Abstract
The aim of this study was to formulate salbutamol sulfate (SS), a model drug, as mucoadhesive in situ gelling inserts having a high potential as nasal drug delivery system bypassing the first-pass metabolism. In situ gelling inserts, each containing 1.4% SS and 2% gel-forming polymer, hydroxypropyl methylcellulose (HPMC), carboxymethylcellulose sodium (CMC Na), sodium alginate (AL), and chitosan (CH) were prepared. The inserts were investigated for their different physicochemical properties. The weight of inserts was 16–27 mg, drug content was 3.9–4.2 mg, thickness ranged between 15 and 28 μm and surface pH was 5–7. Cumulative drug released from the inserts exhibited extended release for more than 10 h following the decreasing order: CH > AL > CMC Na > HPMC. The drug release from CMC Na and AL inserts followed zero-order kinetics while HPMC and CH inserts exhibited non-Fickian diffusion mechanism. The inserts exhibited different water uptake (7–23%) with the smallest values for CH. Differential scanning calorimetry study pointed out possible interaction of SS and oppositely charged anionic polymers (CMC Na and AL). The mucoadhesive in situ gelling inserts exhibited satisfactory mucoadhesive and extended drug release characteristics. The inserts could be used for nasal delivery of SS over about 12 h; bypassing the hepatic first-pass metabolism without potential irritation.
KEY WORDS: in situ gelling inserts, mucoadhesion, nasal delivery, salbutamol sulfate
INTRODUCTION
Solid dosage forms are rarely investigated as nasal drug delivery device. Attempts have been made to combine the advantages of a solid, single unit dosage for nasal drug delivery by using carrier systems that hydrate quickly after contact with mucosa. The proper choice of polymers can allow mucoadhesion and controlled drug release and due to dissolution of the gel and/or mucociliary removal towards the nasopharynx, there would be no need to remove the insert mechanically after it is depleted of drug (1). Therefore, nasal inserts based on mucoadhesive, release controlling polymers which gel in situ, have high potential as nasal drug delivery. Lyophillization technology has been applied to the preparation of mucoadhesive nasal insert with prolonged peptide drug delivery (2). Bertram et al. studied the effect of polymers and absorption enhancers in loading influenza vaccine in in situ gelling nasal inserts for vaccination (3). McInnes et al. (4) studied the use of nasal inserts which rapidly rehydrate upon administration to form a more viscous gel and thus enhancing nasal residence time of nicotine. Many polymers have been explored for developing nasal drug delivery systems. Various studies have demonstrated the efficacy of HPMC in mucoadhesive nasal drug delivery in the form of freeze dried powder (5,6), gel (7), spray dried microspheres (8), and lyophilized insert (2,4,9,10). CMC Na has also been investigated for nasal drug administration (11). Natural mucoadhesive polymers candidates include chitosan (CH) and various gums such as guar, xanthan, gellan, pectin, and alginates (12). Alginates (AL) are classified as anionic mucoadhesives and therefore utilized as a potential delivery vehicle to enhance effectiveness and bioavailability of biomolecules applied to mucosal tissues (13). Many authors have reported the favorable characteristics of chitosan including mucoadhesive property (5,14), safety (15), and strong permeation-enhancing capabilities (16). It has been applied as mucoadhesive carrier for many drugs, e.g., influenza A toxin (17), carvedilol (18), and insulin (19).
In the present study, salbutamol sulfate was chosen as a model drug since it is well-absorbed orally; however, it suffers from poor bioavailability due to first-pass metabolism. Systemic bioavailability of salbutamol sulfate is about 50% due to extensive presystemic metabolism in the GIT and liver, the metabolite possesses little or no-adrenergic activity. The plasma half-life of this drug is 4 to 6 h and thus it requires multiple dosing a day (20).
The aim of this work was to formulate and characterize in situ gelling mucoadhesive inserts for systemic drug delivery of the model drug SS via the nasal route employing the simple film casting method of aqueous solutions of the drug and different mucoadhesive polymers, namely HPMC, CMC Na, AL, and CH were used. The in situ gelling inserts were evaluated for content uniformity, thickness, surface pH, water uptake, in vitro drug release, mucoadhesion capabilities, and thermal properties by differential scanning calorimetry (DSC).
MATERIALS AND METHODS
Salbutamol sulfate and HPMC 4,000 cp were kindly provided by Pharco Pharmaceutical Co., Alexandria, Egypt. High viscosity CMC Na (1% aqueous solution 1,500 ± 400 cp) and sodium chloride were purchased from BDH Chemical Ltd., Poole, England. High molecular weight AL was obtained from Sisco Research Laboratories, Mumbai, India. Medium molecular weight CH and Mucin were obtained from porcine stomach, Type II, Sigma, St Louis, USA. Glycerol, calcium chloride, potassium chloride, and agar were procured from ADWIC, EL-Nasr Pharmaceutical Chemicals Co., Cairo, Egypt. All other chemicals were of analytical grade.
