters, derivatized, and analyzed using HPLC as described in the previous sections. This method is a modification of the screening method described in literature (18,25,26). The enhancement factor, EFnail, gives the improvement in CPO penetration into nail clippings relative to that in the control.
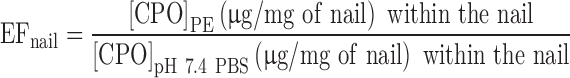 |
Where [CPO]PE and [CPO]pH 7.4 PBS are the concentrations of CPO within the nail clippings in the presence of PEs and pH 7.4 PBS (control), respectively.
The study is approved for use of human nail clippings by the Temple University Institutional Review Board (protocol number 13671).
The In Vitro Transungual Permeation of CPO
The nail penetration study helps to screen different PEs; however, the results have to be confirmed with an in vitro transungual permeation study. The in vitro transungual permeation of CPO was performed using only the PEs which showed promise in the nail penetration screen. The human cadaver toenails (Anatomy Gifts Registry) were washed with Nanopure water and blotted with tissue paper. The weights and thicknesses of the toenails were measured. The human cadaver toenails (Fig. 2a) were hydrated at 100% RH for 24 h, prior to mounting on Franz diffusion cell nail adapters (Fig. 2b). The nail adapters (3 mm orifice diameter) with toenails were placed between the donor and receiver compartments of the Franz cells (Fig. 2c). The receiver compartment was filled with 3 mL of pH 7.4 PBS containing 0.1% w/v gentamicin sulfate. Gentamicin sulfate was added to prevent microbial growth in the receiver compartment. The donor compartment was dosed daily with 21.4 μL of saturated solution of CPO in the potential enhancer from the nail penetration experiment. The temperature of 32 ± 1°C was maintained for the duration of 27 days. The study was performed under occlusion. On days 5, 10, 15, 18, 21, 24, and 27, 0.5 mL of the receiver compartment solution was sampled and analyzed for drug content. Additionally, on day 27, the nails were dismounted from the nail adapters and the drug content was quantified by separating the nail just below the orifice and the nail surrounding the orifice (Fig. 2d). The drug quantification was performed using the process described in the sample preparation.
Fig. 2.
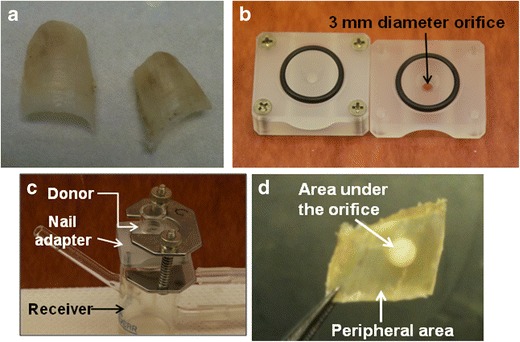
a Human cadaver toenails. b Neoprene nail adapters with orifice diameter of 3 mm. c Franz diffusion cell showing the donor compartment, receiver compartment, and the nail adapter. d Area under the orifice of nail adapter and the peripheral nail
In Vitro Skin Permeation of CPO
The in vitro skin permeation of CPO was performed using the PermeGear Franz diffusion apparatus with the internal volume of 3 mL and orifice diameter of 9 mm. The human cadaver skin (AlloSource) was placed between the donor and the receiver compartments. The receiver compartment was filled with 3 mL of pH 7.4 PBS and the skin was allowed to equilibrate with the receiving medium for 60 min, prior to drug dosing. Twenty microliters of the saturated solution of the drug in the penetration enhancers was added to the donor compartment. The study was performed at 32 ± 1°C for a period of 24 h under occlusion. Each of the penetration enhancers were tested in triplicate and the data were compared with the control. The control cells were dosed with 20 μL of saturated solution of CPO in 10 mM pH 7.4 PBS. At 2, 4, 6, 8, 22, and 24 h, 0.5 mL of the receiver compartment solution was withdrawn and replaced with 0.5 mL of fresh buffer to maintain sink conditions. The drug content in the samples was analyzed using the HPLC method. The flux (J) for permeation (in micrograms per square centimeters per hour) was obtained from the slope of the linear potion of the plot of cumulative amount of CPO permeating per unit surface area of skin (in micrograms per square centimeter) against time (in hours). The permeability coefficient Kp was calculated from the ratio of the steady-state flux (J) and the concentration in the donor compartment (Cd). Since saturated solutions were used as donor solutions, Cd was the saturation solubility of CPO for all practical purposes of the calculation. The enhancement in flux for skin permeation (EFfinite flux) was calculated using the equation:
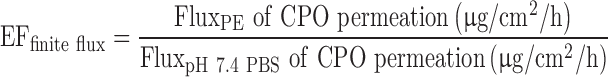 |
where FluxPE and FluxpH 7.4 PBS are the steady-state flux values for the permeation of CPO across the skin in presence of the PEs and pH 7.4 PBS (control), respectively.
