Abstract
β-cyclodextrin (βCD) and methyl-β-cyclodextrin (MβCD) complexes with sulfamethazine (SMT) were prepared and characterized by different experimental techniques, and the effects of βCD and MβCD on drug solubility were assessed via phase-solubility analysis. The phase-solubility diagram for the drug showed an increase in water solubility, with the following affinity constants calculated: 40.4 ± 0.4 (pH 2.0) and 29.4 ± 0.4 (pH 8.0) M−1 with βCD and 56 ± 1 (water), 39 ± 3 (pH 2.0) and 39 ± 5 (pH 8.0) M−1 with MβCD. According to 1H NMR and 2D NMR spectroscopy, the complexation mode involved the aromatic ring of SMT included in the MβCD cavity. The complexes obtained in solid state by freeze drying were characterized by Fourier transform infrared spectroscopy, scanning electron microscopy, and thermal analysis. The amorphous complexes obtained in this study may be useful in the preparation of pharmaceutical dosage forms of SMT.
Keywords: complexation, cyclodextrin, infrared spectroscopy, thermal analysis
INTRODUCTION
The efficacy of drug therapy administered by an extravascular route is influenced by oral drug absorption, which in turn depends on physicochemical, physiological, and dosage form variables (1). SMT (pK1 = 7.4 and pK2 = 2.6; Fig. 1a) (2,3), a short-acting sulfonamide, is frequently administered as a “triple sulfa drug” formulation with sulfadiazine (SDZ) and sulfamerazine (SMR). Sulfonamides are synthetic agents classified as antibacterial compounds, which exerts their bacteriostatic activity by competition with p-aminobenzoic acid that is involved in the enzymatic synthesis of dihydrofolic acid. These synthetic compounds play an important role as effective chemotherapeutics of bacterial and protozoal diseases and also exhibit growth-promoting properties in veterinary medicine. These drugs still exhibit a wide interest in veterinary medicine because of their broad-spectrum of activity and low cost (4–9).
Fig. 1.
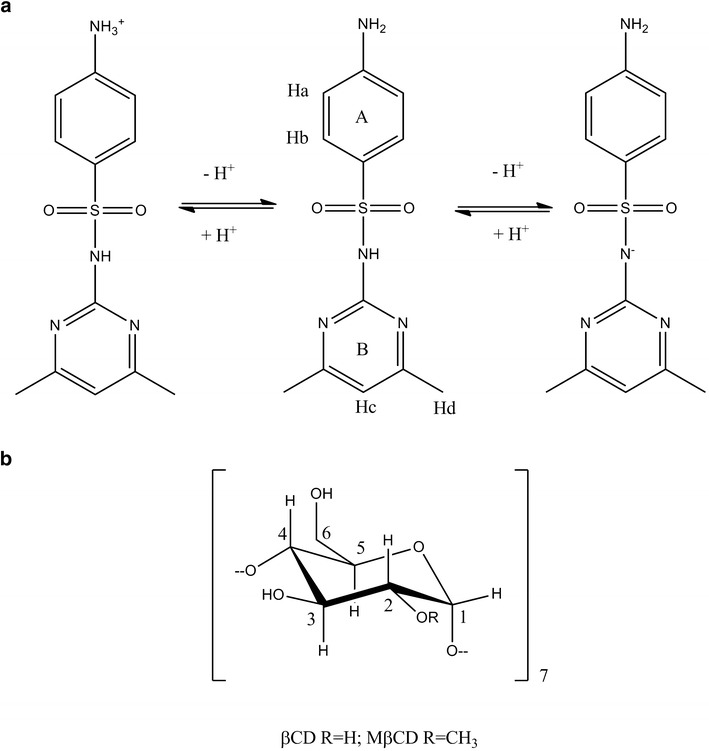
Chemical structure and proton atom numbering scheme of: a SMT (in the protonated, neutral, and anionic forms) and b βCD and MβCD
However, sulfonamides present unfavorable physicochemical properties such as low water solubility, which is a major concern for its therapeutic use and still needs to be investigate. Their therapeutic effects can be influenced by the composition of the formulation, for example cyclodextrins (CDs; Fig. 1b) are useful functional excipients employed in the pharmaceutical industry as complexing agents in order to increase the aqueous solubility of poorly soluble drugs and to improve their bioavailability (10–13).