Preparation of SS Nasal Inserts
In situ gelling inserts were prepared by film casting method. Aqueous solution-I was prepared by dissolving the polymer 2% (CMC Na, HPMC, AL, and CH), in addition to glycerol (1% as a plasticizer) in 10 ml of distilled water (or 1% acetic acid solution for CH) and then stirred (magnetic stirring 1000, Jenway, England) for 2 h. Aqueous solution-II was prepared by dissolving 1.4% SS in 5 ml of water (or 1% acetic acid solution for CH insert). Both solutions I and II were mixed and further stirred for 1 h. The resulting solution was stored at 4°C overnight to allow removal of entrapped air bubbles. The solution was then casted onto a glass Petri dish (5 cm in diameter) and dried in an incubator (Hereaus, Germany) at 40°C for 24 h. The film was carefully removed from the Petri dish, checked visually for any imperfections and circular inserts of 10-mm diameter were cut from the resulting film with a cork borer. The thickness of inserts was measured using a micrometer (Moore and Wright Ltd. Britain Tool, Factory Sheffield, UK) and the inserts were stored in glass vials and kept in a desiccator over anhydrous CaCl2 at room temperature until further investigations.
Differential Scanning Calorimetery
DSC analyses were performed for drug, polymers, physical mixtures (keeping the same ratio between drug and polymer as present in the inserts), and the tested formulations. Samples of 3 mg each were placed in aluminum pan and heated at the rate of 10°C/min to 400°C. The instrument (Perkin Elmer, Germany) was calibrated with Indium and dry N2 was used as carrier gas with a flow rate of 25 ml/min.
Drug Content
Drug content was determined by dissolving the inserts in simulated nasal fluid (SNF of pH 6.5 was composed of 7.45 mg/ml NaCl, 1.29 mg/ml KCl and 0.32 mg/ml CaCl2.2H2O) (21) under continuous shaking for 24 h in a thermostated shaking water bath (GFL Type 1083, Gesellschaft Fur Labortechnik, GmbH &Co., Burgwedel, West Germany) maintained at 37°C. The resulting solution was filtered through a millipore filter 0.45 μm and the amount of SS was then determined spectrophotometricaly (Ultraviolet-Spectrophotometer, Pharmacia LKB Ultrospec III double beam, England) at λ max 276 nm.
Surface pH
The inserts were left to swell for 2 h on the surface of agar plate. Agar solution was prepared by dissolving 2% w/v agar in SNF by heating under stirring, then poured into a Petri dish for gel formation at room temperature. Surface pH, was measured by means of a pH paper placed on the surface of the swollen inserts (22). The measurements were performed in duplicate.
Water Uptake
This was conducted according to the method of Bertram and Bodmeier (9). A sponge (7 × 3.5 × 3 cm, house hold sponge, China) was fully soaked in the hydration medium (SNF) and placed in a container filled with the same medium to a height of 1 cm in order to keep the sponge soaked during the experiment. Square filter paper (3 × 3 cm) was also soaked in the medium and positioned on the top of the sponge. This experimental setup was equilibrated for 30 min and accurately weighed inserts were placed on the filter paper and the water uptake was determined at regular 1-h time intervals for 8 h. The weight increase of the insert was calculated as the weight of hydrated insert and wet filter paper minus weight of wet filter paper. Percent Water uptake of inserts was calculated using the following equation:
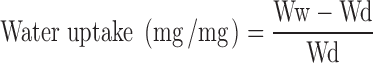 |
Where Ww is the weight of swollen insert after time t and Wd is the original weight of the insert at zero time.
Hydrophilicity of Inserts
The hydrophilicity of the in situ gelling inserts was tested by measuring their moisture absorption capacity. Preweighed inserts were placed above a saturated solution of ammonium chloride at room temperature (relative air humidity of 79%). The inserts were weighed after 48 h and moisture content calculated from the weight increase.
In Vitro Mucoadhesion Testing
Mucoadhesion was performed by adapting the displacement method of Bertram and Bodmeier (9). The inserts were placed on the top of the agar/mucin gel, casted on a glass plate and was inclined (angle 60°) in an incubator at 37°C. The displacement (downwards movement of the insert) in centimeter was measured hourly up to 9 h. The adhesion potential is thus inversely related to the displacement of the insert.