Finite Dose Skin Penetration of CPO
The skin used in the finite dose permeation study was further analyzed for drug content by separating the epidermis and the dermis using a modified heat separation method (23,24). Post the finite dose skin permeation experiment, the skin was dismounted from the Franz cell and washed with Nanopure water thrice on both the epidermal and the dermal sides to remove any surface drug. The skin was then blotted with tissue paper; the area exposed to the drug was separated from the peripheral skin and wrapped in aluminum foil. Each of the samples was heated in the oven at 45°C for 10 min. The epidermis was then peeled from the dermis using sharp forceps. The thickness of the epidermis and the dermis was measured using an electronic digital micrometer (Marathon Management). The two layers were then placed in separate glass vials containing 1 mL of the mobile phase each, for 24 h to extract CPO. The concentration of the drug in the extracting medium was quantified using HPLC. The drug levels were reported in micrograms per milliliter to determine if the minimum inhibitory concentration (MIC) was achieved in the epidermis and the dermis. The enhancement factors, EFepidermis and EFdermis, give the increase in accumulation of CPO in the epidermis and dermis relative to the control.
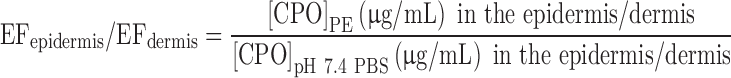 |
Where [CPO]PE and [CPO]pH 7.4 PBS are concentrations of CPO in the epidermis/dermis in presence of PEs and pH 7.4 PBS (control), respectively.
Analysis of Results
Relationship plots with EFnail, EFflux, EFepidermis, or EFdermis on the y-axis and EFsol on the x-axis were prepared to understand how the different enhancement factors were affected by change in solubility. These plots also helped to identify the mechanism of action of the PEs in the two different biological membranes—skin and the nail. Each of the relationship plots was divided into four quadrants (I to IV) by drawing lines parallel to the x- and y-axes, passing though enhancement factor 1 on each axes. The EF of 1 stands for the control sample used in the determination of the respective enhancement factor. This method of analysis is a modification of the reported method (25).
Statistical Analysis of Experimental Data
All the experiments were performed in triplicate and the data were reported as mean value (± standard deviation). A one-way ANOVA (Microsoft Excel 2007) was used to determine statistical significance, where the p value of less than 0.05 was considered as statistically significant.
RESULTS
High Pressure Liquid Chromatography
A linear response was attained in the range of 0.09 to 46.73 μg/mL with a correlation coefficient of 0.9996. The retention time, limit of quantification, and limit of detection for the derivatized CPO were 4.6 min, 90 ng/mL, and 25 ng/mL, respectively.
Recovery of CPO from Human Nail
A recovery of 86–97% was obtained for CPO in the concentration range of 0.25 to 64.7 μg/mg nail after dissolution in 1 M NaOH and the pre-column derivatization process.
Recovery of CPO from Epidermis and Dermis
Of ciclopirox olamine, 74.5% to 95.6% was recovered when epidermis with a diameter of 9 mm was treated with 5.2 to 80 μg of CPO after the extraction of the epidermis. The recovery of CPO from the dermis was greater than 95%.
Saturation Solubility of CPO in PEs
Figure 3 shows that the solubility of CPO was significantly higher in PG, OA, TCL, NMP, and thiourea (TU) compared to the control (pH 7.4 PBS). The nail penetration enhancer urea-hydrogen peroxide (UH) did not significantly increase the solubility of CPO. Thioglycolic acid (TA) caused precipitation of the free acid form of CPO. An increase in solubility of CPO in penetration enhancer increases concentration gradient across the skin. Determination of CPO solubility in the enhancers will also help to elucidate any correlation between improvement in permeation and/or penetration into the target tissues.
Fig. 3.
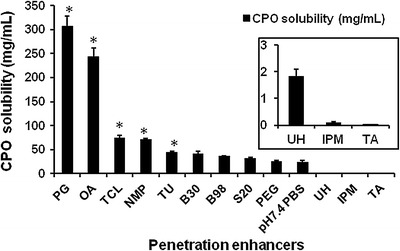
Solubility (in milligrams per milliliter) of CPO in the PEs. The asterisk indicates that the increase in solubility is statistically significant compared to the control (p value < 0.05)
The In Vitro Nail Penetration of CPO
A 5% w/w TU solution was the only agent to increase the penetration of CPO into the nail clippings compared to the control (Fig. 4a). The other nail PEs, TA, and UH did not improve the penetration of CPO into the nail clippings. None of the skin PEs had any effect on the penetration of CPO into the nail. The results from our preliminary studies (unpublished data) showed that this effect of TU is concentration dependent and greater levels of the drug penetrated at higher concentrations of TU (1% w/w). However, it was decided to continue the nail studies with 5% w/w TU because the higher concentrations made the nail very soft. This could be a clinically significant disadvantage. The plot of enhancement factors for nail penetration and solubility (Fig. 4b) shows that 5% w/w TU is the only agent in the third quadrant of the analysis plot which indicates improvements in solubility and nail penetration.