In previous studies, we evaluated the ability of CDs to increase the solubility of different sulfonamides, including SMT, SDZ, and SMR (14–19). The affinity and three-dimensional structure of these complexes were determined by experimental and molecular modeling techniques. However, a detailed description of SMT/CD complex, justifying their inclusion in pharmaceutical formulations of triple sulfa drugs, remains uninvestigated. Therefore, the main purpose of this study was to further investigate the behavior of this complex in aqueous solution and solid state, taking into account the effects of pH and complexation with the βCD derivative MβCD. In this way, the influence of the ionization state of the drug and the substitution of the native CD in the solubility of the drug was assessed. It is also important to note that Sourbaji et al. (20) has shown that the preparation of SMT/βCD complex, in solid state, applying the kneading method was unsuccessful on the basis of DSC studies. Therefore, it is necessary to study a different preparation method such as freeze-drying method for this system to obtain a real inclusion complex in solid state.
MATERIALS AND METHODS
Materials
Sulfamethazine sodium was procured from Parafarm (Argentina), and the SMT base was obtained by neutralization with aqueous HCl solution and subsequent recrystallization from ethanol, m.p. 198°C. The D2O 99.9 at.% D used in NMR studies was purchased from Sigma, while βCD (MW = 1,135) and MβCD (MW = 1,190) were kindly supplied by Roquette (France). All other materials and solvents were of analytical reagent grade. A Milli-Q Water Purification System (Millipore, Billerica, MA) generated the water used in these studies. In addition, the H2KPO4/HNa2PO4 at pH 2.0 and H2KPO4 at pH 8.0 buffers were used (pH-meter HI 255, HANNA Instrument, Romania), with their ionic strengths being adjusted to 0.5 by addition of NaCl and KCl, respectively.
Phase Solubility Studies
The effects of βCD and MβCD on the solubility of SMT were studied as follows: an excess of SMT was added to aqueous buffered solutions of pH 2.0, pH 8.0, or water containing different amounts of βCD (0–13.2 mM) or MβCD (0–105 mM for water and 0–84 mM for pH 2.0 and 8.0). The resulting suspensions were sonicated in an ultrasonic bath for 1 h and placed in a 25.0°C (±0.1) thermostatized water bath (Circulators HAAKE F3-K, Germany) for 72 h. After equilibrium was reached, the suspensions were filtered through a 0.45-μm membrane filter (Millipore) and analyzed by UV–Vis spectrophotometry. Each experiment was repeated at least three times and the results reported were the mean values. The affinity constant (KC) values for the corresponding SMT/CD complex were calculated from the slope of the phase-solubility diagrams (PSD) and intrinsic solubility (S0) according to Eq. (1):
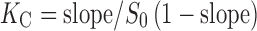 |
1 |
The quantitative determinations of SMT were performed spectrophotometrically (Shimadzu UV-Mini 1240 spectrophotometer) at 262 nm. The stability of the drug was determined in water at 25°C with no drug degradation being found after 72 h of incubation.
NMR Studies
All experiments were performed on a Bruker Avance II High Resolution Spectrometer equipped with a broad band inverse probe and a variable temperature unit (VTU). The spectra were measured at 298 K. The complex was prepared in D2O and the concentration was 1 mM.
1H NMR
These spectra were obtained at 400.16 MHz, with the chemical shift of the residual solvent at 4.8 ppm used as an internal reference. Induced changes in the 1H chemical shifts for SMT, βCD, and MβCD (Δδ) originated due to their complexation were calculated using Eq. 2:
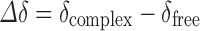 |
2 |
2D ROESY—the geometry of the inclusion complex was studied by two-dimensional rotating frame Overhauser experiments (2D ROESY). The pulse sequence was: roesygpph19; 2D ROESY with cw spinlock for mixing; phase sensitive and water suppression using a 3-9-19 pulse sequence with gradients; p15 (f1 channel), pulse for ROESY spinlock (200,000 μs); d1/2 s, d19 (delay for binomial water suppression), d19 = (1/(2*d)), d = distance of next null (in hertz) use gradient ratio: gp 1/gp 2 (30:30); for z-only gradients, gpz1, 30% and gpz2, 30% use gradient files; gpnam1, SINE.100 and gpnam2, SINE.100. Before Fourier transformation, the matrix was zero filled to 4,096 (F2) by 2,048 (F1), and Gaussian apodization functions were applied in both dimensions.
