Abstract
This study aimed to develop and evaluate a novel multi-unit tablet that combined a pellet with a sustained-release coating and a tablet with a pulsatile coating for the treatment of circadian rhythm diseases. The model drug, isosorbide-5-mononitrate, was sprayed on microcrystalline cellulose (MCC)-based pellets and coated with Eudragit® NE30D, which served as a sustained-release layer. The coated pellets were compressed with cushion agents (a mixture of MCC PH-200/ MCC KG-802/PC-10 at a ratio of 40:40:20) at a ratio of 4:6 using a single-punch tablet machine. An isolation layer of OpadryII, swellable layer of HPMC E5, and rupturable layer of Surelease® were applied using a conventional pan-coating process. Central-composite design-response surface methodology was used to investigate the influence of these coatings on the square of the difference between release times over a 4 h time period. Drug release studies were carried out on formulated pellets and tablets to investigate the release behaviors, and scanning electron microscopy (SEM) was used to monitor the pellets and tablets and their cross-sectional morphology. The experimental results indicated that this system had a pulsatile dissolution profile that included a lag period of 4 h and a sustained-release time of 4 h. Compared to currently marketed preparations, this tablet may provide better treatment options for circadian rhythm diseases.
KEY WORDS: central composite design, circadian rhythm diseases, in vitro dissolution study, multi-unit tablet, sustained-release and pulsatile coating
INTRODUCTION
Circadian rhythms are a characteristic feature of all human beings and often result in similar physiological phenomena over a period of time. Circadian rhythms can affect many systems within the body, including the cardiovascular and respiratory systems. Circadian rhythm diseases are disorders that are based on biological circadian rhythms and often occur during a predictable period of time. Many systemic disorders often occur according to circadian rhythms, including angina pectoris and asthma (1). The goal in treating circadian rhythm diseases is to cure the diseases while reducing the side effects associated with therapy (2). However, during the use of conventional therapies, which include injections and commonly available oral tablets or capsules, the drug is released rapidly after medication and the blood concentrations may quickly reach toxic levels. An important determinant of a medication’s safety and efficacy is the amount of time the drug is available, which is affected by the route of administration, including injection, ingestion, and other means (3). Thus, there is an unmet clinical need for modified release dosage formulations that are suitable for use in the treatment of these diseases.
A sustained-release preparation is one type of modified release dosage formulation that is prepared by either polymer coating, the use of matrix materials or osmotic technology (4), in which the drug can be slowly released to allow the curative effects of the drug to be maintained over a long period of time (5). Although sustained-release preparations extend the release time of the drug, biological systems do not consistently react to these release systems because the drug concentration may not always be high enough during the appropriate stage of the circadian rhythm. The circadian timing of medication delivery can be very important for patient survival in aggressive medical conditions. Pulsatile preparations allow for sudden drug release after a time gap or predetermined lag time that corresponds to the circadian rhythm of a particular disease state. The lag time can be controlled either by osmosis or by the use of an erodible, soluble, or rupturable membrane (2). The lag time can be incorporated into many dosage formulations, including hard gelatin capsules (6), tablets (7), or pellets (8). Although they can release the drug at a predetermined point in time, pulsatile preparations cannot provide steady plasma concentrations over a long period of time.
To meet the biological requirements and cover the entire disease period accurately, we combined the two types of modified-release mechanisms to design a novel, multi-unit drug-delivery system. The pulsatile layer was designed using the expansion-rupture system, which consists of an inner swelling layer and an outer rupturable layer (6). The pulsatile layer was prepared on the surface of the tablet, and the sustained-release layer was prepared on the surface of the pellets. The two layers each consisted of a polymer coating and were controlled by the thickness of the coating. Their mechanisms were individually based on elasticity and tensile strength. With the application of time-controlled release technology, the sustained-release pellets used in this system can completely disintegrate into small units while being taken for a predetermined period of time, after which the drug can be fully released from the pellets (Fig. 1). As compared with a single-unit system based on the same principle, the drug can achieve better bioavailability in the system employing the enlarged pellet surface (9). The multi-unit system can also prevent dose-dumping because a few failed units do not have a serious impact on the drug release (10). The pulsatile layer and sustained-release layer can also be prepared and coated onto the surface of the pellets together, and the coated pellets can be encapsulated to form another type of multi-unit system (11). However, the predetermined time associated with the independent units may differ and therefore may not match the circadian rhythm. In addition, the pulsatile layer may affect the drug release from the sustained-release pellets because the pulsatile layer is located in close proximity to the sustained-release layer during rupture.
Fig. 1.
