Abstract
HIV-infected individuals constitute a population highly susceptible to opportunistic infections, particularly oral candidiasis caused by the most pathogenic human fungal species Candida albicans. Host-produced salivary antimicrobial peptides are considered to be an important part of the host innate immune system involved in protection of the oral cavity against colonization and infection by microbial species. Histatin-5 (Hst-5) specifically has exhibited potent anti-candidal properties in vitro. However, its importance in protecting the oral mucosa against candidal colonization and importantly, its contribution to the observed enhanced susceptibility of HIV-infected individuals to candidiasis has not been previously investigated. To that end, a novel immunoassay was used to demonstrate significant decrease in salivary Hst-5 levels in HIV+ individuals concomitant with enhanced candidal prevalence. Further, saliva’s anti-candidal potency was found to be proportional to Hst-5 concentration and significantly compromised in HIV+ subjects compared to controls. The key role for Hst-5 was further confirmed upon exposure to the Hst-5-specific antibody where saliva’s initial killing activity was substantially compromised. Combined, these findings identify Hst-5 as a key anti-candidal salivary component and demonstrate its decreased levels in HIV infection providing new insights into oral Innate immune defense mechanisms and the enhanced susceptibility of HIV+ individuals to oral candidiasis.
Keywords: HIV/AIDS, Opportunistic infections, Oral candidiasis, Innate immunity, Salivary antimicrobial peptide, Histatin-5, C. albicans
Introduction
In Human Immunodeficiency Virus-infected (HIV+) individuals, oral candidiasis (OC) is considered an AIDS-defining opportunistic infection with up to 80% of these individuals suffering recurrent episodes during the course of their HIV disease progression, despite the introduction of HIV therapy [1–3]. Candida albicans, the etiologic agent is a commensal fungus commonly colonizing human mucosal surfaces [4]. However, under conditions of immune dysfunction, colonizing C. albicans can become an opportunistic pathogen causing recurrent mucosal and life-threatening disseminated infections with high mortality rates [1,5]. Although it is generally considered that susceptibility to OC is enhanced under reduced CD4+ T-cells, the status and efficiency of local host defenses appear to play a key role and hence the prevalence of OC may also depend on the status of secondary defenses [1]. Therefore, identifying the mechanisms and co-factors behind the enhanced susceptibility to OC in HIV+ individuals, would serve as a major breakthrough in our understanding of the pathogenesis of HIV infection and OC.
In the oral cavity, the oral mucosa serves as an important barrier to the myriad of microbial species present in this complex environment. However, saliva, a complex mix of fluids from salivary glands plays an important role in maintaining the balance between health and disease in the oral cavity [6–8]. Specifically, saliva contains a set of antimicrobial peptides (AMPs) produced by the host which are considered to be an important part of the innate immune system [9,10]. Surprisingly, the important role of these innate antimicrobials in the protection of the oral cavity from the constant exposure to microbial challenges, and particularly their potential as therapeutic agents is only just beginning to be appreciated [11,12]. Most notable among the AMPs are the histatins, a family consisting of small molecular-weight histidine-rich cationic proteins. The major proteins in the family are histatins-1, 3 and 5 with histatin-1 (Hst-1) and histatin-3 (Hst-3) encoded by two genes, HTN1 and HTN3, respectively and histatin-5 (Hst-5) being the proteolytic product of Hst-3 [13]. Histatins are secreted by human parotid and submandibular-sublingual glands and reported to be present in saliva in healthy adults at estimated concentrations of 14–47 μg/ml [13–16]. Histatins show killing activities against numerous oral bacteria however, Hst-5 is the most active candidacidal member of the family in killing the yeast and hyphal forms of Candida [16–18]. In contrast to the classical pore-forming peptides, Hst-5-induced killing is proposed to involve several sequential steps beginning with binding and translocation into the cytoplasm in a non-lytic manner where it targets the mitochondria leading to membrane damage and cell death [19–24].
In C. albicans the transition from harmless commensal to pathogen is finely balanced and attributable to a repertoire of virulence determinants and its aptitude to adapt and evade host immune defenses [25]. This capability was validated by our recent findings demonstrating C. albicans ability to degrade and deactivate Hst-5 via a secreted proteolytic enzyme, identifying a novel immune evasion strategy for C. albicans that may be involved in its transition from commensal to pathogen [26]. Importantly, the ability of C. albicans to degrade Hst-5 was found to be proportional to its cell density and inversely proportional to Hst-5 concentration affirming that maintenance of oral health is highly dependent on the fine balance between pathogen and host innate immune factors.