Preparation of Complexes in Solid State
Solutions of SMT with βCD or MβCD (1:1 molar ratio) were prepared in water. The resulting solutions were placed in an ultrasonic bath for 1 h, before being incubated at 25.0°C (±0.1) in a thermostatic water bath (Circulators HAAKE F3-K, Germany) for 48 h. Then, the solutions were frozen at −40°C and freeze drying was started (Freeze Dri 4.5 Labconco Corp., Kansas City, MI).
Fourier Transform Infrared Spectroscopy (FT-IR)
The Fourier transform infrared spectroscopy (FT-IR) spectra of SMT and its complexes with βCD and MβCD were measured as potassium bromide disks on a Nicolet 5 SXC FT-IR Spectrometer. The FT-IR spectra of complexes were compared with those of the corresponding 1:1 molar ratio physical mixture and also with pure SMT, βCD, and MβCD. All spectra were obtained and processed using EZ OMNIC E.S.P v.5.1 software.
Scanning Electron Microscopy Studies
Microscopic morphological structures of the raw materials and the binary systems were investigated and photographed using a scanning electron microscope LEO Model EVO 40XVP. The samples were fixed on a brass stub using double-sided aluminium tape and then made electrically conductive by employing gold coating in a vacuum by a sputter coater PELCO Model 3. The magnification selected was sufficient for appreciating in detail the general morphology of the samples under study.
Differential Scanning Calorimetry and Thermogravimetric Analysis
Differential scanning calorimetry (DSC) measurements of the pure materials and binary systems were carried out using a DSC TA 2920 (TA Instruments, Inc., New Castle, DE). The thermal behavior was studied by heating 1–3 mg of samples in a sealed aluminum pan, from 25°C to 350°C at a rate of 10°C/min, under nitrogen gas flow, using a sealed empty pan as the reference. Indium (99.98%, mp 156.65°C; Aldrich, Milwaukee, WI) was used as standard for calibrating the temperature.
Thermogravimetric analysis (TGA) curves of the different samples were recorded on a TGA 2950 (TA Instruments, Inc.), using the same conditions as in the DSC studies. The TG temperature axes were calibrated with the Curie point of Ni (353°C). In both cases, data were obtained and processed using TA Instruments Universal Analysis 2000 software.
RESULTS AND DISCUSSION
Phase Solubility Studies
As discussed above, SMT is an ampholyte that can exist in the protonated, neutral, or anionic forms depending on the pH of the aqueous solutions. Consequently, in order to verify the pH effect on SMT interaction with the CD cavity, the PSD were determined in water and in phosphate buffer solutions (pH 2.0 and 8.0). For all pH values, the increase in solubility occurred as a linear function of CD concentration, corresponding to the AL-type profile defined by Higuchi & Connors (21) where slope values less than unity indicate the occurrence of soluble complexes of 1:1 mol/mol stoichiometries. The corresponding KC, S0, maximum solubility (Smax) and efficiency of solubility (Smax/S0) values were calculated from each PSD and are presented in Table I.
Table I.
Data from the Phase-Solubility Curves of SMT with βCD and MβCD at Different pH Values
| Solvent | CD | K C (M−1) | Final pH | S 0 (mg/ml) | S max (mg/ml) | ES |
|---|---|---|---|---|---|---|
| Watera | βCD | 101 ± 4 | 6.6 | 0.42 ± 0.02 | 0.93 ± 0.03 | 2 |
| Buf. pH 2 | 40.4 ± 0.4 | 2.1 | 1.1 ± 0.2 | 1.63 ± 0.02b | 1.5 | |
| Buf. pH 8 | 29.4 ± 0.4 | 8.4 | 1.93 ± 0.06 | 2.6 ± 0.1b | 1.3 | |
| Water | MβCD | 56 ± 1 | 6.4 | 0.42 ± 0.02 | 7.7 ± 0.1c | 18 |
| Buf. pH 2 | 39 ± 3 | 2.2 | 1.1 ± 0.2 | 4.4 ± 0.1d | 4 | |
| Buf. pH 8 | 39 ± 5 | 8.4 | 1.93 ± 0.06 | 3.5 ± 0.5d | 2 |
aZoppi et al. (15)
bSMT solubility in βCD (13.2 mM) solutions
cSMT solubility in MβCD (105 mM) solutions
dSMT solubility in MβCD (85 mM) solutions
In water, the affinity constant of the MβCD complex was lower than that of βCD. This lower affinity may be due to the absence of a hydrogen bond interaction between the guest molecule and the substituted OH moiety in the MβCD, or alternatively to a steric hindrance effected by the methyl groups in the substituted CD which in turn impedes the inclusion of the guest molecule. Although SMT/MβCD systems presented low KC values compared with those containing βCD (except at pH 8.0), the overall increase in the solubility of the drug was higher because it was possible to add larger amounts of MβCD due to its higher aqueous solubility compared with βCD. On analyzing the effects of pH, it can be observed that the values of KC for the complexes with both CDs were lower when the drug was ionized, which is not favorable for complex formation. Finally, in the case of the complexes with βCD, the Smax was higher in aqueous buffered solutions, because of the combined effect of the complex formation and the existence of the drug as an ionized species. However, for the complexes with MβCD, the higher value of Smax was obtained in water because it was possible to add larger amounts of MβCD than in buffer solution.