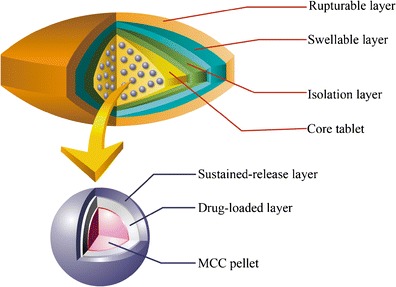
The schematic diagram of the novel multi-unit tablet for treating circadian rhythm diseases
In this study, we chose angina pectoris as a model disease, which has a significant association with circadian rhythm. Angina pectoris in general and variant angina attacks in particular usually have a higher frequency from 2:00 to 6:00 a.m. during ischemic episodes (12). According to the latest survey, approximately 10.2 million people in the USA are suffering from angina, and the number of new cases increases by 500,000 every year (13). We selected isosorbide-5-mononitrate (5-ISMN) as a model drug for use in this system because it has better oral absorption, a wide range of therapeutic concentrations (14) and is cleared from the body by metabolism (15). We designed the system to have a lag time of 4 h and a sustained-release time of 4 h, which accurately coincides with the angina pectoral rhythm and avoids nitrate tolerance (16). 5-ISMN was sprayed on microcrystalline cellulose (MCC)-based pellets that were coated with Eudragit® NE30D, which was used as a sustained-release layer. The pellets were compressed with cushioning agents, including MCC PH-200, MCC KG-802, and PC-10, using a single-punch tablet machine. Pulsatile systems work based on an expansion-rupture system. A conventional pan-coating method is applied to the coating process for the swellable layer of HPMC E5, and the rupturable layer is composed of Surelease®. OpadryII is added as an isolation layer inside the tablets, which can reinforce the core tablets during the coating process. A three-factor, five-level, central composite design (CCD) was used to obtain the ideal coating formulation. Drug release studies were carried out to confirm the designed release time and release mechanism. The morphology of the pellets and tablets was characterized using SEM analysis.
MATERIALS AND METHODS
Materials
Isosorbide-5-mononitrate (5-ISMN) was obtained from the Shijiazhuang Pharmaceutical Group, China. Microcrystalline cellulose spheres 507 (CP-507), microcrystalline cellulose PH-200 (MCC PH-200), microcrystalline cellulose KG-802 (MCC KG-802), and pregelatinized starch 10 (PC-10) were obtained from the Asahi Kasei Corporation, Japan. Eudragit® NE30D was obtained from the Röhm GmbH Chemische Fabrik Company, Darmstadt, Germany. OpadryII, hydroxypropyl methyl cellulose E5 (HPMC E5) and Surelease® were obtained from Colorcon Inc., Shanghai, China. All of the chemicals used in this study were of reagent grade or higher.
Preparation of Drug-Loaded Pellets
A spraying technique was used to prepare the drug-loaded pellets. 5-ISMN was dissolved in an adhesive solution, which was then deposited on commercial MCC pellets. Microcrystalline cellulose spheres (CP-507), ranging in size from 500 to 700 μm, were selected because spheres of this size can be efficiently coated using a spraying technique. The results obtained from several trials indicated that a 2% HPMC E5 water solution was a desirable binder solution to adequately load 5-ISMN on the pellets. Two grams of HPMC E5 was dissolved in 98 ml of purified water for use as an binder solution. Then, 5 g of 5-ISNM was slowly added to the binder solution, which was stirred until the 5-ISNM had thoroughly dissolved. The final concentration of sprayed solution was 50 mg/ml. The spraying process was performed in a fluid-bed coater (JHQ-100, Shenyang, China). Ten grams of MCC pellets was fluidized when the outlet temperature reached 40°C. A nozzle (0.5 mm) attached to a peristaltic pump (BT100-2J, Baoding, China) was used to apply the bottom-sprayed solution to the MCC pellets, with a spray rate of 1.0 ml/min, an air flow rate of 100–150 l/min and an atomizing air pressure of 1.5–1.6 bar. After the layering of the drug had been finished, a coating was applied to the dry pellets at a temperature of 40°C for another 10 min. The amount of drug loaded onto the pellets was approximately 300 mg/g.
Preparation of Sustained-Release Pellets
Eudragit® NE30D, which consists of methyl methacrylate and ethyl acrylate monomers at a ratio of 2:1, is an aqueous polymer dispersion. Due to its superior flexibility and ductility, Eudragit® NE30D is suitable for the coating of pellets that will be compressed into tablets (17). The coating process was conducted using a fluid-bed coater (JHQ-100, Shenyang, China). Five percent GMS and 40% Tween-80 based on the Eudragit® NE30D dry polymer weight were added to the hot purified water at 70–80°C, and homogenized for 10 min with a high-speed homogenizer to form an emulsion. Then the emulsion was added to the Eudragit® NE30D aqueous dispersion while being stirred. The final concentration of the Eudragit® NE30D aqueous dispersion was 10%. The bottom-sprayed solution that was used to coat the MCC pellets was then applied using a peristaltic pump (BT100-2J, Baoding, China) with a 0.8-ml/min spray rate, a 100–150 l/min air flow rate, and a-1.5–1.6 bar atomizing air pressure. The weight gain of drug-loaded pellets was 8%, 13%, and 15%. The coating temperature was set at 25°C according to the film formation temperature of Eudragit® NE30D. In order to form a continuous and uniform film, the water needed to be evaporated. Therefore, it took 24 h for the pellets to form an intact film at 40°C after coating (18).