Given the important role of saliva in maintaining oral health, it is conceivable therefore, that alterations in salivary gland secretion and/or composition contribute to the markedly enhanced predisposition of HIV+ individuals to OC. Yet despite the important clinical implications, studies confirming these observations have been lacking, most likely due to the lack of feasible and sensitive methods for measuring salivary Hst-5 concentrations. To that end the purpose of this study was to accurately measure and analyze salivary Hst-5 levels within the context of HIV infection with the overall goal of providing new insights into oral innate defense mechanisms that play a role in the enhanced susceptibility of HIV+ individuals to OC.
Materials and Methods
Patient population and clinical samples
Thirty two adult patients diagnosed as HIV+ that were being evaluated for oral care at the University of Maryland HIV PLUS Program and a control group of 32 healthy volunteers were included in the study. No patient identifiers were used and IRB approval and informed consent were obtained. Subjects ranged in age between 22–84 years with 93.75% males and 6.25% females in the HIV+ group and 50% males and 50% females in the control group. Complete medical history, including CD4 counts were obtained for each HIV+ patient. Oral evaluation and sample collection were performed by an oral medical professional. All samples were collected at the University of Maryland Dental School where analysis was performed. Samples were collected between 10 am–12 pm to minimize circadian effect, using the Salivette collection systems according to manufacturer directions (Sarstedt, Newton, NC). A standardized method of 2-minute whole saliva sample collection was used to measure salivary flow rate (SFR) where subjects were asked to chew on a cotton tampon provided with the Salivette collection system for 2 minutes to stimulate saliva production. The saliva absorbed was recovered by centrifugation (10,000 xg for 10 min at 4°C) in the tubes provided. The volume of each saliva sample was measured and SFR was calculated as ml/minute (ml/min) [27].
Samples were immediately cultured on yeast peptone dextrose (YPD) agar (SDA; Difco Laboratories, Detroit, MI) and plates were incubated at 35°C for 24–48h. Assessment of fungal presence was based on colony forming unit counts (CFUs) and speciation was performed using standard mycological procedures including colony color on yeast chromogenic media, CHROMagar (BBL, Sparks, MD). Patients were classified as C. albicans negative, or colonized based on CFUs (cells/ml) on primary culture with “heavily colonized” if CFU counts >1×102 cell/ml saliva and minimally colonized” if CFUs < 1×102 cell/ml. This number was taken as an arbitrary cut-off point based on previous statistical analysis (P<0.0001) [28]. For ELISA, following clarification, recovered samples were analyzed or aliquoted and immediately frozen at −86°C until analysis. For testing of anti-candidal activity, clarified saliva was filter-sterilized to remove interfering commensal strains.
Peptide and antibody production
A high purity Hst-5 peptide was synthesized by the University of Maryland Protein Core based on the Hst-5 amino acid sequence (DSHAKRHHGYKRKFHEKHHSHRGY). A rabbit polyclonal antibody specific for Hst-5 was designed and produced (Lampire Biological Laboratories, Inc. Pipersville, PA). Prior to immunization, the peptide was covalently linked to the carrier protein KLH (keyhole limpet hemocyanin) using glutaraldehyde conjugation. Two rabbits were immunized on a schedule designed to maximize immune response. During immunization, test bleeds were taken at 30 and 50 days post-immunization and ELISA performed to evaluate quality of immune response and optimum time to administer a pre-production boost and collect the antiserum. A polyclonal response was confirmed demonstrating direct binding of the antibody to multiple epitopes on Hst-5. Animals were sacrificed and the polyclonal antiserum IgG fraction specific for the peptide immunogen was separated from the total fraction and purified by affinity chromatography using a protein-G affinity column. ELISA titers for the anti-peptide specific IgGs were calculated using a saturating amount of peptide (1 μg/well) in a 96-well microplate format.