NMR Studies
1H NMR Experiments
Figure 2 shows the SMT and MβCD spectra obtained before and after complexation, from which the corresponding chemical shifts (δ) and chemical shift displacements (Δδ) were determined (Tables II and III). The protons lying in the interior of the MβCD hydrophobic cavity (H3 and H5) exhibited displacements in their δ values in the presence of the guest molecule, suggesting the formation of an inclusion complex. Both protons showed a marked shielding effect, indicative of the inclusion of an electron rich moiety. In addition, all drug protons suffered marked displacements, with the protons of the methyl group showing broader peaks in the spectrum of the complex than in the free drug (Fig. 2). This behavior was probably associated with a decrease in the mobility of a moiety of this drug, caused by the complex formation with MβCD.
Fig. 2.
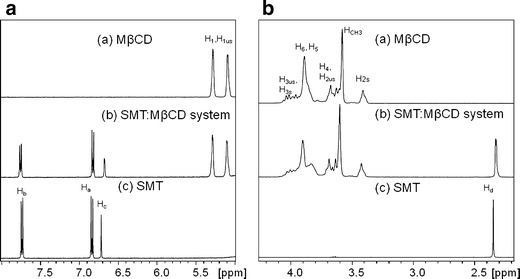
1H NMR Spectra of a MβCD, b SMT/MβCD system, and c SMT. a 2.0–4.5 and b 5.0–8.0 ppm
Table II.
Chemical Shifts for the Protons of SMT in the Free and Complex Forms
| Proton | SMT (ppm) | SMT/MβCD (ppm) | Δδ (ppm) |
|---|---|---|---|
| Ha | 6.8380 | 6.8263 | −0.0117 |
| Hb | 7.7374 | 7.7575 | 0.0201 |
| Hc | 6.7181 | 6.6789 | −0.0392 |
| Hd | 2.3474 | 2.3272 | −0.0202 |
Table III.
Chemical Shifts for the Protons of MβCD in the Free and Complex Forms
| Proton | SMT (ppm) | SMT/MβCD (ppm) | Δδ (ppm) |
|---|---|---|---|
| H 1s | 5.2815 | 5.2874 | 0.0059 |
| H 1us | 5.0880 | 5.1049 | 0.0169 |
| H 3us | 4.0369 | 4.0054 | −0.0315 |
| H 3s | 3.9591 | 4.0054 | 0.0463 |
| H 4–H 2us | Overlapped | ||
| H 6 | 3.8898 | 3.9026 | 0.0128 |
| H 5 | 3.7849 | 3.8325 | 0.0476 |
| H 4 | 3.6280 | 3.6593 | 0.0313 |
| H CH3 | 3.5806 | 3.6002 | 0.0196 |
| H 2s | 3.4111 | 3.4239 | 0.0128 |
2D ROESY Experiments
This experiment was carried out to further investigate the inclusion of SMT into the MβCD cavity and to determine the mode of interaction. Figure 3 shows partial contour plots of 2D ROESY spectra for the SMT/MβCD complex. Intermolecular cross-peaks can be seen between Ha and Hb protons of guest molecules and H5 and H6 protons of MβCD, again revealing the formation of an inclusion complex and suggesting that the ring A of SMT was inserted into the MβCD hydrophobic cavity. These studies show that the inclusion mode of the complex with MβCD was equivalent to the complex obtained with βCD, as was previously reported (15).