Preparation of Drug-Loaded Tablets
Preparation of Tablet Cushioning Agents
Cushioning agents are used to absorb compression forces and avoid damage to the coated pellets during the compression process. The cushioning agents used in the present study consisted of ideal filler materials. MCC was considered to be an ideal filler material due to its optimal protection and compressibility properties (19). Several commercial companies have developed MCC using various methods and used them as the cushioning agents. In the present study, we chose MCC PH-200, MCC KG-802, and PC-10 for use as filler materials (20). MCC PH-200 has better mobility than common MCC. MCC KG-802 has excellent formability and can form the tablets at a lower pressure. PC-10 can disintegrate tablets quickly and improve the quality of the granules. After several trials, the use of cushioning agents at a ratio of 40% PH-200, 40% KG-802, and 20% PC-10 pellets was found to cause the least amount of damage to the drug-loaded pellets according to the dissolution release profile. The cushioning agents must be formed into granules by the wet granulation to improve the mobility and uniform distribution of the pellets. Twenty grams of PH-200, 20 g of KG-802, and 10 g of PC-10 were formed into damp particles by passing them with water through 30 mesh sieves and were then dried for 12 h at 60°C. The dried granules were screened using 18–24 standard mesh sieves to obtain a narrow particle size range and the granulation yield was 83.91%.
Preparation of Core Tablets
The core tablets were prepared using a single-punch tablet machine that produced a concave punch 11 mm in diameter. Forty grams of coated pellets, 60 g of cushioning granules, and 0.5 g of magnesium stearate were placed into a plastic bottle and mixed uniformly. Five hundred milligrams of the mixture was weighed and manually filled into the die cavity of the tablet machine (TDP-1.5T, Shanghai China). The hardness was maintained at 5 kg using a hardness tester (78X-2, Shanghai, China) because the results obtained from several trials indicated that the drug release rate can be increased significantly with an increase of pressure force. The weight of each tablet was maintained within the range of 500 ± 3 mg, and the drug load was 50 mg.
Preparation of Coated Tablets
Isolation Layer Coating
Ten grams of OpadryII was dispersed in 90 ml of purified water to form an isolation layer coating solution and was continuously stirred to prevent deposition. The isolation layer was coated using a pan-coating process. The temperature of the inlet air was 40°C, the spray rate was 2 ml/min and the pan-rotation rate was 8 rpm. The coated tablets were dried at 50°C for 12 h to remove the residual water. The weight gain was 2.5%.
Swelling Layer Coating
Eight grams of HPMC E5 was dissolved in 92 ml of purified water, which was then heated to 80°C and used as a swelling layer coating solution. The swelling layer was coated using a pan coater with the temperature of the inlet air of 45°C, the spray rate of 2 ml/min and the pan-rotating rate of 10 rpm. During the entire coating process, the coating solution was stirred continuously. It took 12 h for the dual-coated tablets to dry at 45°C. The weight gain was 10%.
Rupturable Layer Coating
Based on the dry polymer weight of the coating solution that was used to form the rupturable layer, the Surelease® aqueous dispersion was diluted with purified water to 10% (w/w). A conventional pan-coating process was used form the rupturable layer coating, which was continuously stirred to prevent deposition during the coating process. The temperature of the inlet air was 40°C, the spray rate was 2 ml/min and the pan-rotation rate was 10 rpm. It took 12 h for the coated tablets to dry. The drying process was used to remove residual water during the formation of the multi-unit tablets. The weight gain was 6.5%.
In Vitro Dissolution Studies
Determination of 5-ISMN Concentrations
The quantitative detection of 5-ISMN was performed using high-performance liquid chromatography (HPLC, Shimadzu LC-20A, Kyoto, Japan) equipped with an ultraviolet detector that was set at 210 nm (21). The separation was performed using an XTerra® MS C18 column (5 μm, 250 × 4.6 mm, Waters, USA) at 30°C. The mobile phase used was a mixture of methanol and water at a ratio of 25:75. The injection volume was 20 μl with a flow rate of 1.0 ml/min. The linearity of the method was verified in the concentration range of 6–60 μg/ml (r = 0.9998). The RSD values of the intraday and interday precision for 5-ISMN were less than 2%. The recovery rates for 5-ISMN were in the range of 98–102%, and the RSD values were below 2%.
Pellet and Tablet Dissolution Tests
Dissolution experiments were carried out according to the USP 27 XXIII paddle method using a dissolution apparatus (ZRS-8G dissolution apparatus, Tianjin, China). The release medium used was 900 ml of distilled water and the rotation rate was 100 rpm. Pellets or tablets were immersed in dissolution medium, the temperature of which was maintained at 37 ± 0.5°C. At predetermined time intervals, 5 ml samples were taken and another 5 ml of fresh medium was added immediately. The samples were filtered using a 0.22 μm filter and injected into the HPLC for analysis, as described above. Dissolution experiments were carried out in six groups. The average dissolution profiles and standard deviations were calculated.
Experimental Design and Data Analyses
Central Composite Design
Three independent variables were measured in the present study: weight gain of OpadryII (X1), weight gain of HPMC E5 (X2) and weight gain of Surelease® (X3). Table I lists the experimental range of each factor based on preliminary experimental results. The critical responses were calculated as the square of the difference between the disintegration time and 240 min (Y), as this system was developed to have a lag time of nearly 4 h. The experiments were designed using Design-Expert 7.5 software; Table II presents the experimental layout.
Table I.
Independent Variables and Levels for the Central Composite Design
| Factor | Factor level in coded form | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |
| X 1 (%) | 1.66 | 2.00 | 2.50 | 3.00 | 3.34 |
| X 2 (%) | 4.95 | 7.00 | 10.00 | 13.00 | 15.05 |
| X 3(%) | 3.98 | 5.00 | 6.50 | 8.00 | 9.02 |
Independent variables: X 1 weight gain of OpadryII, X 2 weight gain of HPMC E5, X 3 weight gain of Surelease®
α = 1.682
Table II.
