Abstract
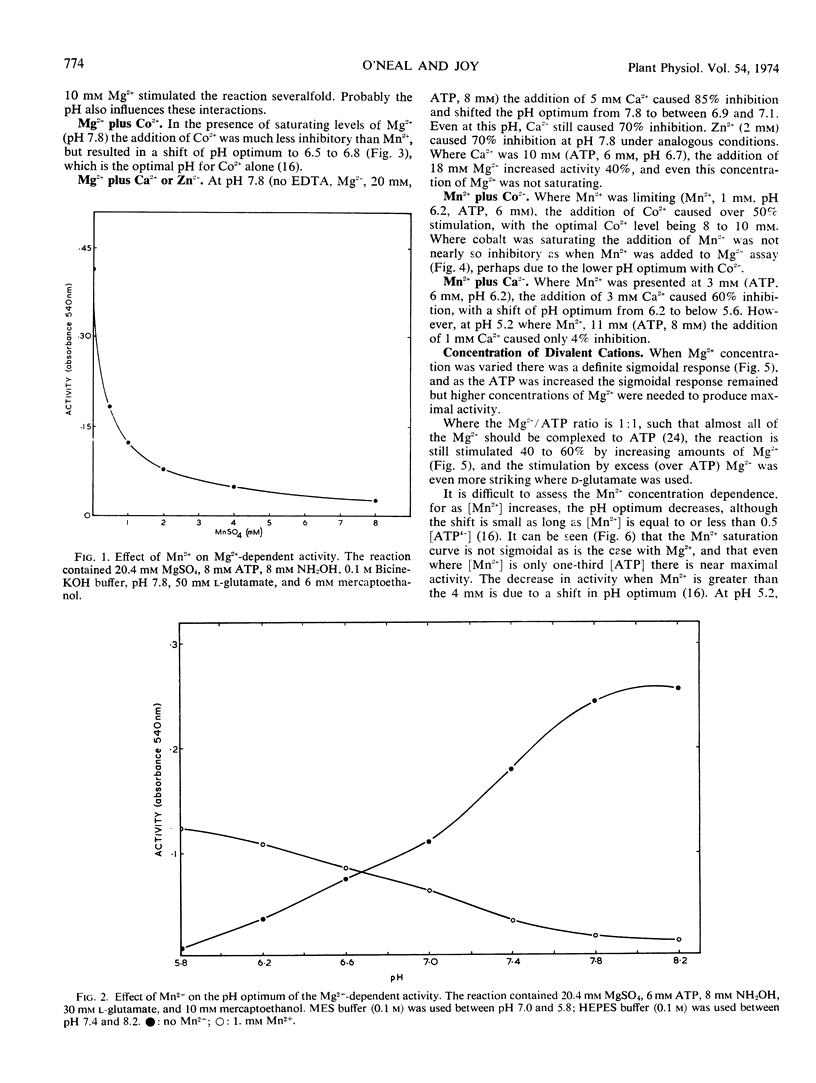
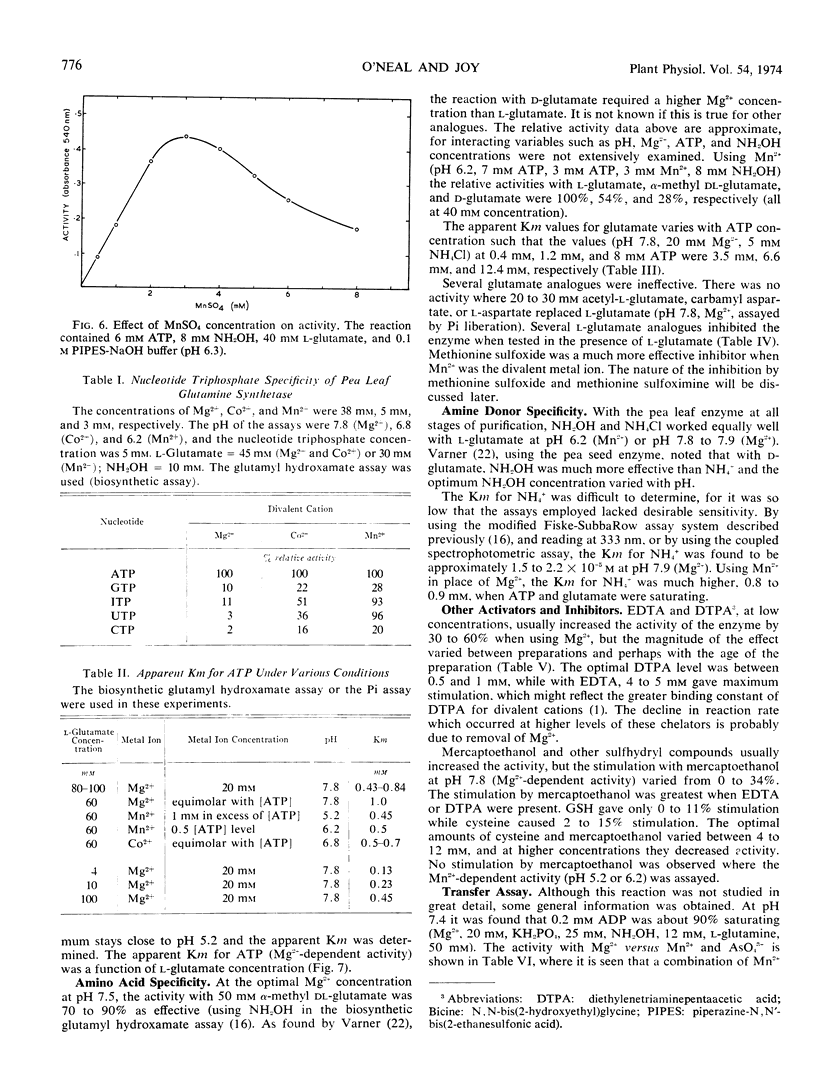
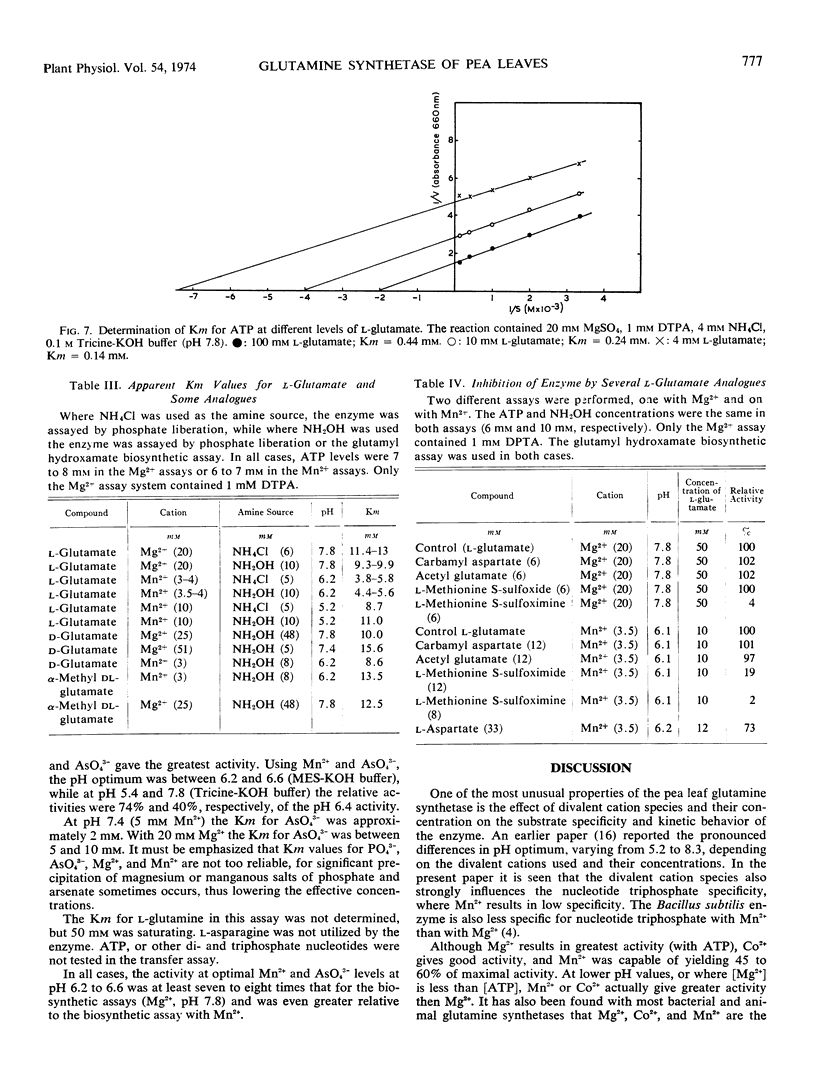
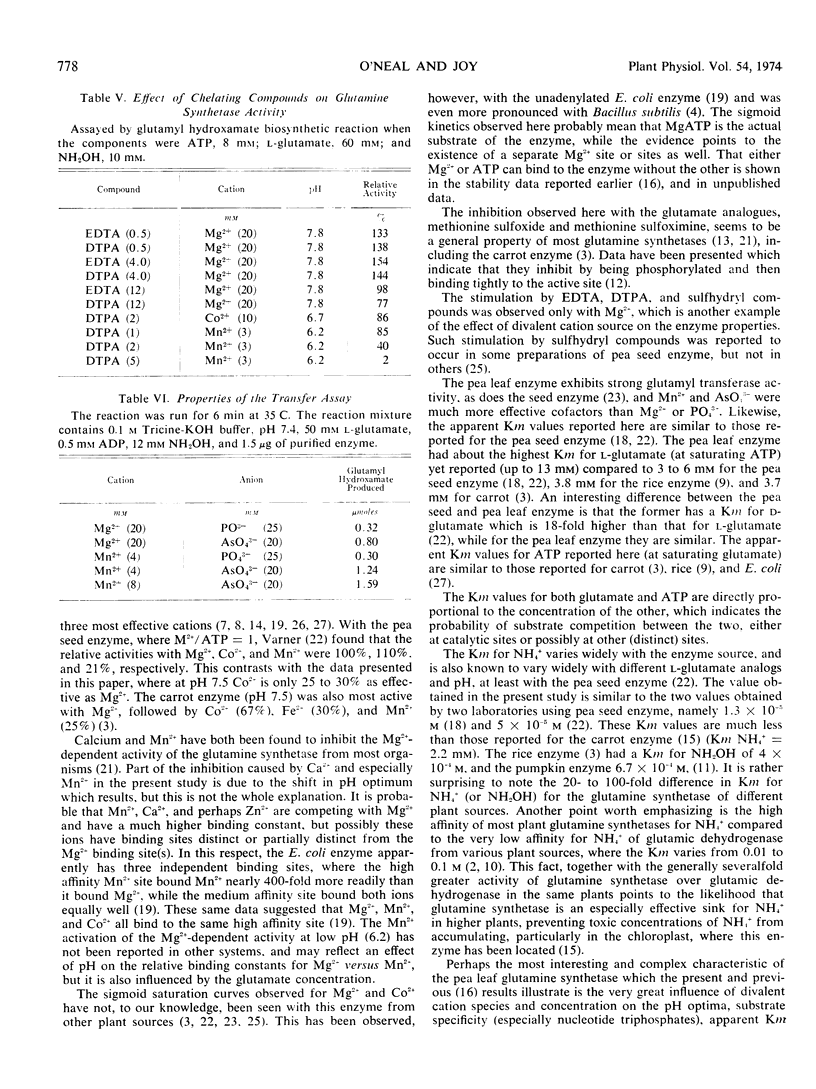
Purified glutamine synthetase from pea seedlings was most active with Mg2+ as the metal activator, but Mn2+ and Co2+ were 45 to 60% and 30 to 45% as effective, respectively, when assayed at the optimal pH for each cation. The Mg2+ saturation curve was quite sigmoid, and evidence indicates that MgATP is the active ATP substance. Co2+ also gave a sigmoidal saturation curve, but when Mn2+ was varied only slightly sigmoidal kinetics were seen. Addition of Mn2+, Ca2+, or Zn2+ at low concentrations sharply inhibited the Mg2+ -dependent activity, partially by shifting the pH optimum. Addition of Co2+ did not inhibit Mg2+-dependent activity. The nucleotide triphosphate specificity changed markedly when Co2+ or Mn2+ replaced Mg2+. Using the Mg2+-dependent assay, the Michaelis constant (Km) for NH4+ was about 1.9 × 10−3 M. The Km for l-glutamate was directly proportional to ATP concentration and ranged from 3.5 to 12.4 mm with the ATP levels tested. The Km for MgATP also varied with the l-glutamate concentration, ranging from 0.14 mm to 0.65 mm. Ethylenediaminetetracetic acid activated the enzyme by up to 54%, while sulfhydryl reagents gave slight activation, occasionally up to 34%.