Fig. 4.
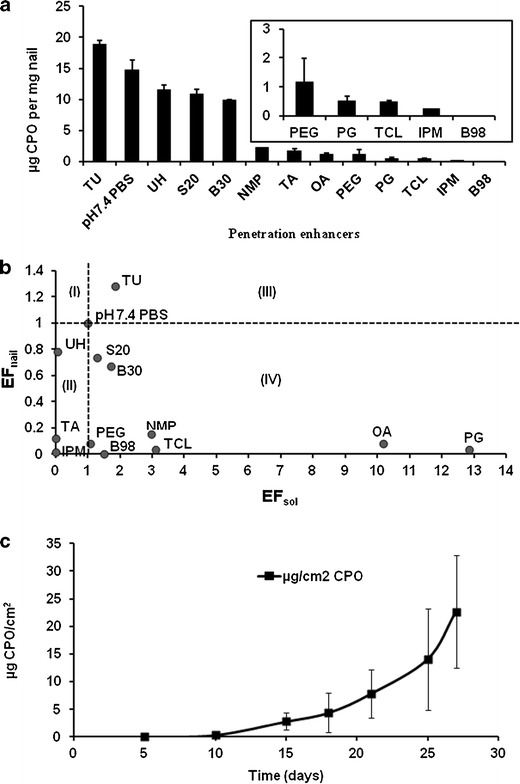
a Concentration (in micrograms per milligram nail) of CPO in the nail clippings in the presence of PEs. b Relationship between EFnail and EFsol for the PEs. c Transungual permeation of CPO through human nail in the presence of 5% w/w TU
The In Vitro Transungual Permeation
The ability of TU in enhancing drug penetration into the nail was confirmed in the in vitro transungual permeation of CPO through human cadaver toenails (Anatomy Gifts Registry). The toenails, obtained from a female donor (55 years of age), were smooth on the surface, normal and devoid of any symptoms (excessive thickness, dystrophy, and discoloration) of onychomychosis. The average thickness of the toenails was 0.96 ± 0.014 mm. Figure 4c shows the permeation profile of ciclopirox olamine through human cadaver nail. A permeation flux of 1.56 μg/cm2/day and a lag time of 14.5 days were observed. Of the CPO, 188.44 ± 39.83 and 6.81 ± 1.24 μg were attained in the orifice and the periphery of the toenails, respectively. These concentrations were significantly greater than the MIC of 0.03–0.25 μg/mL needed for CPO efficacy (27).
The In Vitro Skin Permeation
The permeation parameters are shown in Table II. The permeation of CPO through the skin increased 2.8-, 2.7-, and 1.5-fold in the presence of S20, B30, and NMP, respectively. S20 and B30 were found to be potential penetration enhancers for transdermal delivery of CPO. The permeation of CPO was lower than the control in presence of TCL and PG (Table II). The relationship between the enhancements in flux and solubility of CPO in presence of the penetration enhancers (Fig. 5) shows that S20, B30, B98, TU, and NMP in quadrant III which indicates both an increase the solubility of CPO and permeation through the skin. TCL, PG, and OA in quadrant IV significantly improve the solubility multiple folds but do not show a corresponding increase in the flux of permeation. IPM in quadrant II does not improve solubility or permeation. In topical drug delivery, the enhancers in the quadrants II and IV will be beneficial in minimizing the higher drug concentration reaching the systemic circulation.
Table II.
Permeation Parameters for In Vitro Skin Permeation of CPO in Presence of PEs
| PE | Flux (μg/cm2/h) | Lag time (h) | Q 24 h (μg/cm2) | Permeability coefficient, Kp (×10−5) (cm/h) |
|---|---|---|---|---|
| S20 | 4.40 | 4.3 | 97.71 | 14.34 |
| B30 | 4.23 | 4.1 | 92.03 | 10.26 |
| NMP | 2.43 | 3.2 | 50.39 | 3.41 |
| B98 | 2.03 | 4.6 | 42.78 | 5.59 |
| TU | 1.92 | 3.4 | 42.67 | 4.32 |
| pH 7.4 PBS | 1.58 | 2.3 | 38.89 | 6.60 |
| OA | 1.15 | 3.3 | 24.19 | 4.72 |
| IPM | 1.03 | 4.3 | 23.39 | 1,226.19 |
| TCL | 0.73 | 3.7 | 15.71 | 0.99 |
| TUPG | 0.047 | 1.7 | 1.16 | 0.017 |
| PG | 0.036 | 4.8 | 0.85 | 0.012 |
Fig. 5.
