Abstract
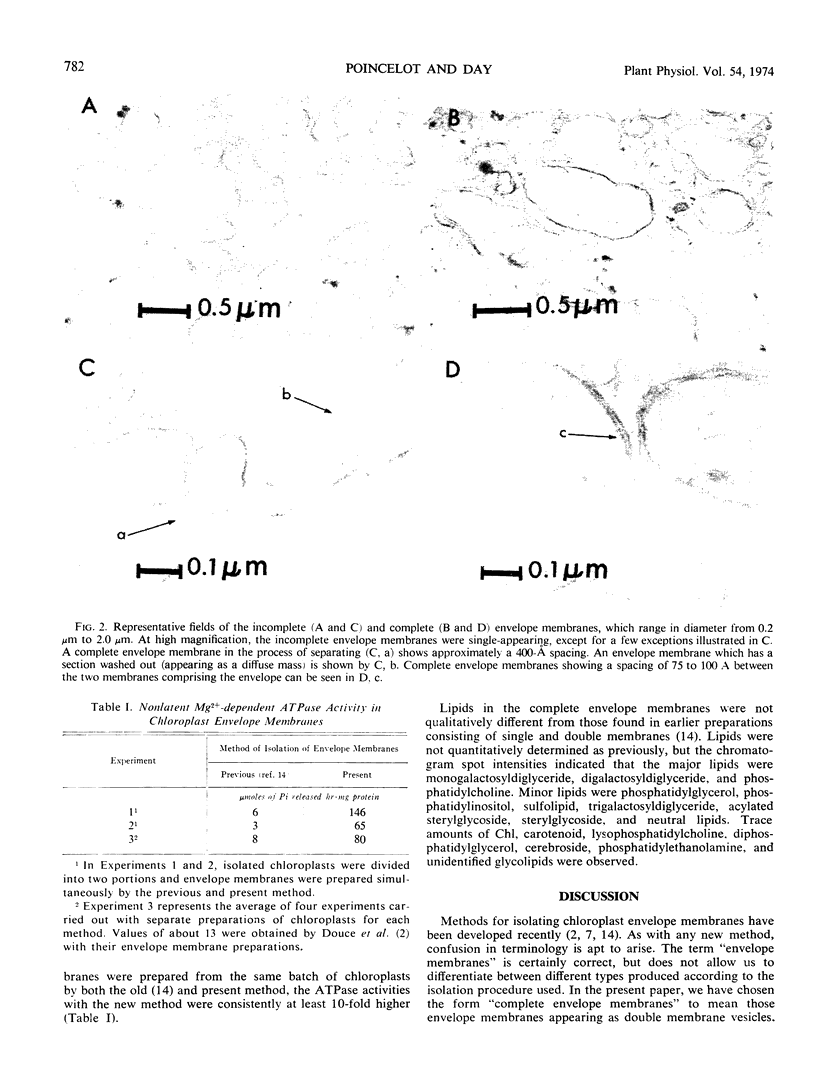
A three-phase, discontinuous sucrose gradient yielded two distinct fractions of envelope membranes from spinach (Spinacia oleracea L.) chloroplasts. Their buoyant densities were 1.08 g cm−3 and 1.11 g cm−3. Electron micrographs showed the lighter and heavier fractions to consist primarily of single and double membranes, respectively. The milligrams of lipid-milligrams of protein ratio for the complete envelope membrane (double membrane fraction) was 1.74. Thin layer chromatograms showed that the lipids of the complete envelope membranes were similar to those found in earlier preparations which consisted of single and double membranes. This isolation procedure is superior to earlier methods in that the percentage of complete envelope membranes is greater and the yield is almost three times as great. Enzymatic and chemical analyses and microscopic examination showed the complete envelope membranes were free of bacterial, fungal, microsomal, mitochondrial, and lamellar membrane contamination as well as stromal contamination. The specific activities of nonlatent Mg2+ -dependent ATPase (80 μmoles of phosphate released hr−1 mg protein−1) were about 10-fold higher than those values found with earlier preparations consisting of single and double membranes, indicating that the ATPase is largely lost in preparations containing single membranes. These higher values show that the ATPase is located in the double membrane and probably functions in the transport processes of the envelope membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackender R. O., Leech R. M. The Galactolipid, Phospholipid, and Fatty Acid Composition of the Chloroplast Envelope Membranes of Vicia faba. L. Plant Physiol. 1974 Mar;53(3):496–502. doi: 10.1104/pp.53.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. XI. Magnesium-adenosine triphosphatase properties of heat-activated coupling factor I from chloroplasts. J Biol Chem. 1972 Oct 25;247(20):6506–6510. [PubMed] [Google Scholar]
- PARK R. B., PON N. G. Chemical composition and the substructure of lamellae isolated from Spinacea oleracea chloroplasts. J Mol Biol. 1963 Feb;6:105–114. doi: 10.1016/s0022-2836(63)80126-6. [DOI] [PubMed] [Google Scholar]
- PARK R. B., PON N. G. Correlation of structure with function in Spinacea oleracea chloroplasts. J Mol Biol. 1961 Feb;3:1–10. doi: 10.1016/s0022-2836(61)80002-8. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P. Differences in lipid composition between intact and membrane-stripped spinach chlorplasts. Biochim Biophys Acta. 1971 Jun 8;239(1):57–60. doi: 10.1016/0005-2760(71)90192-5. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P. Intracellular distribution of carbonic anhydrase in spinach leaves. Biochim Biophys Acta. 1972 Feb 28;258(2):637–642. doi: 10.1016/0005-2744(72)90255-0. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P. Isolation and lipid composition of spinach chloroplast envelope membranes. Arch Biochem Biophys. 1973 Nov;159(1):134–142. doi: 10.1016/0003-9861(73)90437-2. [DOI] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
- Sabnis D. D., Gordon M., Galston A. W. Localization of adenosine triphosphatase activity on the chloroplast envelope in tendrils of Pisum sativum. Plant Physiol. 1970 Jan;45(1):25–32. doi: 10.1104/pp.45.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]