In Vitro Drug Release
The method was a modification of that previously mentioned by Bertram and Bodmeier (9) and Varshosaz et al. (23). A self-made diffusion cell was used for the drug release studies mimicking humidity properties of nasal mucosa. The lower end of polyethylene tube (inner diameter is 14 mm) was closed with a tightly stretched thin sponge (cosmetic sponge, China) after the insert was placed interiorly on the thin sponge and placed vertically into a 5-ml SNF as the release medium in a 25-ml glass beaker. Accordingly, the tube was adjusted exactly to the height of release medium surface so that the sponge was wetted but not submersed. The release experiments were subjected to horizontal shaking (75 rpm) in a thermostated controlled water bath at 37°C. At specified time intervals, the 5 ml of the release medium was taken as a sample and replaced by pre-warmed fresh 5 ml of SNF at 37°C. SS content of the samples was analyzed by U.V spectrophotometry at λ max 276 nm and the concentration of drug was determined from a previously constructed calibration curve. Placebo inserts were also subjected to the drug release test to quantitate the contribution of polymers to U.V. absorption. The actual drug loading of inserts was determined by dissolving the inserts in SNF followed by U.V analysis. All measurements were performed in triplicates (mean ± SD). % Dissolution efficiency (%DE) as a parameter for the comparison of the release profiles was calculated from the area under the curve at time t (using the trapezoidal rule) and expressed as a percentage of the area of the rectangle described by 100% dissolution in the same time (24).
Analysis of Drug Release Data
The data obtained from the in vitro release experiments were analyzed by the following commonly used exponential equation of Korsmeyer et al. (25):
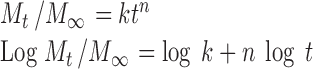 |
Where Mt/M∞, the fraction of released drug at time t; k, release constant and is depending on structural and geometric characteristics of the drug/polymer system; and n, release exponent indicative of the release mechanism.
RESULTS AND DISCUSSION
Film casting method was adopted in this study because it is simple, less expensive, and is easily prepared on laboratory scale. Preliminary trials pointed out that polymer concentrations above 3% w/w resulted in highly viscous gel unsuitable for film formation. Glycerin was added as a plasticizer since it was found compatible with the drug and polymers used and gave soft and flexible inserts compared to the brittle ones without glycerin. The final formulations containing 1.4% w/w SS, 2% w/w polymer and 1% glycerol were assessed for the following attributes.
Thermal Analysis
DSC has been widely used as a rapid thermal method for examining drug–excipient compatibility. SS in the pure form showed a sharp endothermic peak at 168.58°C with transition melting range between 166.00°C and 171.82°C. Examination of the thermograms (figures not shown) revealed that the peak transition endotherms in the presence of crystalline drug in the physical mixtures were reduced and broadened indicating a decrease in crystalline form of the drug. This reduction could be attributed to the partial transformation of drug particles from crystalline to amorphous form or due to the existence of the drug in the form of fine crystallites (26). Kim et al. (27) pointed out that the thermograms of physical mixtures with excipients will display the characteristic features of the active compound if no interaction occurs. Comparing the thermograms of the tested formulae and their respective physical mixtures indicated disappearance of the endothermal peak corresponding to the crystalline drug in the thermograms of the in situ gelling formulations of CMC Na and AL, indicating that SS may have transformed to the amorphous state and dissolved in the polymeric matrix. This finding could be attributed to the possible electrostatic interaction between the cationic drug and the anionic polymers (Figures not shown). On the contrary, the drug peaks in the thermograms of physical mixtures and those for HPMC and CH inserts appeared with reduced intensities, which may confirm the chemical integrity of the drug. Scans for physical mixture of CH and SS could not be done because CH was in the form of flakes and could not be ground to give a homogeneous powder mixture.
The enthalpy of the pure drug was found to be 148.56 j/g and the corresponding values for the individual polymer and the physical mixtures ranged between 35.68 and 108.82 j/g (data not shown). The percentage of relative enthalpy is frequently employed as a criterion for the extent of drug–polymer interactions and for predicting the transformation of a crystalline to an amorphous form (27). The decrease in enthalpy, as indicated by the lower percentage of relative enthalpy change (∆H%) of all formulated inserts, relative to the pure drug (24.04–59.30%) (Data not shown) confirms variable extents of reduction in crystallinity of the drug.
Drug Content, Weight Uniformity, and Thickness of the Inserts
All formulae were prepared with 1.4% SS so as to contain 4 mg per circular insert of 10-mm diameter. This size was found to be suitable for handling and nasal insertion. Physical examination of the prepared inserts revealed smooth appearance with no visible cracks for each of the individual formulations. As shown in Table I, drug content, weight, and thickness of the inserts were constant within each formulation, as evident by low standard deviation values.
Table I.