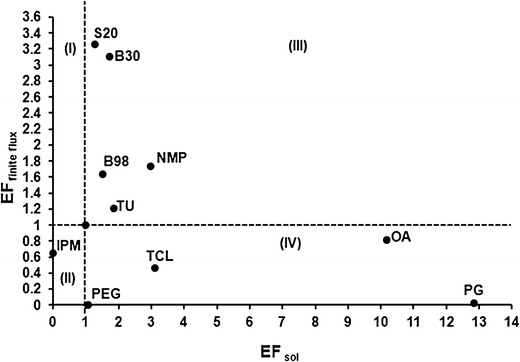
Relationship between EFfinite flux and EFsol for the PEs
In Vitro Skin Penetration
The concentration of CPO in the target tissue—epidermis of the skin—is the final criterion for selection of penetration enhancers for the topical formulation. Figure 6a shows the accumulation of CPO in epidermis and dermis of the skin in presence of the enhancers. The plot does not show standard deviation because the EFepidermis and EFdermis were calculated using the average concentrations of CPO within the skin in the presence of PEs and pH 7.4 PBS. PG, TCL, IPM, and OA showed 3.2-, 2.2-, 1.6-, and 1.03-fold enhancement in drug levels in the epidermis, respectively. The same agents showed 0.5-, 2.3-, 5.6-, and 0.6 fold improvements in drug levels within the dermis, respectively. The penetration enhancers in the quadrant III of Fig. 5c, NMP, B30, S20, and TU, showed 5.9-, 2.1-, 1.6-, and 0.6-fold accumulation in the epidermis. Though NMP and B30 show reasonable drug accumulation in the epidermis, the increase in transdermal permeation would increase the amount of drug in the systemic circulation, which could lead to the adverse effects. PG was the only agent which showed accumulation of CPO selectively in the epidermis and a low permeation flux of 0.04 μg/cm2/h. TCL did not cause any specific accumulation of CPO in a particular layer of the skin. Figure 6b also shows the relationship between EFepidermis and EFsol. PG has now moved to the third quadrant, showing a 12-fold improvement in solubility and a 3.2-fold increase in CPO levels in the epidermis.
Fig. 6.
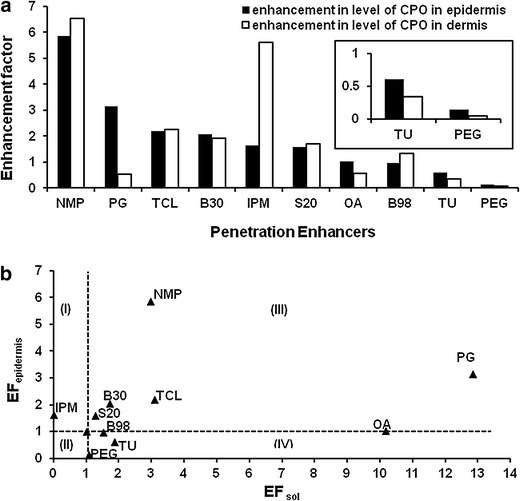
a The enhancement factor (EFepidermis and EFdermis) for the levels of CPO in the presence of the PEs. b The relationship between EFepidermis and EFsol for the PEs
Combination of PEs
Since two different PEs were selected for the nail and the skin, a combination of the two agents 5% TU and PG was studied for nail penetration, skin permeation, and skin penetration. The combination of 5% TU in PG (TUPG) showed 11.7-, 0.03-, 0.03-, and 1.1-fold enhancement in solubility, nail penetration, skin permeation flux, and level in epidermis for CPO. The permeation parameters for this combination are shown in Table II. Though the combination improved solubility and minimized transdermal permeation, it was not beneficial in improving drug levels within the nail and the epidermis. The individual desirable affects of 5% TU and PG were absent when 5% TU was prepared in PG (TUPG).
DISCUSSION
The concentration gradient is the driving force for the passive diffusion of drug molecules across a membrane. As shown in Table I, PEs act by various mechanisms: one mechanism is by increasing the concentration gradient of the drug across the membrane by increasing the solubility of the drug. Higher levels of solubilized form of CPO will ensure maintenance of high concentration gradient across the nail plate and the nail folds (skin). PG, NMP, TCL, and OA are known to be good solubilizing agents for various drug molecules.
