Abstract
Objectives
To assess the utility of dobutamine cardiovascular magnetic resonance (DCMR) results for predicting cardiac events in individuals with reduced left ventricular ejection fraction (LVEF).
Background
It is unknown whether DCMR results identify a poor cardiac prognosis when the resting LVEF is moderately to severely reduced.
Methods
Two-hundred consecutive patients aged 30 to 88 (average 64) years with a LVEF ≤55% that were poorly suited for stress echocardiography, underwent DCMR in which LV wall motion score index (WMSI), defined as the average wall motion of the number of segments scored, was assessed at rest, during low dose, and after peak intravenous infusion of dobutamine/atropine. All participants were followed for an average of 5 years after DCMR to ascertain the post testing occurrence of cardiac death, myocardial infarction (MI), and unstable angina or congestive heart failure warranting hospitalization.
Results
After accounting for risk factors associated with coronary arteriosclerosis and MI, a stress induced increase in WMSI during DCMR was associated with future cardiac events (p< 0.001). After accounting for resting LVEF, a DCMR stress induced change in WMSI added significantly to predicting future cardiac events (p=0.003), but this predictive value was confined primarily to those with a LVEF >40%.
Conclusions
In individuals with mild to moderate reductions in LVEF (40% to 55%), dobutamine induced increases in WMSI forecast MI and cardiac death to a greater extent than an assessment of resting LVEF. In those with a LVEF < 40%, a dobutamine induced increase in WMSI does not predict MI and cardiac death beyond the assessment of resting LVEF.
Keywords: magnetic resonance imaging, cardiac prognosis, myocardial ischemia, dobutamine stress imaging
Introduction
Dobutamine cardiovascular magnetic resonance (DCMR) exhibits high clinical utility for identifying myocardial ischemia in patients with chest pain suspected to have flow limiting epicardial coronary arterial narrowings (1,2). This noninvasive stress imaging modality exhibits low interobserver variability for the interpretation of left ventricular (LV) stress induced wall motion abnormalities (WMA) (3), and in patients with a resting left ventricular ejection fraction (LVEF) >40%, DCMR stress induced WMA serve as additive, independent predictors of future myocardial infarction (MI) and cardiac death (4). At present, however, the prognostic utility of DCMR stress induced LV WMA in patients with a LVEF <40% at rest has not been determined. This question is important to address because many patients presenting for cardiovascular care exhibit a moderately to severely reduced LVEF due to pre-existing coronary arteriosclerosis (5,6), or they exhibit poor image quality during other forms of noninvasive testing (2). This study was performed to determine the prognostic utility of DCMR stress induced LV WMA in patients with resting LV dysfunction that were poorly suited for dobutamine stress transthoracic echocardiography.
Methods
Study population
The study was approved by the Institutional Review Board at the Wake Forest University School of Medicine, and all participants provided informed consent. Patients with contraindications to DCMR (implanted pacemakers or defibrillators, or intracranial metal), or to receiving dobutamine or atropine were excluded from enrollment. From 1997 to 2004, 300 patients underwent DCMR to diagnose inducible ischemia with a LVEF <55%, resting segmental WMA, and ≥ 6 LV myocardial segments not visualized during second harmonic stress echocardiography with or without microbubble contrast.
To be eligible for inclusion in this study, a patient had to successfully complete the low, (7.5mcg/kg/min) and high doses of dobutamine and atropine (administered to achieve 80% of the maximum predicted heart rate response for age), and must not have experienced a coronary arterial revascularization procedure within 45 days of the DCMR. Of 300 referred, 31 did not receive intravenous dobutamine/atropine due to the following: dissection or aneurysm of the aorta (n=3), newly diagnosed large LV mobile thrombus (n=11), new valvular vegetation due to endocarditis (n=1), severe ventricular ectopy preventing adequate gating (n=3), inability to understand the instructions associated with the exam (n=2), newly diagnosed severe hypertension (n=2), inability to lie flat because of decompensated congestive heart failure (n=2), anxiety (n=5), metal artifacts associated with prior surgical procedures (n=1), or newly diagnosed congenital heart disease (n=1). Two hundred sixty-nine subjects received dobutamine with 229 receiving both a low (7.5mcg/kg/min) and high (40–50mcg/kg/min) dose. This latter high dose of dobutamine was not completed in 40 patients because of ventricular ectopy, an atrial arrhythmia, or an early inducible LV wall motion abnormality with the low dose infusion. Immediately after the test results were obtained, 29 individuals were referred for coronary artery revascularization within 45 days. As a result, 200 subjects formed the study population. Out if these, 80 participants were also included in the 2-year follow-up of a population previously reported from our group (4). Comprehensive demographic data regarding health status and medication use were collected at the time of testing. For the purposes of data collection, prior myocardial infarction (MI), hypertension, diabetes, and hypercholesterolemia were defined according to previously published criteria (4).