Experimental Design for Three Factors and Experimental Response
| No. | X 1 (%) | X 2 (%) | X 3 (%) | Y |
|---|---|---|---|---|
| 1 | 2.00 | 7.00 | 8.00 | 20,377.56 |
| 2 | 3.00 | 13.00 | 8.00 | 54,639.06 |
| 3 | 3.34 | 10.00 | 6.50 | 517.56 |
| 4 | 2.50 | 10.00 | 6.50 | 1,314.06 |
| 5 | 2.50 | 10.00 | 6.50 | 1,314.06 |
| 6 | 2.50 | 15.05 | 6.50 | 7,876.56 |
| 7 | 3.00 | 13.00 | 5.00 | 15,314.06 |
| 8 | 2.00 | 13.00 | 8.00 | 689.06 |
| 9 | 1.66 | 10.00 | 6.50 | 16,256.25 |
| 10 | 2.00 | 7.00 | 5.00 | 43,890.25 |
| 11 | 2.50 | 10.00 | 3.98 | 52,098.06 |
| 12 | 3.00 | 7.00 | 5.00 | 45,156.25 |
| 13 | 2.50 | 4.95 | 6.50 | 58,685.06 |
| 14 | 2.50 | 10.00 | 6.50 | 1,314.06 |
| 15 | 2.50 | 10.00 | 6.50 | 1,314.06 |
| 16 | 2.50 | 10.00 | 6.50 | 1,314.06 |
| 17 | 3.00 | 7.00 | 8.00 | 9,264.06 |
| 18 | 2.00 | 13.00 | 5.00 | 826.56 |
| 19 | 2.50 | 10.00 | 6.50 | 1,314.06 |
| 20 | 2.50 | 10.00 | 9.02 | 19,600 |
Factors: X 1 weight gain of OpadryII, X 2 weight gain of HPMC E5, X 3 weight gain of Surelease®, responses: Y square of the difference value between the disintegration time and 240 min
Response values: average, n = 6
Optimization of the Formulation
The multiple linear model and second-order polynomial model were fitted to the response individually using Design-Expert 7.5 software. To evaluate the fit of each equation, F tests, with P > 0.5, were selectively deleted to simplify the model. Graphs of response against the two factors analyzed in the response were plotted using Design-Expert 7.5 software.
Release Kinetic Analyses of Dissolution Data
5-ISMN release kinetics were analyzed using various mathematical models. Seven kinetic models, including the zero-order, first-order, Higuchi matrix, Peppas-Korsmeyer, Hixson-Crowell, Weibull, and Baker-Lonsdale release equations, were applied to the in vitro release data. Regression analyses were performed, and best fits were calculated. The evaluation of the model based on the correlation factors is presented as r2.
Characterization of 5-ISMN Pellets and Tablets
A scanning electron microscope (XL30-ESEM, PHILIPS Company, Holland) was used to examine the shape, surface, and cross-sectional morphology of 5-ISMN-coated pellets and tablets. To facilitate observation, a double-sided adhesive tape was used to fix the samples to a brass specimen stage.
RESULTS AND DISCUSSION
Evaluation of Sustained-Release Pellets
The dissolution curves of pellets with different sustained-release layer weight gains are shown in Fig. 2. Pellets with a weight gain of 8% displayed faster release than the other two pellets and a cumulative release of more than 90% over a 150-min period. Pellets with a weight gain of 18% displayed the slowest release and a cumulative release of more than 90% over a 300-min period. According to the designed sustained-release times described above, a weight gain of 13% was found to be required to obtain an ideal dissolution curve and a cumulative release of more than 90% over a 240 min period.
Fig. 2.
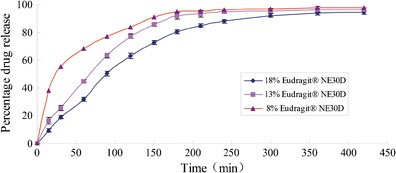
Release profiles of coated pellets in water with different weight gain of Eudragit® NE30D (mean ± SD, n = 6)
Evaluation of the Ratio of Pellets to Cushioning Agents in Multi-Unit Tablets
Different ratios of coated pellets to cushioning granules, including 70:30, 50:50, and 40:60 ratios, were prepared. Figure 3 shows the dissolution curves for these tablets and uncompressed pellets. As compared to the uncompressed pellets, a ratio of 40:60 showed the highest correlation with dissolution rate, and the other two ratios demonstrated lower correlations, illustrating that the rate of drug release increases as the amount of coating on a pellet increases. This relationship is due to the fact that the area of contact of coated pellets increased sharply due to reductions in the size of cushioning granules during the compression process. The tablet structure displayed elastic recovery when the tablet was ejected from the tablet press mold. During ejection, some of the film was pulled-off of the surface of the coated pellets and adhered to the surfaces of other coated pellets. The film on the drug-loaded pellets was damaged, and drug release increased (22). While the amount of cushioning granules increased, the release rate of the drug from the tablets decreased. A possible explanation for this process may be that more cushioning granules separate the pellets effectively and prevent contact with the interface during compression. Therefore, coated pellets and cushioning granules at a ratio of 40:60 displayed the ideal properties.
Fig. 3.