Physicochemical Parameters and % Moisture Uptake for the In Situ Gelling Nasal Inserts of Salbutamol Sulfate
| Formulation | Thickness (μm ± SD) (n = 10) | Drug content/insert (mg ± SD) (n = 6) | Weight (mg ± SD) (n = 10) | Surface pH (n = 2) | % Moisture uptakea |
|---|---|---|---|---|---|
| CMC Na | 15.1 ± 0.9 | 4.2 ± 1.3 | 18.5 ± 0.8 | 6–7 | 16.9 ± 0.02 |
| HPMC | 20.3 ± 0.5 | 4.3 ± 0.2 | 16.4 ± 1.4 | 6–7 | 12.8 ± 0.03 |
| AL | 26.6 ± 0.7 | 3.9 ± 0.5 | 19.8 ± 3.6 | 6–7 | 22.9 ± 0.02 |
| CH | 27.1 ± 0.8 | 4.1 ± 0.7 | 27.5 ± 0.9 | 5–6 | 7.0 ± 0.03 |
HPMC hydroxypropyl methylcellulose, CMC Na carboxymethylcellulose sodium, AL sodium alginate, CH chitosan, SD standard deviation
aMoisture uptake by the in situ gelling nasal inserts after 48 h at room temperature and 79.3% RH
Variation in thickness and weight between different formulations may be due to differences in density, molecular weight, and viscosity of the different polymers (28).
Table I point out the small thickness (15–27 μm) and weight values (16–27 mg) of the prepared inserts, which is considered advantageous for nasal application. After preliminary evaluation in human volunteers, the dry and hydrated inserts with nasal mucosa did not result in foreign body sensation and was augmented by the observed reasonable pH of the tested inserts (Table I).
Surface pH of Inserts
The determined pH values of the tested formulations were found to be in the range of 6–7 for all inserts except CH inserts (pH 5–6) due to the use of acetic acid as a solvent for chitosan. pH values of inserts were within the range of the reported pH of physiological nasal fluid (29).
Hydrophilicity of Inserts
The absorption of water vapor from the surrounding air by polymers can be used as a measure for insert hydrophilicity. Examining the inserts under such condition was considered of importance in characterizing a formulation which could be subjected to a high humidity environment in the nasal cavity. It is hypothesized that initial moisture content acts as the deciding factor in moisture absorption (30). The results in Table I revealed that the %moisture absorption (7–23%) followed the order AL > CMC Na > HPMC > CH.
Water Uptake
The ability of different hydrogels to absorb water is due to presence of hydrophilic groups such as –COOH, –OH, and –OSO3H (31). Uptake of water by the in situ gelling inserts as a function of time exhibits different attitudes, depending on the type of polymer in the insert (Fig. 1). After 30 min from the start of study period, all formulae except CH showed similar water uptake. The similar initial water uptake could probably be due to slow initial hydration of most inserts due to their complex structure and the insufficient voids capable of accommodating water molecules. After initial hydration phase, polymers differentiate into different profiles depending on water affinity of the polymers as well as the expansion and mobility of the polymer chain that lead to enhanced penetration process and thus marked water uptake. Therefore, this initial hydration phase was followed by a significant variation in water uptake starting at 2 h in the rank of CMC Na > AL > HPMC.
Fig. 1.
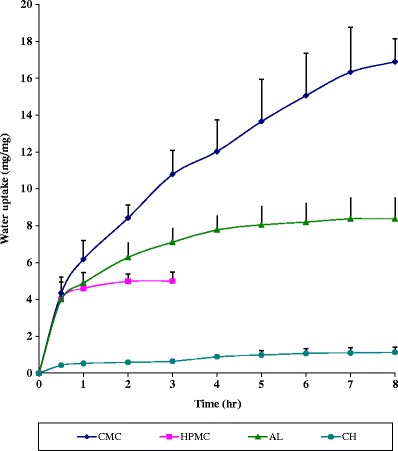
Water uptake profiles of the in situ gelling salbutamol sulfate nasal inserts prepared from different polymers at room temperature
The marked higher water uptake observed in Fig. 1 for CMC Na and AL inserts could be greatly attributed to the ionic nature of the polymers, allowing high tendency towards water uptake. The non-ionic HPMC inserts continue to swell to a measurable diameter till 2 h, and then irregular immeasurable diameter associated with mass loss was observed when it reached an equilibrium state of hydration. This mass loss of HPMC inserts could be due to the swelling tendency of this polymer and the consequence could be the formation of empty spaces within the insert matrix (32).
CH inserts showed the least water uptake (Fig. 1) which could be due to its weak aqueous solubility that limits its water uptake and swelling (33). In addition, it has been reported that the water uptake capacity of CH films is dependent on the solvent used, and it increases with decreasing pH due to protonation of the primary amino group (34). Since the water uptake study was investigated using SNF which has almost neutral pH 6.5, CH inserts exhibited the lowest water uptake. The plateau seen in the water uptake profile of CH may be due to either the solvent front on each surface meeting in the center of the insert (thus there was no further unhydrated polymer to hydrate and expand) or the protective gel coat allowing only a small quantity of water to diffuse into the inner core (35).