The in vitro nail penetration screen identifies PEs that improve the drug concentration in the nail. However, the results of this study have to be confirmed with the in vitro transungual study because both the dorsal and the ventral surfaces of the nail clippings are exposed to the drug and the PE in the nail penetration study. Nevertheless, the nail penetration study is a high-throughput experiment to select potential nail penetration enhancers. One of the screening methodologies in literature for transungual delivery of antifungal agents shows that 5% w/v of TA significantly improves the in vitro penetration of CPO into human nail clippings (28). In the report, CPO was prepared as a 1 mg/mL solution using 0.5% SLS in pH 7.4 PBS containing TA. We found that CPO had low solubility of 2.5 μg/mL in 5% TA prepared in pH 7.4 PBS. Therefore, TA was not used in our further permeation studies using human cadaver nails. TA was however studied for its property to disrupt the nail barrier, where the nail clippings were first treated with 5% TA followed by saturated solution of CPO in pH 7.4 PBS (unpublished data). We found that pretreatment with 5% TA showed 3.9-fold higher accumulation of CPO within the nail clippings and it was the best enhancer for CPO if used as a pretreatment. This observation is in alignment with the report showing 3.8-fold higher flux of permeation of caffeine through human nail, pretreated with 5% TA (18). One of the long-term goals of the current project was to select enhancer(s) which could be incorporated within the transungual formulation containing CPO. There are literature reports (18,29) and our observation showing significant improvement in drug penetration when TA was used for pretreatment of nails. However, inclusion of TA with CPO in a formulation could lead to incompatibility and precipitation of CPO. Therefore, TA was not studied further in the transungual permeation studies. A higher concentration of TU (10% w/w) was studied as part of a preliminary study (unpublished data) and it showed 2.5-fold higher accumulation of CPO in the nail plate. However, 5% w/w was used in the subsequent studies because the nails treated with 10% w/w TU appeared soft and fragile. Also, it was speculated that a high concentration of TU in a formulation could result in incompatibility and instability of the drug. The 5% w/w TU showed significant improvement in solubility (Fig. 3) and 1.2-fold higher accumulation of CPO in the nail clippings. This solubility enhancement in presence of TU could be a drug-specific phenomenon. Thiourea is a strong reducing agent, which reduces the disulfide linkage of nail keratin and thereby improves drug penetration into the nail (18). It can therefore be concluded from Fig. 4b that 5% TU works by two mechanisms on the nail plate—improving levels of solubilized CPO and reducing the disulfide bonds of nail keratin. The use of 5% TU results in good transungual permeation of CPO within 27 days.
The reported MIC of CPO against the dermatophytes is in the range of 0.004 to 1.0 μg/mL (17,27,30–32). The concentration of CPO attained in the receiver medium at the end of 27 days was 1.79 ± 1.0 μg/mL. The concentration of CPO within the nail was calculated as milligrams per milliliter using the volume of the nail (product of nail thickness and area exposed to CPO treatment). Thus, the concentration of CPO within the nail was 27.71 ± 5.98 mg/mL and is 4 orders of magnitude higher than the reported MIC values for CPO (17,27,30–32). The nail-minimum fungicidal concentration in presence of nail powder (MFC) of CPO was determined against seven strains of Trichophyton rubrum growing (33). The nail-MFC for CPO was in the range of 16–32 μg/mL (33). The concentration attained in the nail is significantly greater than the nail-MFC of CPO. This high accumulation of CPO within the nail will also serve as a reservoir for drug release after termination of the therapy and prevent relapse or reinfection. Five percent TU was therefore selected as the PE for the nail.
The main goal of topical delivery is to maximize skin exposure and minimize the systemic exposure of the drug, thereby reducing the chances of adverse side effects and drug–drug interactions. The enhancers which increase the flux through the skin are beneficial for transdermal applications. The analysis plot in Fig. 5 shows that S20, B30, B98, NMP, and TU belong to quadrant III because they improve both solubility and permeation of CPO. TU, B98, S20, and B30 showed <2-fold increase in solubility, while the enhancement in permeation for B98 to S20 was 1.6- to 3.4-fold. The data suggest that improved solubility is not the only mode for increased permeation. These nonionic surfactants are speculated to alter the barrier properties by solubilizing the lipids in the stratum corneum and emulsifying the sebum (19). NMP increases both the drug solubility and permeation across the skin. Thus, S20, B30, B98, and NMP are potential PEs for the transdermal delivery of CPO. Hence, these agents cannot be incorporated in the topical formulation containing CPO. In case of topical formulations, it is important to select penetration enhancers which increase the levels of the drug in the target organ, skin (34). With the objective to identify one single enhancer for the skin and the nail plate, 5% TU was also tested for its action on transdermal permeation and skin penetration of CPO. In favor of topical delivery, TU did not remarkably increase the skin permeation. However, no significant drug accumulation was achieved in the epidermis in the presence of TU.