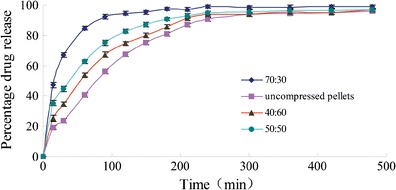
Release profiles of tablets in water with different radio of the coating pellets to the cushioning agents (mean ± SD, n = 6)
Evaluation of the Pulsatile Layer
Another isolation layer was added to the dual layers in this study on the basis of common pulsatile systems based on expansion-rupture systems. The rupturable system of the novel tablets was composed of three layers: an isolation layer, a swelling layer, and a control layer. Coating in a pan coater required the core tablets to have a higher strength, but the release rate of tablets increased sharply due to the damaged film on the coated pellets that occurred at a higher pressure. When used as an isolation layer, OpadryII reinforced the strength of the core tablets during the coating process. HPMC E5 was used as a swellable layer due to its expansion properties. Surelease®, an aqueous polymer dispersion composed of plasticized ethylcellulose, was used as a rupturable coating and easily ruptured in the dissolution medium.
Analysis of Central Composite Design
Multiple linear and second-order polynomial models were used to fit the response, and the obtained models were verified using ANOVA. Higher F and R values (correlation coefficient) were the standards used to identify the fit of the model for the response. The second-order polynomial model was determined to be a better fit for the response based on the results shown in Table III.
Table III.
Regression Coefficients and Statistical Analysis of the Two Models
| Model fitting | Factor | Factor coefficient | P value | ANOVA |
|---|---|---|---|---|
| Multiple linear model (square of the difference value between the disintegration time and 240 min) | Intercept | 17,653.74 | 0.2673 | |
| Weight gain of OpadryII | 2,351.99 | 0.6734 | F = 1.44 | |
| Weight gain of HPMC E5 | −9,714.44 | 0.0952 | R 2 = 0.2130 | |
| Weight gain of Surelease® | −5,482.40 | 0.3319 | ||
| Second-order polynomial model (square of the difference value between the disintegration time and 240 min) | Intercept | 1,428.91 | 0.0049 | |
| Weight gain of OpadryII | 2,351.99 | 0.4640 | ||
| Weight gain of HPMC E5 | −9,714.44 | 0.0104 | F = 5.99 | |
| Weight gain of Surelease® | −5,482.40 | 0.1063 | R 2 = 0.8436 | |
| (Weight gain of OpadryII) (weight gain of HPMC E5) | 9,785.63 | 0.0358 | ||
| (Weight gain of OpadryII) (weight gain of Surelease®) | 3,385.38 | 0.4212 | ||
| (Weight gain of HPMC E5) (weight gain of Surelease®) | 12,324.05 | 0.0122 | ||
| (Weight gain of OpadryII)2 | 1,750.02 | 0.5735 | ||
| (Weight gain of HPMC E5)2 | 10,551.34 | 0.0056 | ||
| (Weight gain of Surelease®)2 | 11,459.35 | 0.0034 |
Influence of Various Factors on the lag Time of the System
Y increased slightly after an increase in X1, as shown in Fig. 4 A and B, due to the fact that the isolation layer, OpadryII, was mainly used to isolate moisture and reinforce the tablets to allow them to be easily coated. The low percentage of weight gain cannot affect the disintegration time of the system. Figure 4 A and C illustrates that Y decreased when X2 increased due to the fact that the more weight HPMC E5 gained, the lower the difference between the disintegration time and 240 min due to the increasing swelling ability of the swellable layer. However, Y increased when X2 increased after a minimum point due to the fact that as the coating weight increased, the swelling time of HPMC E5 increased. Figure 4 B and C shows that Y tended to decrease first and then increased as X3 increased. When the amount of Surelease® weight gain was low, the disintegration time was short for the rupturable coating and the swellable layer easily burst. As Surelease® weight gain increased, the time period leading up to bursting was nearly 240 min. Values of Y at this point were at their lowest. When the Surelease® continued to be weighed, the rupturable coating did not easily burst at 240 min, and Y then increased as X3 increased.
Fig. 4.
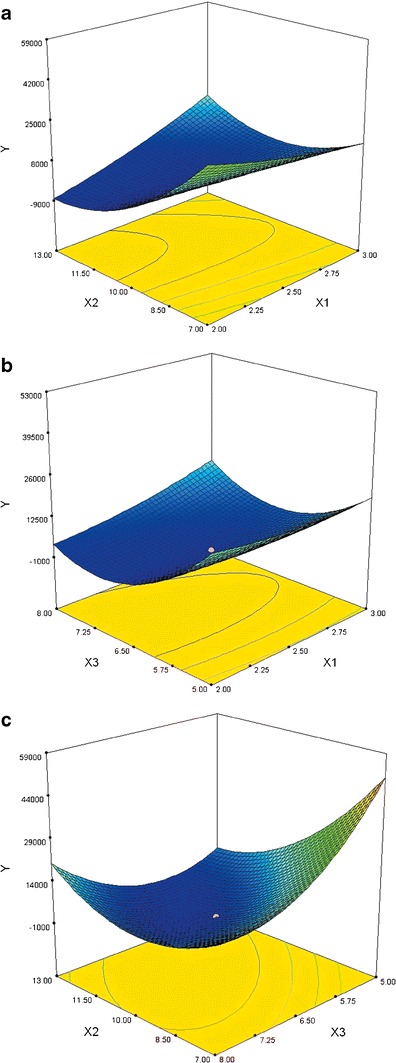
Response surfaces for square of the difference value between release times over a 4-h time period (Y) as a significantly influential factor
Optimization of the Coating Formulation
The optimum values of Y, as displayed in Fig. 4, were as follows: X1, 2.46; X2, 12.33; and X3, 6.66. To verify the adequacy of the model for prediction, we sought three points at which to test the accuracy of the prediction results, including one near the optimum point (formulation A) and two additional random points (formulations B and C). The compositions, actual values, and responses of formulations A through C are listed in Table IV, which shows that there is a correlation between the predicted and actual results.