In Vitro Mucoadhesion Test
The in situ gelling inserts once administered into the nasal cavity, have to adhere to the nasal mucosa to take up water and to transform into a gel. Therefore, the presence of water is a prerequisite for mucoadhesion, which is a key factor for a successful prolonged nasal drug delivery. Hydration is required for a mucoadhesive polymer to expand and create a proper macromolecular mesh of sufficient size and also to induce mobility in the polymer chains in order to enhance the interpenetration process between polymer and mucin (36). The mucadhesion properties were tested by displacement method. The displacement (downwards movement of the insert) in cm was measured hourly and up to 9 h. No displacement was observed for all inserts except for HPMC (Fig. 2).
Fig. 2.
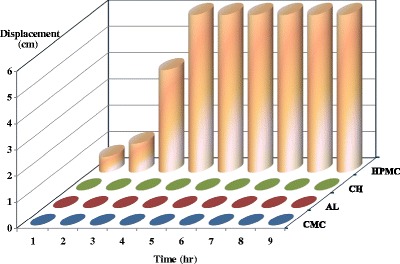
Adhesion profiles of the in situ gelling mucoadhesive nasal inserts prepared from different polymers as determined by the displacement method
CH, a positively charged polymer, formed a thin film on the agar/mucin gel due to its opposite charge to mucin and agar. A positive mucoadhesion performance of the negatively charged polymers CMC Na and AL may be related to their good balance between available hydrogen bonding sites and the open expanded conformation (37).
In Vitro Drug Release
The release of drugs from nasal inserts prepared from different polymers is a complex phenomenon composed of multiple single processes such as solubility of the drug, physical state of drug in the polymeric inserts, electrostatic drug–polymer interactions, viscosity of the hydrated inserts resulting from polymer molecular weight, water uptake, and polymer mass loss during hydration, and spreading of the gel with subsequent increase of the release area (9,38).
The release profiles of SS in SNF pH 6.5 from the different hydrogel formulations are shown in Fig. 3. All formulae exhibited extended and prolonged drug release for at least 10 h. The amount released from CH inserts after 1 h was 24.83% ± 8.5 which is at least double that of other polymers namely; AL (12.01% ± 1.1), HPMC (10.85% ± 2.6), and CMC Na (7.5% ± 2.9).
Fig. 3.
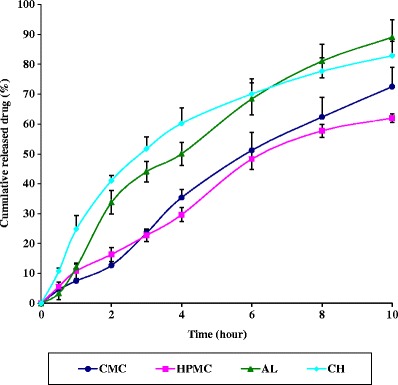
Drug release profiles from the in situ gelling nasal inserts prepared from different polymers in simulated nasal fluid at 37°C
The highest release profile of SS was from the CH insert could be attributed to the pH of SNF (pH 6.5) which results in partial protonation of CH (pka = 6.3) (35) rendering it and the drug (pka = 9.5) predominantly positively charged. The resultant repulsive forces would force the drug molecules into the dissolution medium and thus enhancing the release rate from CH inserts. Following exposure to the release medium, the polymer hydrates forming a viscous gel layer, which gradually starts to undergo attrition or erosion. The drug is then liberated by a combination of diffusion through the viscous gel layer as well as erosion.
Drug release from CMC Na was relatively slow (Fig. 3) with a small % DE value (Table II). This may be explained by referring to the water uptake data, where CMC Na exhibited the highest water uptake (Fig. 1). Although the marked increase in surface area during swelling can promote drug release, the increase in diffusional path length of the drug may paradoxically delay such release. Further, the thick gel layer formed on the swollen insert surface is capable of preventing matrix disintegration and controlling additional water penetration (39). In addition, SS is expected to be partially ionized in the dissolution medium (pH 6.5), where the reported pka value of SS is 9.5 (40). Therefore, the predominantly positively charged drug may bind to the anionic polymer CMC Na through an electrostatic attraction resulting in a reduced diffusion rate through the gel layer, and consequently reduced and retarded drug release.
Table II.
Release Kinetic Parameters and % Dissolution Efficiency of the Release Data of Salbutamol Sulfate from the Prepared In Situ Gelling Mucoadhesive Nasal Inserts
| Formula | (n) values | Mechanism of release | % Dissolution efficiency (% DE ± SD) |
|---|---|---|---|
| CMC Na | 0.99 | Zero order | 39.68 ± 3.1 |
| HPMC | 0.83 | Non Fickian | 36.86 ± 2.1 |
| AL | 1.12 | Zero order | 55.16 ± 3.8 |
| CH | 0.69 | Non Fickian | 58.56 ± 2.9 |
HPMC hydroxypropyl methylcellulose, CMC Na carboxymethylcellulose sodium, AL sodium alginate, CH chitosan, DE dissolution efficiency, SD standard deviation
As shown in Fig. 3, the release of SS from HPMC inserts exhibited the maximum prolonged release profile. This could be attributed to the ability of HPMC to form a thick gel and its progressive swelling would lead to some structural changes among which are changes in the porosity and tortuosity. Diffusion of the drug through the swollen polymer may be retarded due to clogging of the pores and increased path length for the drug (41).