Development and evaluation of ELISA
The purified Hst-5 peptide was reconstituted in PBS and a standard curve was performed with each assay using Hst-5 at concentrations ranging from 0.5–500 μg/ml. Briefly the wells of high-binding 96-well microtiter plates (Corning incorporated, Corning, NY) were coated with 100 μl of each Hst-5 concentration or 1/100 dilution of saliva. Following overnight incubation at 4°C, wells were blocked with 5% dry milk in PBS and plates were incubated for 1h at room temperature. Anti-Hst-5 antibody (1/1000) (100 μl) was added to each well and plates were incubated for 1h at 37°C. Following washing steps with PBS with 0.1% Tween, 100 μl of an HRP-labeled goat anti-rabbit secondary antibody (1/5000) (Lampire) was added to each well and plates were incubated for 1h at 37°C. Wells were washed with PBS and 100 μl of ABTS Peroxidase Substrate (KPL, Inc., Gaithersburg, Md.) was added and plates incubated for 20 min at room temperature in the dark. The reaction was stopped by the addition of 50 μl of Stop Solution (Thermo Scientific, Waltham, MA) and optical density (OD) was measured at 405nm using a microtiter plate reader (Dynateck Laboratories, UK). A standard curve was plotted with each run and the average Hst-5 concentration for each sample was calculated in μg/ml saliva.
For each plate, a linear standard curve was established using Hst-5 standards. The coefficient of variation is the ratio of standard deviation to the mean. The value is expressed as a percentage of variance to the mean and therefore indicates any inconsistencies and inaccuracies in the results (the larger the variance, the more inconsistency and error). The lowest Hst-5 concentration could be measured with a coefficient of variation below 15%. The functional sensitivity was determined from the between-lot assay variation of saliva samples (n=7). The intra- and inter-assay variations for saliva samples were determined by repeated measurements of same samples. In order to maximize signal-to-noise ratio, a spike recovery assay was performed to determine the effect of the constituents of the sample on the results. A known amount of Hst-5 was added to the saliva sample (spiked sample) in 1:1 ratio and Hst-5 concentration was compared to that of unspiked sample. The percent recovery was calculated according to the following equation:
To assess the precision of assay results for samples tested at different dilutions in each saliva sample, a linearity-of-dilution assay was performed where three saliva samples were serially diluted. In order to cover the whole range, five different dilutions were made of each sample and Hst-5 levels were measured along with neat (undiluted) samples. Measured Hst-5 levels were corrected for dilution factors and reported as observed Hst-5 level. Assay recovery was evaluated by comparing observed vs. expected values based on neat samples. The antibody was raised to be specific to Hst-5 by only recognizing the modified sequence. In order to confirm the specificity, the antibody was tested in ELISA for cross-reactivity with Hst-1 and Hst-3. In addition, Western analysis was also performed to test the reactivity of the antibody with salivary proteins.
Hst-5 concentrations in HIV+ and control subjects
Purified saliva samples recovered from all subjects were processed for Hst-5 levels by ELISA as described above. Hst-5 concentrations were analyzed among subjects in terms of HIV status, CD4 counts, subjects’ demographics, fungal colonization and other variables.
Salivary candidacidal and neutralization assays
In order to assess the anti-candidal potency of saliva with respect to Hst-5 concentration, saliva from subjects from each HIV+ and control groups were pooled and Hst-5 concentration of the pooled samples was determined by ELISA measurement. For candidacidal assays, the standard C. albicans SC5314 strain was used [29]. Cultures were grown in YPD broth (Difco Laboratories) overnight at 30°C and cells were equilibrated in fresh media to an optical density of 1.0 at OD600. Cells were washed with sterile PBS (1mM) and resuspended in PBS. Saliva from six different subjects from each group with Hst-5 concentrations representing each of the categories (H, M, L) were selected and pooled for testing for anti-candidal potency. The arbitrary ranges of Hst-5 concentrations in the 3 categories were designated (based on preliminary observations) as: high (H) > 9 μg/ml; medium (M) 5–8 μg/ml and low (L) < 5 μg/ml. Since no concentrations higher than 5 μg/ml were measured in the HIV+ group, only two pooled saliva samples were tested from that group representing the (M) and (L) categories. Hst-5 purified peptide reconstituted in PBS was used as a positive control. A dose-dependent killing curve for Hst-5 was performed in order to determine the optimal concentration to be used in the killing assay. The final concentration of 5 μg/ml was chosen as this concentration consistently resulted in significant killing (~ 90%). For the candidacidal assay, C. albicans cells were used at final cell density of 1×104 cells/ml in the wells of a 96-well microtiter plate with Hst-5 to a final volume of 100 μl per well. Alternatively, C. albicans cells were added to each of the saliva samples (no Hst-5). Negative controls with cells and PBS alone were included and plates were incubated for 1 h at 37°C with shaking. Aliquots from reactions were diluted and plated on YPD agar and incubated for 24–48 h at 35°C. The number of single colonies on each plate was counted and percent cell killing was calculated based on drop in CFU counts compared to the control (PBS).