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A. The isolation and characterization of glutamic dehydrogenase from corn leaves. Arch Biochem Biophys. 1956 May;62(1):173–183. doi: 10.1016/0003-9861(56)90100-x. [DOI] [PubMed] [Google Scholar]
- Bailar J. C., Jr Some coordination compounds in biochemistry. Am Sci. 1971 Sep-Oct;59(5):586–592. [PubMed] [Google Scholar]
- Deuel T. F. Bacillus subtilis glutamine synthetase. Specific catalytic changes associated with limited sulfhydryl modification. J Biol Chem. 1971 Feb 10;246(3):599–605. [PubMed] [Google Scholar]
- Deuel T. F., Lerner A., Albrycht D. Regulatory properties of rat liver glutamine synthetase. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1419–1425. doi: 10.1016/0006-291x(72)90871-6. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Stadtman E. R. Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem. 1970 Oct 25;245(20):5206–5213. [PubMed] [Google Scholar]
- Hubbard J. S., Stadtman E. R. Regulation of glutamine synthetase. II. Patterns of feedback inhibition in microorganisms. J Bacteriol. 1967 Mar;93(3):1045–1055. doi: 10.1128/jb.93.3.1045-1055.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. B., Ginsburg A. Some kinetics of the interaction of divalent cations with glutamine synthetase from Escherichia coli. Metal ion induced conformational changes. Biochemistry. 1972 Sep 26;11(20):3723–3735. doi: 10.1021/bi00770a010. [DOI] [PubMed] [Google Scholar]