The drug release profile from AL inserts exhibited also delayed release pattern with % DE value of 55.16. During dissolution, AL absorbs a significant amount of water to hydrate, swells and forms a stable hydrogel upon exposure to the divalent cations Ca+2 present in SNF. The gel formation in the presence of Ca+2 has been explained through the egg box model. The drug which is embedded in the alginate insert is now immobilized in the polymer matrix because of the cross linked gelation. Therefore, the in situ gel-forming insert acts as a reservoir which release drug from the matrix depending on the pore size of the Ca-alginate gel (42).
Kinetic Analysis of Release Data
Table II presents the kinetic analysis of the in vitro release data of SS from mucoadhesive in situ gelling inserts, according to the exponential equation of Korsmeyer et al. (25). The calculated n values were found between 0.69 and 1.16 for the tested formulations. These values suggest that more than one mechanism may be involved in the release kinetics.
In case of CMC Na in situ gelling inserts, the release exponent n was found to be 0.99, indicating zero-order release kinetics, where drug release involves polymer relaxation and chain disentanglement (43,44). The release was thus controlled by the viscoelastic relaxation of the matrix during solvent penetration as well as the diffusivity of the drug in the gel layer formed as the insert swells. In this case, the relative rates at which the swelling and eroding fronts moved relative to each other were synchronized and a constant diffusional path length (concentration gradient) was obtained. This result was in agreement with Nafee et al. (22), where they reported that zero-order release kinetics obtained due to the electrostatic interaction between CMC Na backbone and the drug cations; resulting in inhibition of drug diffusion which is normally more rapid than gel erosion.
On the other hand, drug release mechanism expected from AL inserts followed super case-II transport where n value was 1.12 (45). In such system, the rate of dissolution medium uptake into the polymer is largely determined by relaxation of the polymer chains. In general, the relaxation contribution was higher for the formulations with higher n values (46).
The release exponent n was found to be 0.83 and 0.69 for HPMC and CH in situ gelling inserts respectively, indicating anomalous (non-Fickian) release. Anomalous transport kinetics prevails if the difference between the penetration of the diffusion front and the erosion front is not too high (47). When swelling is predominant, drug diffusion probably occurs through the solvent-filled pathways of the swollen insert. Erosion of the matrix can also influence the drug release from this polymer matrix and a relative contribution of erosion and diffusion to the overall release mechanism is suggested. Studying the release kinetics of a water soluble drug, Cetylpyridinium chloride, from CH patches resulted in n values ranging from 0.637 to 0.767, indicating also a non-Fickian release behavior (35).
CONCLUSION
The formulated mucadhesive in situ gelling inserts were smooth in appearance, uniform in regard to thickness, weight, and drug content, as well as non-irritating to nasal mucosa. The developed nasal inserts exhibited satisfactory mucoadhesive characteristics, water uptake, and extended drug release. Therefore, the inserts could be used for nasal delivery of SS over about 12 h; bypassing the hepatic first-pass metabolism.
The inserts made of CMC Na or AL seems to be superior over HPMC or CH with respect to water and moisture uptake, in vitro mucoadhesion, and drug release. Regarding drug release retardation, CMC Na and AL inserts exhibited better retardation of drug release than CH, but expectedly to a lesser extent than HPMC inserts.