IPM in quadrant II and TCL, OA, and PG in quadrant IV (Fig. 5) do not increase the permeation of CPO. Relative to the other PEs, the lag time for CPO permeation (Table II) is the highest in presence of PG at 4.8 h. This effect is attributed to the increased tortuosity of the stratum corneum in the presence of 100% PG, caused by dehydration of the epidermis (35). Though PG shows the greatest solubility for CPO, it shows lowest flux for its transdermal permeation. This phenomenon is a concentration-dependent effect of PG. PG at higher concentrations is reported to reduce the transdermal flux of the permeant (36,37) due to an increase in viscosity and dehydration of the stratum corneum (35). Moreover, at higher concentrations of PG, there is extensive water loss from the polar head regions in the epidermal layer, an increase in viscosity, and a decrease in partition coefficient of the drug (35).
This indicates that in presence of 100% PG, the contribution of EFsol of CPO towards its EFfinite flux is minimal.
The skin penetration study quantifies the levels of the drug in the epidermal and dermal layers of the skin. One of the target sites for the topical antifungal therapy is the skin, specifically the epidermis. The final selection criterion for the optimum enhancers is their ability to enhance the drug accumulation in the epidermis. The drug levels in the epidermis and dermis of the skin along with the flux values in the finite dose skin studies will serve as a better criterion for the selection of penetration enhancers for the skin. Inspite of higher drug accumulation in the epidermis and dermis in the presence of NMP, BRIJ 30, and Span 20, these penetration enhancers are not preferred for the formulation development because they also increase the permeation of CPO through the skin which could lead to other toxicities. Therefore, it is important to choose penetration enhancers which show greater accumulation in the layers of the skin with minimum flux for the drug permeation. IPM showed 1.6- and 5.3-fold accumulation in the epidermis and the dermis, respectively (Fig. 6a). Since IPM was not selective in CPO penetration into the epidermis, it was not selected as the skin PE of CPO. Similarly, OA was also eliminated as the skin PE because of minimal accumulation within the epidermis. PG and TCL both showed good accumulation of CPO within the epidermis of the skin. PG and TCL are reported to improve drug penetration into the skin while reducing the transdermal permeation (19,38–40). Additionally, in the presence of PG, 5.29 ± 0.19 mg/mL of CPO was quantified within the epidermis. This value is significantly higher than the reported MIC of 0.004–1.0 μg/mL (17,27,30–32). PG was chosen as the best enhancer for the skin penetration of CPO because it showed much lower permeation flux than the control and enhanced the levels of CPO, selectively within the epidermis.
The combination of 5% TU in PG did not yield an additive effect with respect to nail penetration, skin permeation, and skin penetration. The loss of the individual effects of 5% TU and PG in the combination was not investigated further. It was concluded that the formulation has to be designed to incorporate both the enhancers but without losing the individual positive effects of TU and PG. This poses additional challenge in designing and developing a formulation with these attributes.
CONCLUSION
In passive delivery of the drug, the concentration gradient is the driving force for drug diffusion. It is therefore important to maintain a high concentration gradient between the formulation and the target tissues (nail folds and nail). The inclusion of tissue-specific PE was hypothesized to enhance drug delivery of CPO into the nail fold (skin) and the nail. The strategic method of selection of the PEs for transungual drug delivery of CPO has been described. The nail PE 5% TU showed an increase in the solubility, in vitro nail penetration, and transungual nail permeation of CPO. PG showed the ideal properties of increased skin penetration and reduced transdermal permeation of CPO, to be selected as the skin PE. One single PE with positive effects on the skin and the nail was not identified. Also, the combination of the two selected PEs (TU and PE) did not function as expected in the two tissues. The analysis plots gave good insight into the probable mechanisms of each of the PEs in the skin and the nail. Incorporating these two PEs into a single topical formulation is challenging and discussion of the development of such a formulation is beyond the scope of this article.