- Manning J. M., Moore S., Rowe W. B., Meister A. Identification of L-methionine S-sulfoximine as the diastereoisomer of L-methionine SR-sulfoximine that inhibits glutamine synthetase. Biochemistry. 1969 Jun;8(6):2681–2685. doi: 10.1021/bi00834a066. [DOI] [PubMed] [Google Scholar]
- Meister A. The specificity of glutamine synthetase and its relationship to substrate conformation at the active site. Adv Enzymol Relat Areas Mol Biol. 1968;31:183–218. doi: 10.1002/9780470122761.ch5. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Localisation of glutamine synthetase in chloroplasts. Nat New Biol. 1973 Nov 14;246(150):61–62. doi: 10.1038/newbio246061a0. [DOI] [PubMed] [Google Scholar]
- PAMILJANS V., KRISHNASWAMY P. R., DUMVILLE G., MEISTER A. Studies on the mechanism of glutamine synthesis; isolation and properties of the enzyme from sheep brain. Biochemistry. 1962 Jan;1:153–158. doi: 10.1021/bi00907a023. [DOI] [PubMed] [Google Scholar]
- Pahlich E., Joy K. W. Glutamate dehydrogenase from pea roots: purification and properties of the enzyme. Can J Biochem. 1971 Jan;49(1):127–138. doi: 10.1139/o71-018. [DOI] [PubMed] [Google Scholar]