REFERENCES
- 1.Werner U. In situ gelling nasal inserts for prolonged drug delivery. Berlin: Fachbereich Biologie, Chemie, Pharmazie der Freien Universität Berlin; 2003. [Google Scholar]
- 2.Thapa P, Baillie AJ, Stevens HN. Lyophilization of unit dose pharmaceutical dosage forms. Drug Dev Ind Pharm. 2003;29:595–602. doi: 10.1081/DDC-120018648. [DOI] [PubMed] [Google Scholar]
- 3.Bertram U, Bernard MC, Haensler J, Maincent P, Bodmeier R. In situ gelling nasal inserts for influenza vaccine delivery. Drug Dev Ind Pharm. 2010;36:581–93. doi: 10.3109/03639040903382673. [DOI] [PubMed] [Google Scholar]
- 4.McInnes FJ, Thapa P, Baillie AJ, Welling PG, Watson DG, Gibson I, et al. In vivo evaluation of nicotine lyophilised nasal insert in sheep. Int J Pharm. 2005;304:72–82. doi: 10.1016/j.ijpharm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Garmise RJ, Mar K, Crowder TM, Hwang CR, Ferriter M, Huang J, et al. Formulation of a dry powder influenza vaccine for nasal delivery. AAPS PharmSciTech. 2006;7:E19. doi: 10.1208/pt070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garmise RJ, Staats HF, Hickey AJ. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech. 2007;8:E81. doi: 10.1208/pt0804081. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza R, Mutalik S, Venkatesh M, Vidyasagar S, Udupa N. Insulin gel as an alternate to parenteral insulin: formulation, preclinical, and clinical studies. AAPS PharmSciTech. 2005;6:E184–9. doi: 10.1208/pt060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardeshi CV, Rajput PV, Belgamwar VS, Tekade AR. Formulation, optimization and evaluation of spray-dried mucoadhesive microspheres as intranasal carriers for Valsartan. J Microencapsul. 2012;29:103–14. doi: 10.3109/02652048.2011.630106. [DOI] [PubMed] [Google Scholar]
- 9.Bertram U, Bodmeier R. In situ gelling, bioadhesive nasal inserts for extended drug delivery: in vitro characterization of a new nasal dosage form. Eur J Pharm Sci. 2006;27:62–71. doi: 10.1016/j.ejps.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 10.McInnes FJ, O’Mahony B, Lindsay B, Band J, Wilson CG, Hodges LA, et al. Nasal residence of insulin containing lyophilised nasal insert formulations, using gamma scintigraphy. Eur J Pharm Sci. 2007;31:25–31. doi: 10.1016/j.ejps.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Cho HJ, Balakrishnan P, Shim WS, Chung SJ, Shim CK, Kim DD. Characterization and in vitro evaluation of freeze-dried microparticles composed of granisetron–cyclodextrin complex and carboxymethylcellulose for intranasal delivery. Int J Pharm. 2010;400:59–65. doi: 10.1016/j.ijpharm.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: the next generation. J Pharm Sci. 2000;89:850–66. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Tafaghodi M, Rastegar S. Preparation and in vivo study of dry powder microspheres for nasal immunization. J Drug Target. 2010;18:235–42. doi: 10.3109/10611860903434035. [DOI] [PubMed] [Google Scholar]
- 14.Patil SB, Sawant KK. Chitosan microspheres as a delivery system for nasal insufflation. Colloids Surf B: Biointerfaces. 2011;84:384–9. doi: 10.1016/j.colsurfb.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Singla AK, Chawla M. Chitosan: some pharmaceutical and biological aspects—an update. J Pharm Pharmacol. 2001;53:1047–67. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 16.Cerchiara T, Luppi B, Bigucci F, Zecchi V. Chitosan salts as nasal sustained delivery systems for peptidic drugs. J Pharm Pharmacol. 2003;55:1623–7. doi: 10.1211/0022357022322. [DOI] [PubMed] [Google Scholar]
- 17.Nand KG, Priti T, Vikas S, Vinod KD. Development and characterization of chitosan coated poly-(ε-caprolactone) nanoparticulate system for effective immunization against influenza. Vaccine. 2011;29:9026–37. doi: 10.1016/j.vaccine.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Patil S, Babbar A, Mathur R, Mishra A, Sawant K. Mucoadhesive chitosan microspheres of carvedilol for nasal administration. J Drug Target. 2010;8:321–31. doi: 10.3109/10611861003663523. [DOI] [PubMed] [Google Scholar]
- 19.Dyer AM, Hinchcliffe M, Watts P, Castile J, Jabbal-Gill I, Nankervis R, et al. Nasal delivery of insulin using novel chitosan based formulations: a comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm Res. 2002;19:998–1008. doi: 10.1023/A:1016418523014. [DOI] [PubMed] [Google Scholar]
- 20.Morgan DJ, Paull JD, Richmond BH, Wilson-Evered E, Ziccone SP. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol. 1986;22:587–93. doi: 10.1111/j.1365-2125.1986.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinac A, Filipovic-Grcic J, Voinovich D, Perissutti B, Franceschinis E. Development and bioadhesive properties of chitosan-ethylcellulose microspheres for nasal delivery. Int J Pharm. 2005;291:69–77. doi: 10.1016/j.ijpharm.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int J Pharm. 2003;264:1–14. doi: 10.1016/S0378-5173(03)00371-5. [DOI] [PubMed] [Google Scholar]