Table IV.
The Predicted Values and Actual Results for Optimum Formulation A as well as Two Other Random Formulations B and C
| Formulation | X 1 (%) | X 2 (%) | X 3 (%) | Response | Predicted value | Actual value | Bias (%) |
|---|---|---|---|---|---|---|---|
| A | 2.50 | 12.50 | 6.50 | Y | 659.62 | 691.43 | 4.82 |
| B | 3.30 | 10.00 | 6.30 | Y | 9,883.27 | 10,370.28 | 4.93 |
| C | 2.40 | 8.50 | 5.50 | Y | 22,808.45 | 21,976.32 | −3.65 |
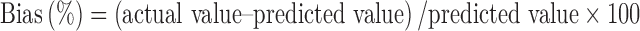
Factors: X 1 weight gain of OpadryII, X 2 weight gain of HPMC E5, X 3 weight gain of Surelease®; responses: Y square of the difference value between the disintegration time and 240 min
Response values: average, n = 6
Evaluation of Novel Multi-Unit Tablets
The cumulative release curves are shown in Fig. 5, including those for the novel tablet with an optimized formulation, a commercially available tablet with a conventional formulation and a commercially available sustained-release tablet. The commercially available tablet with a conventional formulation resulted in a cumulative release exceeding 95% over a 15 min period and the commercially available sustained-release tablet over a 12 h period. The release curve obtained from this novel pulsatile sustained-release tablet indicated that the tablet is able to completely deliver the drug within a period of 8 h, with a lag time of 4 h and a sustained-release of 4 h. The swelling of HPMC E5 resulted in the disintegration of the tablet. The weight gain of HPMC E5 and Surelease® determined the lag time of the system. When the tablet disintegrated, it dispersed into pellets containing the sustained-release layer, and sustained release of the drug began. As compared with the two commercially available tablets, the joint pulsatile and sustained-release actions aligned precisely with the circadian rhythm of angina pectoris and avoided nitrate tolerance for the dose-free interval was more than 14h (16). The whole-system functions were based on the multi-unit pellet tablets and expansion-rupture mechanism. The lag time and sustained-release time were determined during the course of our research. The optimal prescription and preparation are listed in Table V.
Fig. 5.
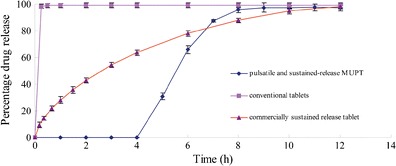
Release profiles of the novel multi-unit tablet, commercially conventional tablet and commercially sustained-release tablet (mean ± SD, n = 6)
Table V.
Process Parameters for Film Coating During Preparation
| Parameter | Sustained release layer | Isolation layer | Swellable coating | Rupturable coating |
|---|---|---|---|---|
| Polymer used | Eudragit® NE30D | OpadryII | HPMC E5 | Surelease® |
| Method | Fluidized bed | Pan coater | Pan coater | Pan coater |
| Inlet air temperature (°C) | 25 | 40 | 45 | 40 |
| Spray rate (ml/min) | 0.8 | 2 | 2 | 2 |
| Spray pressure (bar) | 1.5 | – | – | – |
| Pan speed (rpm) | – | 8 | 10 | 10 |
| Weight gain (%) | 13.00 | 2.45 | 10.20 | 6.84 |
Release Kinetic Analyses of Dissolution Data
The dissolution profiles of the novel tablets (Fig. 5) can be divided into delayed and sustained-release portions. Because there was no release during the delayed portion, the sustained-release portion of the dissolution curve was suitable to fit into drug-release kinetic models (Table VI) so that 5-ISMN release mechanisms at sustained-release portions could be investigated. The results indicated that the sustained-release profile of this novel formulation of 5-ISMN can be adapted to fit the Weibull model. The release mechanism of this model illustrated that the novel formulation was diffusion-controlled rather than being controlled by the intrinsic dissolution rate of 5-ISMN. However, Weibull model was an empirical model which is not deducted from the kinetic fundament. And there were no parameters related to the drug dissolution rate. In addition, Higuchi and First-order model also showed better correlations to the release profile. Higuchi model, a theoretical model, indicated that the sustained-release profile was based on the diffusion-controlled, and yet was not suitable for coated tablets or pellets. First-order model was mainly used to illustrate the matrix tablets/pellets (23). Therefore, it needed a new theoretical model to resolve the release behavior of this novel system including the pulsatile portion in the future work.
Table VI.