In order to demonstrate that Hst-5 is the key salivary component responsible for saliva’s observed anti-candidal activity, 100 μl of the pooled saliva from the healthy group with the highest Hst-5 concentration was subjected to ELISA measurement to determine the final Hst-5 concentration. The saliva sample was then tested in the killing assay as described above in the presence of the anti-Hst-5 specific antibody (1/10). The C. albicans percent killing for the antibody-treated saliva was compared to that obtained for the sample not exposed to the antibody.
Statistical analysis
All statistical analyses were performed using the statistical package SigmaStat version 8.0 (Amonk, NY). Chi-square test was used for comparison of categorical variables and t test for the measured variables in the comparisons between groups. Average mean Hst-5 concentration, % coefficient of variance (CV) and standard deviation for each assay was calculated in the SigmaStat excel file. For the comparisons within groups either Mann-Whitney or Kruskal-Walis tests were used if there were two or more groups respectively. For all analyses, significant levels were considered at p value of <0.05.
Results
Validation of the Hst-5 antibody and ELISA
Results from the evaluation of the analytical performance of ELISA and validation assays are presented in Supplemental Material (Tables S1–S4). The specificity of the antibody to Hst-5 and cross reactivity with Hst-1 and Hst-3 was tested by ELISA and reactivity with salivary proteins by Western analysis. Although with the raw antiserum containing a broader background not captured on the column some level of cross reactivity was seen with the other histatins, the generated purified sequence-specific antibodies were highly specific for Hst-5 with low level cross-reactivity seen with Hst-3 and Hst-1 (Figure S1). Similarly, Western analysis of saliva following electrophoretic separation demonstrated no reactivity with salivary proteins (data not shown).
Hst-5 concentrations in HIV+ and control subjects
Characteristics and results of sample analysis of the HIV+ and HIV− subjects are presented in Supplemental Material (Tables S5 and S6) respectively and analysis of characteristics in Table 1. In the HIV+ group only 4 subjects had Hst-5 concentrations >3 μg/ml (mean 2.45 μg/ml) whereas only 2 HIV− subjects had concentrations <3 μg/ml (mean 6.41 μg/ml) demonstrating 62% decrease in Hst-5 levels in HIV+ group. In contrast, 50% of HIV+ subjects had C. albicans recovered compared to only 15.6% in HIV− group (Table 2). Although low CD4 counts (<500) correlated with fungal presence, no correlation was seen with Hst-5 concentration. In general, no associations could be made between Hst-5 concentrations and gender although the HIV+ group included only two females. However, in the HIV− older subjects exhibited the lowest Hst-5 concentrations; based on distribution into concentration categories, the mean age (years) in the (H) category was 38.4; the mean for the (M) category was 39.5 and for the (L) the mean was 55.5. Of note, the 3 lowest Hst-5 concentrations were for subjects 72–84 years old, 2 of which had a high candidal prevalence and 3 of the highest 4 concentrations were for subjects 30–31 year old (Figure 1). In contrast, since all the subjects in the HIV+ group had very low and comparable concentrations, no associations could be made between Hst-5, age, medications or duration of HIV infection.
Table 1.
Analysis of study population characteristics.
| Parameters HIV+ (n=32), | Mean ± SD |
|---|---|
| HIV− (n=32) | |
| Age range (22–84 y) | 47.65 ± 13.2 |
| Gender (male) | HIV+ (93.75%) |
| HIV− (50%) | |
| CD4 cells/mm3 | 460.22 ± 260.63 |
| HIV therapy (%) | 78.13% |
| C. albicans colonized | HIV+ (50%) |
| HIV− (16%) | |
| CFUs (cells/ml) | HIV+ (16) 1.27×105 |
| ±3.3 x105 | |
| HIV− (5) 2.01×105 ± | |
| 4.46 x105 | |
| Hst-5 (μg/ml) | HIV+ 2.07 ± 0.92, HIV− |
| 6.47 ± 3.0 | |
| SFR (ml/min) | <0.02 HIV+ (50%), |
| >0.02 HIV+ (50%) | |
| >0.02 HIV− (100%) |
Table 2.