Fig. 3.
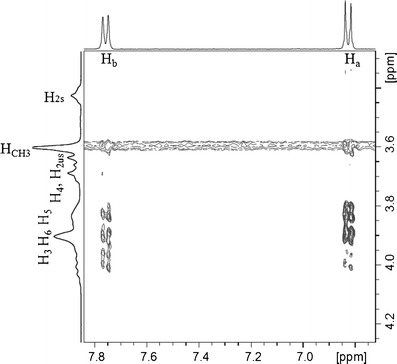
Partial contour plot of the 2D ROESY spectrum of the SMT/MβCD system
Fourier Transform Infrared Spectroscopy
It has been vastly reported that the variation of the shape, shift, and intensity of the FT-IR absorption bands of the guest or host molecule can provide enough information for the occurrence of complexation (22). The infrared spectra of the binary systems taken in the region of 4,000–400 cm−1 were compared with that of the free drug (Figs. 4 and 5). In the SMT spectrum, the bands that appear between 3,500 and 3,400 cm−1 are due to asym (NH2) and sym (NH2) vibrations of the amino (NH2) group of the drug. The band for the sulfonamidic (N–H) group is observed around 3,125 cm−1.On the other hand, the spectral region from 4,000 to 3,000 cm−1 is difficult to analyze for βCD and its complexes due to primary and secondary −OH groups of βCD and water molecules of crystallization (23). For this reason only the SMT bands between 1,640 and 900 cm−1 were used to depict the inclusion complex disposition. Spectra of all binary systems did not show new peaks indicating that no chemical bonds were created in the formed compounds. The spectra for the physical mixtures (Figs. 4c and 5c) consisted of an overlay of the pure compounds spectra. On the other hand, the FT-IR spectra of the complexes prepared by the freeze-drying method (Figs. 4d and 5d) showed appreciable shifts and reduction in intensity of the characteristic SMT bands, evidencing the presence of more or less intense solid-state interactions between the components. The scissoring vibrations for the amino (−NH2) groups that appear at 1,640 cm−1 in the free drug spectra was shifted to a lower frequency (1,630 cm−1) in the complexes prepared by freeze-drying method. Furthermore, a reduction of the intensities of the bands at 1,330 cm−1 (assigned to SO2 asymmetric stretching) and at 967 cm−1 (assigned to S–N stretching) was observed for these systems, probably owing to a restriction of the vibration related to the complexation process. These changes in the characteristic bands of pure drug confirm the existence of the inclusion complexes as new compounds with different spectroscopic bands. It should be noted that these results are in agreement with the inclusion mode previously reported in the 2D ROESY experiments.
Fig. 4.
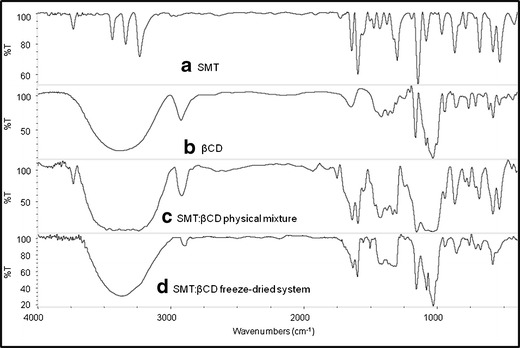
IR spectra of a SMT, b βCD, c SMT/βCD physical mixture system, and d SMT/βCD freeze-drying system
Fig. 5.
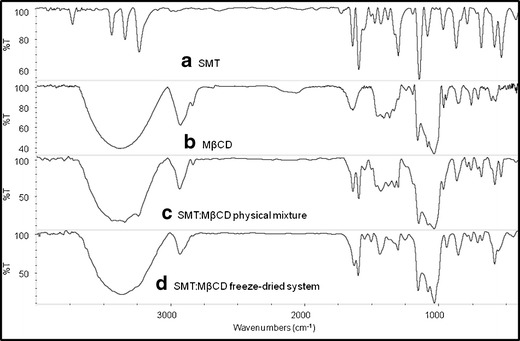
IR spectra of a SMT, b MβCD, c SMT/MβCD physical mixture system, and d SMT/MβCD freeze-drying system
Scanning Electron Microscopy Studies
Scanning electron microscopy (SEM) is used to study the microscopic aspects of the raw materials and their corresponding treated samples. Although this study is inadequate to affirm inclusion complexation, it helps to assess the existence of a single component in the preparations obtained (24).