- Segal A., Stadtman E. R. Variation of the conformational states of Escherichia coli glutamine synthetase by interaction with different divalent cations. Arch Biochem Biophys. 1972 Sep;152(1):367–377. doi: 10.1016/0003-9861(72)90226-3. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Kingdon H. S., Stadtman E. R. Regulation of glutamine synthetase. VII. Adenylyl glutamine synthetase: a new form of the enzyme with altered regulatory and kinetic properties. Proc Natl Acad Sci U S A. 1967 Aug;58(2):642–649. doi: 10.1073/pnas.58.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Leu F. Y., Meister A. Rat liver glutamine synthetase. Preparation, properties, and mechanism of inhibition by carbamyl phosphate. J Biol Chem. 1972 Sep 10;247(17):5312–5321. [PubMed] [Google Scholar]
- VARNER J. E. The optical specificity of glutamine synthetase. Arch Biochem Biophys. 1960 Sep;90:7–11. doi: 10.1016/0003-9861(60)90603-2. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Webster G. C. Studies on the Enzymatic Synthesis of Glutamine. Plant Physiol. 1955 Sep;30(5):393–402. doi: 10.1104/pp.30.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Stadtman E. R. Regulation of glutamine synthetase. IV. Reversible dissociation and inactivation of glutamine synthetase from Escherichia coli by the concerted action of EDTA and urea. Arch Biochem Biophys. 1967 Oct;122(1):174–189. doi: 10.1016/0003-9861(67)90137-3. [DOI] [PubMed] [Google Scholar]