REFERENCES
- 1.Baran R, Hay RJ, Tosti A, Haneke E. A new classification of onychomycosis. Br J Dermatol. 1998;139:567–71. doi: 10.1046/j.1365-2133.1998.02449.x. [DOI] [PubMed] [Google Scholar]
- 2.Drake LA. Impact of onychomycosis on quality of life. J Am Podiatr Med Assoc. 1997;87(11):507–11. doi: 10.7547/87507315-87-11-507. [DOI] [PubMed] [Google Scholar]
- 3.Drake LA, Scher RK, Smith EB, Faich GA, Smith SL, Hong JJ, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38:702–4. doi: 10.1016/S0190-9622(98)70199-9. [DOI] [PubMed] [Google Scholar]
- 4.Lubeck DP. Measuring health-related quality of life in onychomycosis. J Am Acad Dermatol. 1998;38:S64–8. doi: 10.1016/S0190-9622(98)70487-6. [DOI] [PubMed] [Google Scholar]
- 5.Drake LA, Patrick DL, Fleckman P, André J, Baran R, Haneke E, et al. The impact of onychomycosis on quality of life: development of an international onychomycosis-specific questionnaire to measure patient quality of life. J Am Acad Dermatol. 1999;41(2 Part1):189–96. doi: 10.1016/S0190-9622(99)70047-2. [DOI] [PubMed] [Google Scholar]
- 6.Turner RR, Testa MA. Measuring the impact of onychomycosis on patient quality of life. Qual Life Res. 2000;9(1):39–53. doi: 10.1023/A:1008986826756. [DOI] [PubMed] [Google Scholar]
- 7.Gregory N. Special patient populations: onychomycosis in the HIV-positive patient. J Am Acad Dermatol. 1996;35(3 part 2):S13–6. doi: 10.1016/S0190-9622(96)90064-X. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Coutiño G, Arenas R, Reyes-Terán G. Clinical presentation of onychomycosis in HIV/AIDS: a review of 280 Mexican cases. Indian J Dermatol. 2011;56(1):120–1. doi: 10.4103/0019-5154.77577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta AK, Taborda P, Taborda V, Gilmour J, Rachlis A, Salit I, et al. Epidemiology and prevalence of onychomycosis in HIV-positive individuals. Int J Dermatol. 2000;39:749–53. doi: 10.1046/j.1365-4362.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 10.Levy LA. Epidemiology of onychomycosis in special-risk populations. J Am Podiatr Med Assoc. 1997;87(12):546–50. doi: 10.7547/87507315-87-12-546. [DOI] [PubMed] [Google Scholar]
- 11.Gupta AK, Lynde CW, Jain, Sibbald RG, Elewski BE, Daniel CR, et al. A higher prevalence of onychomycosis in psoriatics compared with non-psoriatics: a multicentre study. Br J Dermatol. 1997;136:786–9. doi: 10.1111/j.1365-2133.1997.tb03673.x. [DOI] [PubMed] [Google Scholar]
- 12.Szepietowski JC, Salomon J. Do fungi play a role in psoriatic nails? Mycoses. 2007;50(6):437–42. doi: 10.1111/j.1439-0507.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- 13.Winston JA, Miller JL. Treatment of onychomycosis in diabetic patients. Clin Diabetes. 2006;24(4):160–6. doi: 10.2337/diaclin.24.4.160. [DOI] [Google Scholar]
- 14.Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43:S70–80. doi: 10.1067/mjd.2000.109071. [DOI] [PubMed] [Google Scholar]
- 15.Monti D, Saccomani L, Chetoni P, Burgalassi S, Saettone MF, Mailland F. In vitro transungual permeation of ciclopirox from a hydroxypropyl chitosan-based, water-soluble nail lacquer. Drug Dev Ind Pharm. 2005;31:11–7. doi: 10.1081/ddc-43935. [DOI] [PubMed] [Google Scholar]
- 16.Johnson L. Dermatophytes—the skin eater. Mycologist. 2003;17(4):147–9. doi: 10.1017/S0269915X04004185. [DOI] [Google Scholar]
- 17.Subissi A, Monti D, Togni G, Maillan F. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70(16):2133–52. doi: 10.2165/11538110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Khengar R, Jones S, Turner R, Forbes B, Brown M. Nail swelling as a pre-formulation screen for the selection and optimisation of ungual penetration enhancers. Pharm Res. 2007;24(12):2207–12. doi: 10.1007/s11095-007-9368-3. [DOI] [PubMed] [Google Scholar]
- 19.Williams A, Barry B. Penetration enhancers. Adv Drug Deliv Rev. 2004;56:603–18. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Escarrone AL, Bittencourt C, Laporta L, dos Santos M, Primel E, Caldas S. LC–UV method with pre-column derivatization for the determination of ciclopirox olamine in raw material and topical solution. Chromatographia. 2008;67:967–71. doi: 10.1365/s10337-008-0623-5. [DOI] [Google Scholar]
- 21.Lehr K-H, Damm P. Quantification of ciclopirox by high-performance liquid chromatography after pre-column derivatization. J Chromatogr. 1985;339:451–6. doi: 10.1016/s0378-4347(00)84680-0. [DOI] [PubMed] [Google Scholar]