Figure 6 shows the SEM images of SMT, βCD, MβCD alone, and the binary systems prepared by physical mixture and freeze-drying methods. It can be seen that SMT is present as crystals of irregular shapes and sizes, while βCD exhibits a parallelogram shape and MβCD is composed of spherical particles with amorphous character.
Fig. 6.
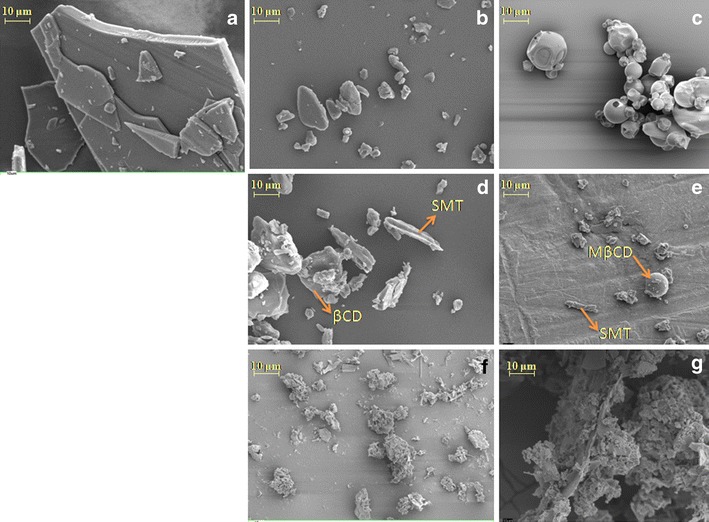
Scanning electron microphotographs of a SMT, b βCD, c MβCD, d SMT/βCD physical mixture system, e SMT/MβCD physical mixture system, f SMT/βCD freeze-drying system, and g SMT/MβCD freeze-drying system
SEM images of the physical mixtures revealed the characteristic SMT crystals, which were mixed with CDs particles, thus confirming the presence of crystalline drug, where residual CDs particles were easily identified.
Finally the morphology of the particles of SMT/βCD and SMT/MβCD system prepared by freeze-drying presents significant changes in their shape and aspect, which is indicative of the presence of a new solid phase. These finding may be simply a consequence of a crystalline habitus change in the systems or may support the evidence of the presence of a new solid complex.
Differential Scanning Calorimetry and Thermogravimetric Analysis
Several thermoanalytical techniques are often used for studying complexation, being TGA and DSC as the most commonly used techniques (13). The DSC and TG curves of pure components and binary systems with βCD and MβCD are shown in Figs. 7 and 8, respectively. The thermal curve of SMT was typical of a crystalline anhydrous substance, with a sharp fusion endothermic event occurring at 198°C corresponding to the melting point of the drug, followed by an exothermal event attributable to its thermal decomposition, as can be observed by comparison with the TG curve (Δm = 52%). Both βCD and MβCD lost water at temperatures between 25°C and 100°C (Δm = 11% and 7%, respectively), with decomposition taking place above 300°C as evidenced by the mass loss observed in the TG curves (Δm = 77% and 83%, respectively).
Fig. 7.

DSC and TG curves of βCD (green), SMT (red), SMT/βCD physical mixture system (SMT/βCD PM; pink), and SMT/βCD freeze-drying system (SMT/βCD FD; blue)
Fig. 8.