Dobutamine/atropine imaging protocol
Images were acquired according to previously published techniques (2,4) either with a 1.5T GE Horizon or a 1.5 T GE CV/I (General Electric Medical Systems, Waukesha, WI) whole body imaging system using a phased array cardiac surface coil placed on the chest. Single-slice, multi-phase gradient-echo images were acquired throughout testing. Patients’ routine use of medications, including β-receptor antagonists, was not altered before testing.
Image analyses
Images were analyzed using a software program designed for display of dobutamine stress MR images in a multi-window synchronized format (2,4). For each of the 17 segments at each of the 3 stages of the protocol (rest, low dose and peak infusion), LV wall motion was assessed with a visual scoring system in which 1=normal, 2=hypokinetic, 3=akinetic, and 4=dyskinetic. Wall motion score index (WMSI) during each stage of the protocol was defined as the cumulative sum of individual segment scores divided by the number of interpreted segments. The change in WMSI from rest to low dose and from low dose to high dose pharmacologic infusion was recorded.
Follow up
Personnel unaware of the study design or stress testing results contacted each subject (or, if deceased, an immediate family member). Any change in physical state, medical condition, or medication use was confirmed by review of the participant’s medical records. Hard events were defined as MI (angina of ≥30 minutes duration, and either ≥2 mm ST segment elevation in 2 consecutive electrocardiogram leads or a rise in serum Troponin I or creatine kinase level and its MB fraction 2 times the upper limit of normal) (8), or cardiac death (death in the presence of acute coronary syndrome, significant cardiac arrhythmia, or refractory congestive heart failure [CHF]) (9). The designation “any events” included hard events along with unstable angina (USA) or CHF warranting hospital admission (4). When available, electrocardiogram, enzymatic, or autopsy data were used to substantiate cardiac mortality. In the case of 2 simultaneous cardiac events, the worst event (cardiac death>MI>USA>CHF) was selected for the purpose of analyses. Time to any event was defined as time to first event. Median follow-up time was calculated according to the method proposed by Schemper and Smith (10).
Statistical Analysis
All grouped data were expressed as mean ± standard deviation. Chi-square and Kruskal-Wallis tests were used to assess differences in categorical and continuous characteristics, respectively, between those with and without any events and between those with and without hard events. Kaplan-Meier methods were used to estimate the probability of cardiac events as a function of follow-up duration, and log-rank tests were used to assess unadjusted differences between groups. Cox proportional hazards regression models were used to identify independent predictors of the time to cardiac events. The risk of a given variable was expressed by a hazard ratio (HR) with corresponding 95% confidence intervals. Variables were considered significant if the null hypothesis of no contribution could be rejected at a two-sided probability value of < 0.05. Secondary analyses to calculate the added benefit of stress induced change in WMSI for strata defined by the LVEF at rest were performed by Cox proportional hazards models. Modeling that considered a nonlinear relationship between LVEF and the log of the hazard of having an event, and nonlinear models estimating possible interactions between the effect of increased WMSI and LVEF were estimated using a Cox proportional model that allowed for spline fitting of continuous factors (S-Plus 7.0 for Windows, Enterprise Developer, 2005 Insightful Corp.). The degrees of freedom for the spline fits were chosen as the largest value that still made the risk of an event to be a monotonic decreasing function of LVEF.
Results
Demographic data regarding all 200 participants are displayed in Table 1. The majority (65%) of participants were men. Eleven of the 200 subjects had atrial fibrillation at rest and throughout pharmacologic stress testing. The hemodynamic data for the study participants are shown in Table 2. Eighty-three percent of participants reached 80% of the MPHRR for age during stress. Contact was made with all 200 participants at a median time of 60.6 months (Interquartile Range: 45.9 – 72.6 months).