- 22.Myoung Y, Choi H-K. Permeation of ciclopirox across porcine hoof membrane: effect of pressure sensitive adhesives and vehicles. Eur J Pharm Sci. 2003;20:319–25. doi: 10.1016/j.ejps.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Frank JD, Manson JM, Cartwright ME. Separation of epidermis from dermis in the rhesus monkey. Exp Dermatol. 1995;4(2):89–92. doi: 10.1111/j.1600-0625.1995.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 24.Kassis V, SØndergaard J. Heat separation of normal human skin for epidermal and dermal prostaglandin analysis. Arch Dermatol Res. 1982;273:301–6. doi: 10.1007/BF00409259. [DOI] [PubMed] [Google Scholar]
- 25.Murthy SN, Vaka SR, Sammeta SM, Nair AB. TranScreen-N: method for rapid screening of trans-ungual drug delivery enhancers. J Pharm Sci. 2009;98(11):4264–71. doi: 10.1002/jps.21743. [DOI] [PubMed] [Google Scholar]
- 26.Manda P, Sammeta SM, Repka MA, Murthy N. Iontophoresis across the proximal nail fold to target drugs to the nail matrix. J Pharm Sci. 2012;101(7):2392–7. doi: 10.1002/jps.23139. [DOI] [PubMed] [Google Scholar]
- 27.Kokjohn K, Bradley M, Griffiths B, Ghannoum M. Evaluation of in vitro activity of ciclopirox olamine, butenafine HCl and econazole nitrate against dermatophytes, yeasts and bacteria. Int J Dermatol. 2003;42(S1):11–7. doi: 10.1046/j.1365-4362.42.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 28.Chouhan P, Saini TR. Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int J Pharm. 2012;436(1–2):179–82. doi: 10.1016/j.ijpharm.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Traynor MJ, Turner RB, Evans CR, Khengar RH, Jones SA, Brown MB. Effect of a novel penetration enhancer on the ungual permeation of two antifungal agents. J Pharm Pharmacol. 2010;62(6):730–7. doi: 10.1211/jpp.62.06.0009. [DOI] [PubMed] [Google Scholar]
- 30.Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149(2):296–305. doi: 10.1046/j.1365-2133.2003.05418.x. [DOI] [PubMed] [Google Scholar]
- 31.Shehata AS, Mukherjee PK, Ghannoum MA. Comparison between the standardized clinical and laboratory standards institute M38-A2 method and a 2,3-Bis(2-methoxy-4-nitro-5-[(sulphenylamino)carbonyl])-2H-tetrazolium hydroxide based method for testing antifungal susceptibility of dermatophytes. J Clin Microbiol. 2008;46(11):3668–71. doi: 10.1128/JCM.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh J, Zaman M, Gupta AK. Evaluation of microdilution and disk diffusion methods for antifungal susceptibility testing of dermatophytes. Med Mycol. 2007;45(7):595–602. doi: 10.1080/13693780701549364. [DOI] [PubMed] [Google Scholar]
- 33.Schaller M, Borelli C, Berger U, Walker B, Schmidt S, Weindl G, et al. Susceptibility testing of amorolfine, bifonazole and ciclopirox olamine against Trichophyton rubrum in an in vitro model of dermatophyte nail infection. Med Mycol. 2009;47:753–8. doi: 10.3109/13693780802577892. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Malick AW, Meleer NM, Mouskountakis JD, Behl CR. Studies of in vitro skin permeation and retention of a leukotriene antagonist from topical vehicles with a hairless guinea pig model. J Pharm Sci. 1992;81(7):631–4. doi: 10.1002/jps.2600810708. [DOI] [PubMed] [Google Scholar]
- 35.Thomas NS, Panchagnula R. Transdermal delivery of zidovudine: effect of vehicles on permeation across rat skin and their mechanism of action. Eur J Pharm Sci. 2003;18(1):71–9. doi: 10.1016/S0928-0987(02)00242-7. [DOI] [PubMed] [Google Scholar]
- 36.Touitou E, Abed L. Effect of propylene glycol, Azone and n-decylmethyl sulphoxide on skin permeation kinetics of 5-fluorouracil. Int J Pharm. 1985;27:89–98. doi: 10.1016/0378-5173(85)90188-7. [DOI] [Google Scholar]
- 37.Bonina FP, Montenegro L. Penetration enhancer effects on in vitro of heparin sodium percutaneous absorption salt. Int J Pharm. 1992;82:171–7. doi: 10.1016/0378-5173(92)90172-X. [DOI] [Google Scholar]
- 38.Lorenzetti OJ. Propylene glycol gel vehicles. Cutis. 1979;23:747–50. [PubMed] [Google Scholar]
- 39.Ritschel W, Panchagnula R, Stemmer K, Ashraf M. Development of an intracutaneous depot of drugs. Skin Pharmacol. 1991;4:235–45. doi: 10.1159/000210957. [DOI] [PubMed] [Google Scholar]
- 40.Puglia C, Bonina F, Trapani G, Franco M, Ricci M. Evaluation of in vitro percutaneous absorption of lorazepam and clonazepam from hydro-alcoholic gel formulations. Int J Pharm. 2001;228(1–2):79–87. doi: 10.1016/S0378-5173(01)00806-7. [DOI] [PubMed] [Google Scholar]