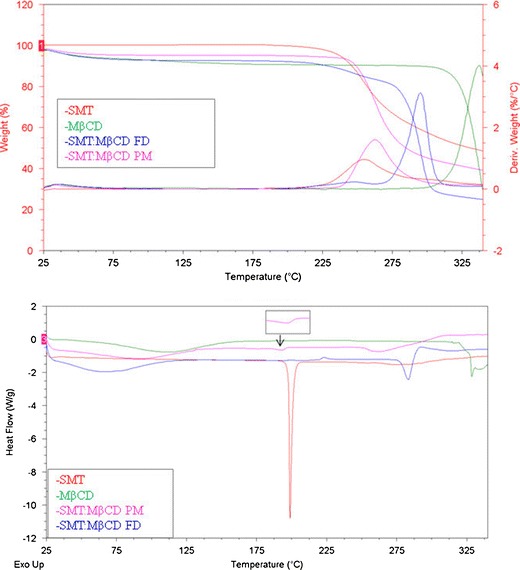
DSC and TG curves of MβCD (green), SMT (red), SMT/MβCD physical mixture system (SMT/MβCD PM; pink) SMT/MβCD freeze-drying system (SMT/MβCD FD; blue)
The curves of the physical mixtures of SMT/βCD and SMT/MβCD presents two events, the first of which corresponds to the dehydration band of CD (Δm = 9% and 3%, respectively), and the second one corresponding to the melting point of SMT, which is reduced and to some extent displaced in the case of MβCD system at (198°C and 193°C, respectively). The peak reduction may be explained by the small SMT content, but its displacement is supposed to be caused by an interaction with the CD during the heating phase. Finally, decomposition takes place above 200°C (Δm = 42% and 56%, respectively).
Regarding the results obtained for the systems prepared by freeze-drying method, the DSC curve shows that the endothermic peak of pure SMT at 198°C is not present, indicating that the melting event did not take place, which might be due to changes in the crystalline form of the solid or to the formation of an inclusion complex. Furthermore, the thermogravimetric profile shows an initial loss of weight corresponding to the dehydration of the complexes (Δm = 4% and 5%, respectively), followed by a decomposition phase that starts above 200°C (Δm = 66% and 67%, respectively).
Taking into account the results obtained by the different techniques in solid state, it is possible to confirm the formation of inclusion complexes between SMT and both CDs when the systems were prepared by applying a freeze-drying method. The amorphous nature of both samples was also confirmed by means of the previously discussed techniques.
CONCLUSIONS
The freeze-drying method employed in this work to prepare the system in solid state resulted in real inclusion of the drug in the CD cavity proved by IR and DSC studies. Simultaneous analysis of complexation behavior using different methods obviously has a lot of advantages. Indeed, analogies found among the SMT/βCD and SMT/MβCD interactions in aqueous solution and in the solid state suggest similar patterns of complexation mechanisms and inclusion modes of the guest molecule in the host cavity. The solubility of SMT was improved by inclusion complexation with CDs. This satisfactory behavior of the SMT/CD complex is potentially useful for its future application in pharmaceutical dosage formulations. Previous studies in our group (14,15) have demonstrated that CDs can form complexes with SDZ and SMR, which are frequently administered as a “triple sulfa drug” with SMT. Therefore, this might have relevant applications in the preparation of an oral dosage product containing complex forms of these three drugs, which could also lead to better bioavailability.
ACKNOWLEDGMENTS
The authors thank the Fondo para la Investigación Científica y Tecnológica (FONCYT) Préstamo BID PICT 1376, the Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba (SECyT), and the Consejo Nacional de Investigaciones Científicas y Tecnológicas de la Nación (CONICET) for financial support. We also thank Ferromet S.A. (agent of Roquette in Argentina) for its donation of βCD and MβCD. We are grateful to Dr. Gloria Bonetto for NMR measurements and for her helpful discussion of the 1H NMR spectra, and to Dr. Paul Hobson, native English speaker, for revision of the manuscript.