- 23.Varshosaz J, Sadrai H, Heidari A. Nasal delivery of insulin using bioadhesive chitosan gels. Drug Deliv. 2006;13:31–8. doi: 10.1080/10717540500309040. [DOI] [PubMed] [Google Scholar]
- 24.Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–9. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- 25.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 26.Etman MA, Nada AH. Evaluation of simple and ball mill ground mixtures of two NSAIDs with different hydrophilc carriers. Alex J Pharm Sci. 1999;13:135–40. [Google Scholar]
- 27.Kim KH, Frank MJ, Henderson NL. Application of differential scanning calorimetry to the study of solid drug dispersions. J Pharm Sci. 1985;74:283–9. doi: 10.1002/jps.2600740312. [DOI] [PubMed] [Google Scholar]
- 28.Doijad RC, Manvi FV, Malleswara Rao VSN, Patel PS. Buccoadhesive drug delivery system of isosorbide dinitrate: formulation and evaluation. Indian J Pharm Sci. 2006;68:744–8. doi: 10.4103/0250-474X.31007. [DOI] [Google Scholar]
- 29.Washington N, Steele RJ, Jackson SJ, Bush D, Mason J, Gill DA, et al. Determination of baseline human nasal pH and the effect of intranasally administered buffers. Int J Pharm. 2000;198:139–46. doi: 10.1016/S0378-5173(99)00442-1. [DOI] [PubMed] [Google Scholar]
- 30.Nappinnai M, Chandanbala R, Balaijirajan R. Formulation and evaluation of nitrendipine buccal films. Indian J Pharm Sci. 2008;70:631–5. doi: 10.4103/0250-474X.45402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peppas NA, Khare AR. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv Drug Deliv Rev. 1993;11:1–35. doi: 10.1016/0169-409X(93)90025-Y. [DOI] [Google Scholar]
- 32.Perioli L, Ambrogi V, Angelici F, Ricci M, Giovagnoli S, Capuccella M, et al. Development of mucoadhesive patches for buccal administration of ibuprofen. J Control Release. 2004;99:73–82. doi: 10.1016/j.jconrel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Lehr C-M, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–8. doi: 10.1016/0378-5173(92)90353-4. [DOI] [Google Scholar]
- 34.Aksungur P, Sungur A, Unal S, Iskit AB, Squier CA, Senel S. Chitosan delivery systems for the treatment of oral mucositis: in vitro and in vivo studies. J Control Release. 2004;98:269–79. doi: 10.1016/j.jconrel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Nafee NA, Boraie MA, Ismail FA, Mortada LM. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta Pharm. 2003;53:199–212. [PubMed] [Google Scholar]
- 36.Gu JM, Robinson JR, Leung SH. Binding of acrylic polymers to mucin/epithelial surfaces: structure–property relationships. Crit Rev Ther Drug Carrier Syst. 1988;5:21–67. [PubMed] [Google Scholar]
- 37.Madsen F, Eberth K, Smart JD. A rheological examination of the mucoadhesive/mucus interaction: the effect of mucoadhesive type and concentration. J Control Release. 1998;50:167–78. doi: 10.1016/S0168-3659(97)00138-7. [DOI] [PubMed] [Google Scholar]
- 38.Bertram U, Bodmeier R. Parameters affecting the drug release from in situ gelling nasal inserts. Eur J Pharm Biopharm. 2006;63:310–9. doi: 10.1016/j.ejpb.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez CF, Bruneau N, Barra J, Alfonso D, Doelker E. Hydrophilic cellulose derivatives as drug delivery carriers: influence of the substitution type on the properties of compressed matrix tablets. In: Wise DL, editor. Handbook of pharmaceutical controlled release technology. New York: Marcell Dekker; 2000. pp. 1–30. [Google Scholar]
- 40.Moffat AC (2004) Salbutamol sulfate,Clarke’s analysis of drug and poisons. In: Galichet LY, editor. London: Pharmaceutical Press.
- 41.Vigoreaux V, Ghaly ES. Fickian and relaxational contribution quantification of drug release in a swellable hydrophillic polymer matrix. Drug Dev Ind Pharm. 1994;20:2519–26. doi: 10.3109/03639049409042655. [DOI] [Google Scholar]
- 42.Mishra DN, Gilhotra RM. Design and characterization of bioadhesive in-situ gelling ocular inserts of gatifloxacin sesquihydrate. DARU. 2008;16:1–8. [Google Scholar]
- 43.Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release. 1985;2:257–75. doi: 10.1016/0168-3659(85)90050-1. [DOI] [Google Scholar]
- 44.Ritger PL, Peppas NA. A simple equation for description of solute release II Fickian and anomalous from swellable devices. J Control Release. 1987;5:37–42. doi: 10.1016/0168-3659(87)90035-6. [DOI] [PubMed] [Google Scholar]
- 45.Rao V, Shyale S. Preparation and evaluation of ocular inserts containing norfloxacin. Turk J Med Sci. 2004;34:239–46. [Google Scholar]
- 46.Peppas NA, Sahlin JJ. A simple equation for the description of sustained release. III Coupling of diffusion and relaxation. Int J Pharm. 1989;57:169–72. doi: 10.1016/0378-5173(89)90306-2. [DOI] [Google Scholar]
- 47.Lindner WD, Lippold BC. Drug release from hydrocolloid embeddings with high or low susceptibility to hydrodynamic stress. Pharm Res. 1995;12:1781–5. doi: 10.1023/A:1016238427313. [DOI] [PubMed] [Google Scholar]