Average Hst-5 concentration in C. albicans-colonized and non-colonized subjects.
| Status | n | Average Hst-5 (μg/ml) | % decrease |
|---|---|---|---|
| HIV+ | 16 | 2.65 ± 1.04* | 62.46 |
| colonized HIV+ not | 16 | 2.26 ± 0.80** | 68 |
| colonized HIV− | 5 | 3.36 ± 0.85* | 52.4 |
| colonized HIV− non-colonized | 27 | 7.02 ± 2.90 | N/A |
Percent (%) decrease compared to the HIV− non-colonized group;
P<0.05;
P<0.01
Figure 1. Measured salivary Hst-5 concentrations in the HIV− and HIV+ subjects stratified based on age.
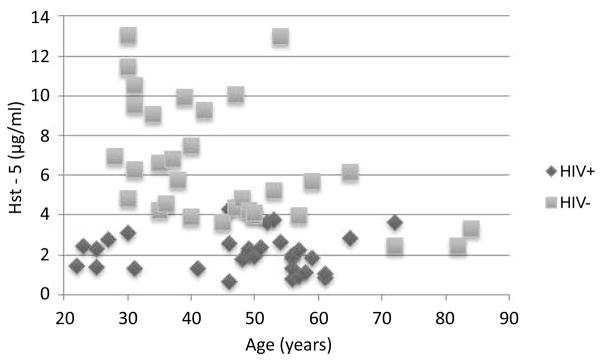
Scatter plot demonstrating clustering of HIV+ subjects below 5μg/ml Hst-5 concentration whereas majority of control subjects above 5μg/ml with older subjects exhibiting the lowest Hst-5.
Salivary flow rate (SFR) was assessed in both groups in accordance with established guidelines (low SFR < 0.2 ml/min) [30]. Based on these parameters, 50% of the HIV+ had low SFR compared to only 2 HIV− subjects (P<0.0001). Since Hst-5 concentrations were comparably low in all of the HIV+ subjects, no correlation could be made between SFR and Hst-5 concentration. Of note however, 11 of the 16 colonized HIV+ subjects had SFR in the low range.
Saliva candidacidal assays
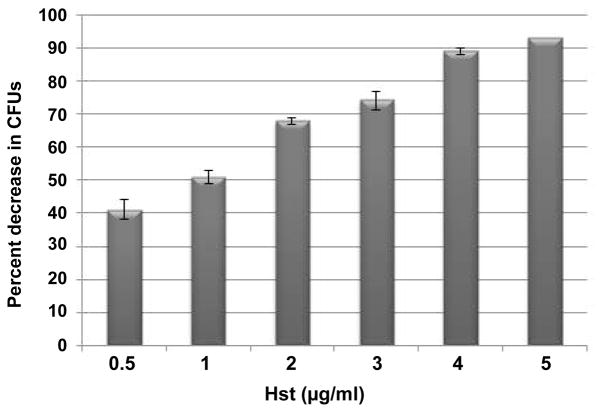
In these experiments, 6 saliva samples were pooled from each population group representing the different ranges of Hst-5 concentrations (H, M, L) (no Hst-5 concentrations for HIV+ subjects fell within H range). Results demonstrated that the candidacidal ability of saliva was proportional to Hst-5 concentration regardless of HIV status with 0% C. albicans killing exhibited by saliva with < 5 μg/ml indicating that a minimum of this concentration is required for candidacidal activity (Table 3). Consistent with values from Hst-5 killing curve, control Hst-5 (5 μg/ml) peptide resulted in over 90% killing of C. albicans (Figure 2).
Table 3.
Anti-candidal potency of characterized pooled saliva from the HIV− and HIV+ groups.
| Saliva | Hst-5 (μg/ml) | % killing |
|---|---|---|
| HIV+ (M) | 4.82* | 18.62 |
| HIV+ (L) | 1.1 | 0 |
| HIV− (H) | 8.67* | 35.86 |
| HIV− (M) | 5.62* | 19.31 |
| HIV− (L) | 2.6 | 0 |
Experiments were performed on three separate occasions. (L) % killing was compared with rest of the groups.
P<0.05
Figure 2. Histatin-5 killing potency of C. albicans.
Histatin-5 viability assays demonstrated a concentration-dependent killing effect on C. albicans. Error bars indicate the standard errors of the means. All experiments were performed in triplicate on more than one occasion.
All results were significant with P<0.05 compared to % killing with PBS.
Antibody neutralization
To identify Hst-5 as the key salivary anti-candidal component, saliva samples from 6 healthy control subjects with highest Hst-5 (8.67 μg/ml) were pooled. Sample was treated with the Hst-5-specific antibody and killing potency assessed. Results demonstrated approximately 35.5% C. albicans killing for the saliva sample prior to antibody exposure. However, following exposure to the Hst-5-specific antibody, the percent killing by the saliva sample dropped to 6% indicating 30% loss of the saliva’s initial anti-candidal activity (Figure 3).