REFERENCES
- 1.Mayersohn M. Principles of drug absorption. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. New York: Marcel Dekker; 2002. pp. 23–66. [Google Scholar]
- 2.Budavari S, O’Neil MJ, Smith A, Heckelman PE. The Merck Index. 13. Whitehouse Station: Merck and Co; 2001. [Google Scholar]
- 3.Papastephanou C, Frantz M. Sulfamethazine. In: Florey K, editor. Analytical profiles of drug substances. New York: Academic; 1982. pp. 401–422. [Google Scholar]
- 4.Reguera C, Ortiz MC, Herrero A, Sarabia LA. Optimization of a FIA system with amperometric detection by means of a desirability function: Determination of sulfadiazine, sulfamethazine and sulfamerazine in milk. Talanta. 2008;75:274–283. doi: 10.1016/j.talanta.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Zhang Y, Wang S. Development of flow-through and dip-stick immunoassays for screening of sulfonamide residues. J ImmunOL Methods. 2008;337:1–6. doi: 10.1016/j.jim.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Tommasino J-B, Renaud FNR, Luneau D, Pilet G. Multi-biofunctional complexes combining antiseptic copper(II) with antibiotic sulfonamide ligands: Structural, redox and antibacterial study. Polyhedron. 2011;30:1663–1670. doi: 10.1016/j.poly.2011.03.033. [DOI] [Google Scholar]
- 7.Hossain GMG, Amoroso AJ, Banu A, Malik KMA. Syntheses and characterisation of mercury complexes of sulfadiazine, sulfamerazine and sulfamethazine. Polyhedron. 2007;26:967–974. doi: 10.1016/j.poly.2006.09.056. [DOI] [Google Scholar]
- 8.Gamba V, Terzano C, Fioroni L, Moretti S, Dusi G, Galarini R. Development and validation of a confirmatory method for the determination of sulphonamides in milk by liquid chromatography with diode array detection. Anal Chim Acta. 2009;637:18–23. doi: 10.1016/j.aca.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Ramos Payán M, López MÁB, Fernández-Torres R, Navarro MV, Mochón MC. Hollow fiber-based liquid phase microextraction (HF-LPME) for a highly sensitive HPLC determination of sulfonamides and their main metabolites. J Chromatogr B. 2011;879:197–204. doi: 10.1016/j.jchromb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62:1607–1621. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 12.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 13.Fromming KH, Szejtli J. Cyclodextrins in pharmacy. Dordrecht: Kluwer Academic; 1994. [Google Scholar]
- 14.Delrivo A, Zoppi A, Longhi MR. Interaction of sulfadiazine with cyclodextrins in aqueous solution and solid state. Carbohydr Polym. 2012;87:1980–1988. doi: 10.1016/j.carbpol.2011.10.025. [DOI] [Google Scholar]
- 15.Zoppi A, Quevedo MA, Delrivo A, Longhi MR. Complexation of sulfonamides with beta-cyclodextrin studied by experimental and theoretical methods. J Pharm Sci. 2010;99:3166–3176. doi: 10.1002/jps.22062. [DOI] [PubMed] [Google Scholar]
- 16.Granero GE, Garnero C, Longhi MR. The effect of pH and triethanolamine on sulfisoxazole complexation with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci. 2003;20:285–293. doi: 10.1016/S0928-0987(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 17.Garnero C, Aiassa V, Longhi M. Sulfamethoxazole:hydroxypropyl-β-cyclodextrin complex: preparation and characterization. J Pharm Biomed Anal. 2012;63:74–79. doi: 10.1016/j.jpba.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Granero GE, Maitre MM, Garnero C, Longhi MR. Synthesis, characterization and in vitro release studies of a new acetazolamide-HP-β-CD-TEA inclusion complex. Eur J Med Chem. 2008;43:464–470. doi: 10.1016/j.ejmech.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Palma SD, Tartara LI, Quinteros D, Allemandi DA, Longhi MR, Granero GE. An efficient ternary complex of acetazolamide with HP-ß-CD and TEA for topical ocular administration. J Control Release. 2009;138:24–31. doi: 10.1016/j.jconrel.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Sourbaji M, Pintye-Hódi K, Novák CS, Szabó-Révész P, Kása P, Jr, Erõs I. A study of sulfadimidine-β-cyclodextrin mixtures. J Incl Phenom. 2000;37:299–307. doi: 10.1023/A:1008110928425. [DOI] [Google Scholar]
- 21.Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 22.Ge X, Huang Z, Tian S, Huang Y, Zeng C. Complexation of carbendazim with hydroxypropyl-β-cyclodextrin to improve solubility and fungicidal activity. Carbohydr Polym. 2012;89:208–212. doi: 10.1016/j.carbpol.2012.02.072. [DOI] [PubMed] [Google Scholar]
- 23.Salustio PJ, Feio G, Figueirinhas JL, Pinto JF, Cabral Marques HM. The influence of the preparation methods on the inclusion of model drugs in a beta-cyclodextrin cavity. Eur J Pharm Biopharm. 2009;71:377–386. doi: 10.1016/j.ejpb.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Naidu NB, Chowdary KP, Murthy KV, Satyanarayana V, Hayman AR, Becket G. Physicochemical characterization and dissolution properties of meloxicam-cyclodextrin binary systems. J Pharm Biomed Anal. 2004;35:75–86. doi: 10.1016/j.jpba.2004.01.003. [DOI] [PubMed] [Google Scholar]