Mathematical Models Fit for the Sustained-Release Portion of the Novel Multi-Unit Tablet (Release Parameters and Regression Coefficients)
| Release model | Parameter | Value | Equation |
|---|---|---|---|
| Zero-order | r 2 | 0.8983 |
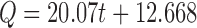
|
| k 0 | 20.07 | ||
| First-order | r 2 | 0.9799 |
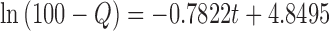
|
| k 1 | −0.7822 | ||
| Higuchi | r 2 | 0.9606 |
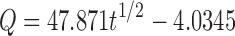
|
| k H | 47.87 | ||
| Peppas-Korsmeyer | r 2 | 0.9092 |
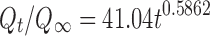
|
| k p | 41.04 | ||
| n | 0.5862 | ||
| Hixson-Crowell | r 2 | 0.6896 |
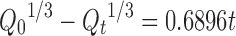
|
| k β | −0.7916 | ||
| Weibull | r 2 | 0.9926 |
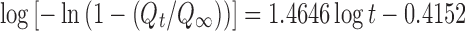
|
| a | 2.061 | ||
| b | 1.464 | ||
| T d | 1.921 | ||
| Baker-Lonsdale | r 2 | 0.6104 |
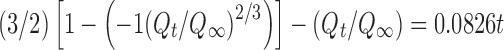
|
| k | 0.0826 |
r 2 correlation coefficients of regression line; k, k 0, k 1, k H, k s, and k p are release rate constants; n diffusion exponent; a scale parameter; b shape
parameter; T d time interval necessary to release 63.2% of drug
Scanning Electron Microscopy
To confirm the mechanism underlying drug release, the internal and external morphology of pellets coated with Eudragit® NE30D, as well as the tablets that were coated with three layers, were studied using scanning electron microscopy (Fig. 6). Observed under a SEM, the 5-ISMN sustained-release pellets were spherical with smooth surfaces (Fig. 6a). The cross-sectional view indicated compact coating of Eudragit® NE30D around the MCC pellet core (Fig. 6b). The cross-sectional view of the tablet prepared using the optimum formulation showed that the membrane was intact, without rupture, or fusion (Fig. 6c). This view also illustrated that the cushioning granules displayed a robust cushioning effect during the compression process. The three layers coating the outside of the tablets—the isolation layer, the swelling layer, and the outer rupturable coating—can be clearly observed in the cross-section (Fig. 6d).
Fig. 6.
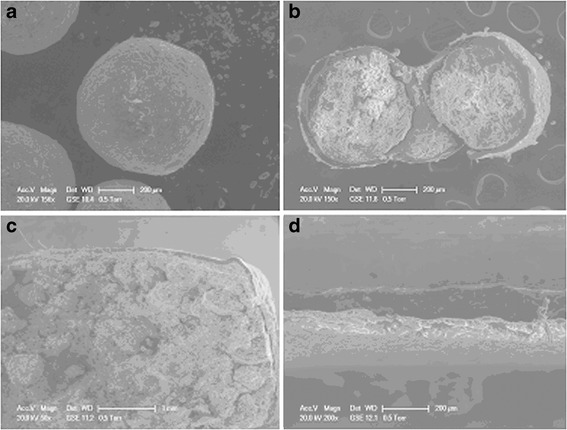
Scanning electron microphotographs of: a surface morphology of the coated pellet at ×156 magnification; b cross-section of the coated pellet at ×150 magnification; c cross-section of the novel multi-unit tablet at ×50 magnification, and d cross-section of the coating for the novel multi-unit tablet at ×200 magnification
CONCLUSION
In this study, a novel multi-unit tablet combined a pellet with a sustained-release coating and a tablet with a pulsatile coating for the treatment of circadian rhythm diseases was developed and evaluated. The expansion-rupture system based on a triple layer structure was used to control the lag time accurately. To coat successfully by using the pan-coating process at a lower hardness, an isolation layer of OpadryIIwas used to make the core tablets stronger. The optimum lag time of the formulation was established using the central composite design-response surface methodology, and the lag time of the system can be modified by weight gains in the swelling and rupturable layers. Accordingly, the sustained-release time can also be altered by weight gains in Eudragit® NE30D and suitable for more circadian rhythm diseases. Release-kinetics analyses showed that the Weibull model fit well with the sustained-release dissolution curve. Moreover, the new theoretical release mechanism for this novel system including the pulsatile portion and how this mechanism behaves in the human body remains to be analyzed further.
ACKNOWLEDGMENTS
This study was financially supported by a program supporting scientific research conducted by college graduates in Jangsu Province (No. CXLX12_0313). We would like to thank the Asahi Kasei, ISP, Evonik and Colorcon Corporations for providing the blank MCC pellets, excipients, and coating materials.
Abbreviations
- 5-ISMN
Isosorbide-5-mononitrate
- MCC
Microcrystalline cellulose
- PC
Pregelatinized starch
- HPMC
Hydroxypropyl methyl cellulose
- h
Hours
- min
Minutes
- rpm
Rotations per minute
- ANOVA
Analysis of variance
- GMS
Glyceryl monostearate
Contributor Information
Jianping Liu, Phone: +86-25-83271923, FAX: +86-25-83271923, Email: liujianpingljp@hotmail.com.
Jiabi Zhu, Phone: +86-25-83271923, FAX: +86-25-83271923, Email: zhujiabicpu@163.com.