Figure 3. C. albicans percent killing by Hst-5 (5μg/ml) and pooled saliva in the absence (S) and presence (S+Ab) of the Hst-5 antibody.
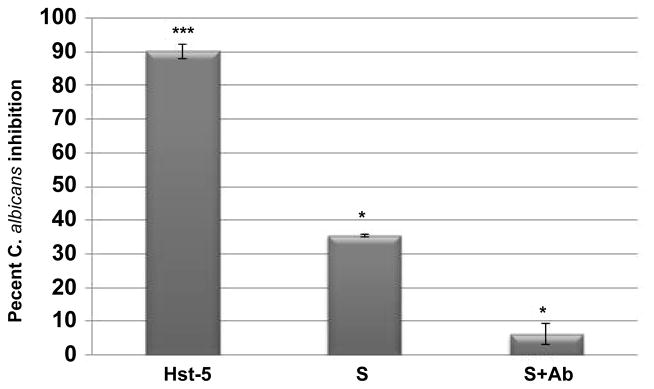
Drastic decrease in saliva’s initial anti-candidal potency in the presence of the antibody indicating loss of activity due to Hst-5 neutralization. Error bars indicate the standard errors of the means. All experiments were performed in triplicate on more than one occasion. *** P<0.001 (Hst-5 compared to PBS); * P<0.05 (S compared to PBS; S+Ab compared to S).
Discussion
In HIV+ individuals, it has been suggested that salivary gland function is adversely affected early in HIV disease [6]. Therefore, it is conceivable that HIV infection compromises the expression and/or secretion of peptides such as Hst-5 in turn contributing to the enhanced prevalence of opportunistic infections and particularly OC [31–34]. Our recent studies had demonstrated the efficacy of Hst-5 in the protection of murine oral tissue against C. albicans colonization and tissue infection [35]. However, the contribution of Hst-5 to saliva’s protective function in the host was not previously investigated, likely due to lack of feasible methods for measuring salivary Hst-5. To that end, the goal of this study was to assess salivary Hst-5 in terms of HIV status, salivary candidacidal potency, candidal prevalence and subject demographics.
The reported concentration of salivary histatins in healthy individuals varies greatly, likely due to the method used with no significant differences in levels noted between whole and glandular saliva [19]. Consistent with these observations, a wide range of Hst-5 concentrations was seen in our HIV− subjects, however concentrations in the HIV+ were across the board low, with approximately 62% decrease in levels compared to control population (P<0.0001) (Figure 1). Although HIV-associated salivary gland disease has been documented, there are discrepancies between studies reporting alterations in reduced salivary flow and composition in HIV+ individuals. Studies by Lin et al. [6] suggested that salivary gland function is adversely affected in HIV infection and based on CD4 counts, the changes occur in the early stages of the disease and do not appear to be compounded by medications. Our findings corroborated these previous observations in that SFR was lower in the HIV+ group and no correlation was found with CD4 counts. Similarly, no associations could be made between Hst-5 concentrations and length of HIV infection status which ranged from 1 to 29 years with 50% of subjects diagnosed over 15 years ago (Table S5).
However, our findings demonstrated an association between Hst-5 concentration and age in the HIV− group consistent with the findings from a previous study by Johnson et al. [36] (Table S6; Figure 1). These age-related findings are of interest as oral candidal infections increase with age. Importantly, in the HIV+ group, the decrease in Hst-5 concentration was found to be concomitant with an enhanced prevalence of candidal colonization (Table 1). However, at the time of sampling only two patients had documented clinical OC and three had documented history of OC. Nevertheless, since all of the HIV+ subjects had low and relatively close Hst-5 concentrations, no clear associations could be made in terms of clinical disease.
Although histatins possess significant in vitro antifungal activity, the extent of candidacidal activity in saliva in vivo is less clear. Whole or glandular saliva was shown to not exhibit the expected level of candidacidal activity in vitro according to the calculated concentrations in saliva. Our findings were consistent with these earlier observations as in candidacidal assays, 5 μg/ml of purified Hst-5 resulted in 90% percent killing of C. albicans (Figure 2), whereas a comparable concentration in saliva as measured by ELISA resulted in approximately 20% killing. The concept of salivary “masking” has been proposed to account for the discrepancy between in vitro and in vivo activity where a study by Flora et al. [15] identified calcium as a potent inhibitor of Hst-5 suggesting that this ion might be involved in the masking effect of saliva. Therefore, in order to confirm lack of interference by salivary salts, we performed candidacidal assays using serially-diluted saliva which resulted in percent killing consistent with neat saliva indicating lack of interference (data not shown). Another important issue to consider is the potential cleavage of the peptide in whole saliva. However, a recent study by Sun et al. [37] investigating the kinetics of histatin proteolysis in saliva demonstrated that the antifungal activity of these proteins is sustained in the proteolytic environment of the oral cavity.