REFERENCES
- 1.Mandal AS, Biswas N, Karim KM, et al. Drug delivery system based on chronobiology—a review. J Control Release. 2010;147(3):314–325. doi: 10.1016/j.jconrel.2010.07.122. [DOI] [PubMed] [Google Scholar]
- 2.Youan BB. Chronopharmaceutics: gimmick or clinically relevant approach to drug delivery? J Control Release. 2004;98(3):337–353. doi: 10.1016/j.jconrel.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Lemmer B. Chronobiology, drug-delivery, and chronotherapeutics. Adv Drug Deliv Rev. 2007;59(9–10):825–827. doi: 10.1016/j.addr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Verma RK, Kaushal AM, Garg S. Development and evaluation of extended release formulations of isosorbide mononitrate based on osmotic technology. Int J Pharm. 2003;263(1–2):9–24. doi: 10.1016/S0378-5173(03)00360-0. [DOI] [PubMed] [Google Scholar]
- 5.Bonn R. Sustained-release isosorbide mononitrate (50 mg): optimization of a once-daily dosage form for long-term treatment of angina pectoris. Am J Cardiol. 1988;61(9):12–14. doi: 10.1016/0002-9149(88)90082-3. [DOI] [PubMed] [Google Scholar]
- 6.Bussemer T, Dashevsky A, Bodmeier R. A pulsatile drug delivery system based on rupturable coated hard gelatin capsules. J Control Release. 2003;93(3):331–339. doi: 10.1016/j.jconrel.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Sungthongjeen S, Puttipipatkhachorn S, Paeratakul O, Dashevsky A, Bodmeier R. Development of pulsatile release tablets with swelling and rupturable layers. J Control Release. 2004;95(2):147–159. doi: 10.1016/j.jconrel.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Mohamad A, Dashevsky A. Development of pulsatile multiparticulate drug delivery system coated with aqueous dispersion Aquacoat ECD. Int J Pharm. 2006;318(1–2):124–131. doi: 10.1016/j.ijpharm.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Clarke GM, Newton JM, Short MB. Comparative gastrointestinal transit of pellet systems of varying density. Int J Pharm. 1995;114(1):1–11. doi: 10.1016/0378-5173(94)00200-O. [DOI] [Google Scholar]
- 10.Bodmeier R. Tableting of coated pellets. Eur J Pharm Biopharm. 1997;43(1):1–8. doi: 10.1016/S0939-6411(96)00028-8. [DOI] [Google Scholar]
- 11.Yadav D, Survase S, Kumar N. Dual coating of swellable and rupturable polymers on glipizide loaded MCC pellets for pulsatile delivery: formulation design and in vitro evaluation. Int J Pharm. 2011;419(1–2):121–130. doi: 10.1016/j.ijpharm.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Cassano GB, Maggini C, Guazzelli M. Nocturnal angina and sleep. Prog Neuro-Psychopharmacol. 1981;5(1):99–104. doi: 10.1016/0364-7722(81)90010-2. [DOI] [PubMed] [Google Scholar]
- 13.Phelps CE, Buysman EK, Gomez Rey G. Costs and clinical outcomes associated with use of ranolazine for treatment of angina. Clin Ther. 2012;34(6):1395.e4–1407.e4. doi: 10.1016/j.clinthera.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Abshagen U, Sporl-Radun S. First data on effects and pharmacokinetics of isosorbide-5-mononitrate in normal man. Eur J Clin Pharmacol. 1981;19(6):423–429. doi: 10.1007/BF00548586. [DOI] [PubMed] [Google Scholar]
- 15.Schaumann W. Pharmacokinetics of isosorbide dinitrate and isosorbide-5-mononitrate. Int J Clin Pharmacol Ther Toxicol. 1989;27(9):445–453. [PubMed] [Google Scholar]
- 16.Jonathan A, Uri E, Udho T, et al. Tolerance: an historical overview. Am J Cardiol. 1998;81(1):3A–14A. doi: 10.1016/S0002-9149(97)00992-2. [DOI] [Google Scholar]
- 17.Bodmeier R, Paeratakul O. Mechanical properties of dry and wet cellulosic and acrylic films prepared from aqueous colloidal polymer dispersions used in the coating of solid dosage forms. Pharm Res. 1994;11(6):882–888. doi: 10.1023/A:1018942127524. [DOI] [PubMed] [Google Scholar]
- 18.Cui Y, Zhang Y, Tang X. In vitro and in vivo evaluation of ofloxacin sustained release pellets. Int J Pharm. 2008;360(1–2):47–52. doi: 10.1016/j.ijpharm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Torrado JJ, Augsburger LL. Effect of different excipients on the tableting of coated particles. Int J Pharm. 1994;106(2):149–155. doi: 10.1016/0378-5173(94)90313-1. [DOI] [Google Scholar]
- 20.Asahi Chemical Corp, Yaginuma Y, Yoshida N (2010) Process for production of tablets containing both crystalline cellulose and granules: WO, WO 2009/028487 A1[P]. 2009-03-05.
- 21.Huang YH, Zhang YL, Wang F. Determination of isosorbide-5-mononitratesustained release capsule by HPLC. HeiLongJiang Med J. 2004;17(4):243–244. [Google Scholar]
- 22.Shajahan A, Anil VC, Sunil BJ. A flexible technology for modified-release drug: multiple-unit pellet system (MUPS) J Control Release. 2010;147(1):2–16. doi: 10.1016/j.jconrel.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Paulo C, José MS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]