Further, candidacidal assays using pooled saliva with different Hst-5 concentrations demonstrated percent C. albicans killing to be proportional to concentration. This strong association between candidacidal effect and Hst-5 concentration was further confirmed by antibody neutralization assays which resulted in significant loss of anti-candidal activity (Figure 3). It is important to note however that the Hst-5 antibody did not result in complete loss of activity, attributing a potential role for other histatins or AMPs in the process, albeit minimal.
In conclusion, recognizing the various factors and conditions that play a role in the progression of candidal colonization to infection in vulnerable populations will contribute to our understanding of the quandary of the enhanced propensity of the HIV+ population to OC. Importantly, the availability of a feasible method conducive for large scale measurement of Hst-5 will allow for prospective clinical studies in order to elucidate the nature of the salivary dysfunction in HIV disease and explore its impact on innate immunity and predisposition to opportunistic infections. These clinical studies are currently underway in our laboratory.
Supplementary Material
Acknowledgments
We would like to thank Dr. Jan Bolscher from the Academic Centre for Dentistry Amsterdam (ACTA) for providing us with histatins 1 and 3 and Dianna Weikel for her assistance with subject recruitment, the informed consent process and the collection of clinical samples. This work was supported by NIH grants DE016257 NIH/NAID and P50 CA97007 NIH/NCI.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Fidel PL., Jr Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis. Adv Dent Res. 2006;19:80–84. doi: 10.1177/154407370601900116. [DOI] [PubMed] [Google Scholar]
- 2.Klein RS, Harris CA, Small CB, Moll B, Lesser M, et al. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 3.de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17:729–759. doi: 10.1128/CMR.17.4.729-759.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderone RA, Clancy CJ. Candida and Candidiasis. 2. ASM Press; Washington DC, USA: 2012. [Google Scholar]
- 5.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin AL, Johnson DA, Stephan KT, Yeh CK. Alteration in salivary function in early HIV infection. J Dent Res. 2003;82:719–724. doi: 10.1177/154405910308200912. [DOI] [PubMed] [Google Scholar]
- 7.Iorgulescu G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J Med Life. 2009;2:303–307. [PMC free article] [PubMed] [Google Scholar]
- 8.Tiwari M. Science behind human saliva. J Nat Sci Biol Med. 2011;2:53–58. doi: 10.4103/0976-9668.82322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auvynet C, Rosenstein Y. Multifunctional host defense peptides: antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J. 2009;276:6497–6508. doi: 10.1111/j.1742-4658.2009.07360.x. [DOI] [PubMed] [Google Scholar]
- 10.Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology. 2011;216:322–333. doi: 10.1016/j.imbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Falla TJ. Potential therapeutic application of host defense peptides. Methods Mol Biol. 2010;618:303–327. doi: 10.1007/978-1-60761-594-1_19. [DOI] [PubMed] [Google Scholar]
- 12.Peters BM, Shirtliff ME, Jabra-Rizk MA. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog. 2010;6:e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad M, Piludu M, Oppenheim FG, Helmerhorst EJ, Hand AR. Immunocytochemical localization of histatins in human salivary glands. J Histochem Cytochem. 2004;52:361–370. doi: 10.1177/002215540405200307. [DOI] [PubMed] [Google Scholar]
- 14.Edgerton M, Koshlukova SE, Lo TE, Chrzan BG, Straubinger RM, et al. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J Biol Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 15.Flora B, Gusman H, Helmerhorst EJ, Troxler RF, Oppenheim FG. A new method for the isolation of histatins 1, 3, and 5 from parotid secretion using zinc precipitation. Protein Expr Purif. 2001;23:198–206. doi: 10.1006/prep.2001.1493. [DOI] [PubMed] [Google Scholar]
- 16.Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, et al. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- 17.Nikawa H, Jin C, Fukushima H, Makihira S, Hamada T. Antifungal activity of histatin-5 against non-albicans Candida species. Oral Microbiol Immunol. 2001;16:250–252. doi: 10.1034/j.1399-302x.2001.160409.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsai H, Bobek LA. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:2224–2228. doi: 10.1128/aac.41.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong J, Vylkova S, Li XS, Edgerton M. Calcium blocks fungicidal activity of human salivary histatin 5 through disruption of binding with Candida albicans. J Dent Res. 2003;82:748–752. doi: 10.1177/154405910308200917. [DOI] [PubMed] [Google Scholar]
- 20.Jang WS, Li XS, Sun JN, Edgerton M. The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob Agents Chemother. 2008;52:497–504. doi: 10.1128/AAC.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmerhorst EJ, Troxler RF, Oppenheim FG. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc Natl Acad Sci U S A. 2001;98:14637–14642. doi: 10.1073/pnas.141366998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyurko C, Lendenmann U, Helmerhorst EJ, Troxler RF, Oppenheim FG. Killing of Candida albicans by histatin 5: cellular uptake and energy requirement. Antonie Van Leeuwenhoek. 2001;79:297–309. doi: 10.1023/a:1012070600340. [DOI] [PubMed] [Google Scholar]
- 23.Koshlukova SE, Lloyd TL, Araujo MW, Edgerton M. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J Biol Chem. 1999;274:18872–18879. doi: 10.1074/jbc.274.27.18872. [DOI] [PubMed] [Google Scholar]
- 24.Mochon AB, Liu H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008;4:e1000190. doi: 10.1371/journal.ppat.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown AJ, Odds FC, Gow NA. Infection-related gene expression in Candida albicans. Curr Opin Microbiol. 2007;10:307–313. doi: 10.1016/j.mib.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Meiller TF, Hube B, Schild L, Shirtliff ME, Scheper MA, et al. A novel immune evasion strategy of candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS One. 2009;4:e5039. doi: 10.1371/journal.pone.0005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navazesh M, Kumar SK University of Southern California School of Dentistry. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc. 2008;139(Suppl):35S–40S. doi: 10.14219/jada.archive.2008.0353. [DOI] [PubMed] [Google Scholar]
- 28.Epstein JB, Pearsall NN, Truelove EL. Quantitative relationships between Candida albicans in saliva and the clinical status of human subjects. J Clin Microbiol. 1980;12:475–476. doi: 10.1128/jcm.12.3.475-476.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 30.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 31.Torres SR, Garzino-Demo A, Meiller TF, Meeks V, Jabra-Rizk MA. Salivary histatin-5 and oral fungal colonisation in HIV+ individuals. Mycoses. 2009;52:11–15. doi: 10.1111/j.1439-0507.2008.01602.x. [DOI] [PubMed] [Google Scholar]
- 32.Challacombe SJ, Naglik JR. The effects of HIV infection on oral mucosal immunity. Adv Dent Res. 2006;19:29–35. doi: 10.1177/154407370601900107. [DOI] [PubMed] [Google Scholar]
- 33.Lal K, Pollock JJ, Santarpia RP, 3rd, Heller HM, Kaufman HW, et al. Pilot study comparing the salivary cationic protein concentrations in healthy adults and AIDS patients: correlation with antifungal activity. J Acquir Immune Defic Syndr. 1992;5:904–914. [PubMed] [Google Scholar]
- 34.Lin AL, Johnson DA, Patterson TF, Wu Y, Lu DL, et al. Salivary anticandidal activity and saliva composition in an HIV-infected cohort. Oral Microbiol Immunol. 2001;16:270–278. doi: 10.1034/j.1399-302x.2001.016005270.x. [DOI] [PubMed] [Google Scholar]
- 35.Peters BM, Zhu J, Fidel PL, Jr, Scheper MA, Hackett W, et al. Protection of the oral mucosa by salivary histatin-5 against Candida albicans in an ex vivo murine model of oral infection. FEMS Yeast Res. 2010;10:597–604. doi: 10.1111/j.1567-1364.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson DA, Yeh CK, Dodds MW. Effect of donor age on the concentrations of histatins in human parotid and submandibular/sublingual saliva. Arch Oral Biol. 2000;45:731–740. doi: 10.1016/s0003-9969(00)00047-9. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Salih E, Oppenheim FG, Helmerhorst EJ. Kinetics of histatin proteolysis in whole saliva and the effect on bioactive domains with metal-binding, antifungal, and wound-healing properties. FASEB J. 2009;23:2691–2701. doi: 10.1096/fj.09-131045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.