Table 1.
Demographic Data
| Any Events | Hard Events | ||||
|---|---|---|---|---|---|
|
| |||||
| Yes | No | Yes | No | ||
|
| |||||
| Patient Characteristics | (n=200) | (n=106) | (n=94) | (n=35) | (n=165) |
| Age, y | 64±11 | 63±11 | 65±11 | 63±10 | 64±11 |
| Men:Women | 130:70 | 68:38 | 62:32 | 26:9 | 104:61 |
| Weight, Kg | 90±20 | 91±21 | 90±19 | 90±20 | 91±20 |
| Height, cm | 170±11 | 170±10 | 171±11 | 170±11 | 170±11 |
| BSA, m2 | 2.06±0.67 | 2.01±0.24 | 2.12±0.99 | 2.01±0.25 | 2.07±0.7 |
| LVEF, % *,** | 43.5±9 | 42±10 | 45±9 | 39±10 | 44±9 |
| WMSI rest ** | 1.71±0.48 | 1.77±0.51 | 1.64±0.45 | 2.01±0.50 | 1.65±0.46 |
| WMSI peak *,** | 1.59±0.50 | 1.68±0.53 | 1.49±0.45 | 1.94±0.52 | 1.52±0.47 |
|
| |||||
| Historical Information | n (%) | n (%) | n (%) | n (%) | n (%) |
| Hypertension | 145(72) | 80(75) | 65(69) | 24(69) | 121(73) |
| Diabetes mellitus | 80(40) | 47(44) | 33(35) | 16(46) | 64(39) |
| COPD | 40(20) | 23(22) | 17(18) | 7(21) | 33(20) |
| Smoking | 89(44) | 48(45) | 41(44) | 16(46) | 73(44) |
| Hypercholesterolemia | 114(57) | 60(57) | 54(57) | 17(49) | 97(59) |
| Prior revascularization (CABG or PTCA) | 114(57) | 59(56) | 55(59) | 19(54 | 95(58) |
| Prior Q-wave MI | 82(41) | 42(40) | 40(43) | 18(51) | 64(39) |
|
| |||||
| Medications | |||||
| B-Blockers | 96(48) | 54(51) | 42(45) | 15(43) | 81(49) |
| Calcium antagonist | 48(24) | 29(27) | 19(20) | 8(23) | 40(24) |
| ASA | 132(66) | 71(67) | 61(65) | 20(57) | 112(68) |
| Nitrate | 78(39) | 43(41) | 35(37) | 14(40) | 64(39) |
| ACE inhibitor * | 75(37) | 49(46) | 26(28) | 16(46) | 59(36) |
| Diuretic * | 102(51) | 64(60) | 38(40) | 23(66) | 79(48) |
Abbreviations: BSA, body surface area; CABG, coronary artery bypass graft; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricule ejection fraction; WMSI, wall motion score index; PTCA, percutaneous transluminal coronary angioplasty;
p<0.05 any events yes vs. no;
p<0.05 hard events yes vs. no
Table 2.
Dobutamine Stress Data
| Hemodynamic measures | n=200 | Any Events | Hard events | ||
|---|---|---|---|---|---|
|
| |||||
| Yes (n=106) | No (n=94) | Yes (n=35) | No (n=165) | ||
|
| |||||
| Rest HR (bpm) | 71 ± 13 | 72± 14 | 70±12 | 73±12 | 71 ± 13 |
| Peak HR (bpm) | 122 ± 18 | 121±18 | 122±19 | 125±14 | 121 ± 19 |
| % Maximum Predicted Heart Rate adjusted for age* | 92 ±14 | 91±14 | 93 ±15 | 94±11 | 92 ± 15 |
| Rest Systolic Blood Pressure (mmHg) | 138 ± 22 | 139±24 | 138±21 | 135±25 | 139 ± 21 |
| Peak Systolic Blood Pressure (mmHg) | 144 ± 27 | 142±28 | 147±27 | 141±27 | 145 ± 27 |
| Rest Diastolic Blood Pressure (mmHg) | 77 ± 13 | 77±14 | 77±12 | 75±16 | 78 ± 12 |
| Peak Diastolic Blood Pressure (mmHg) | 74 ± 15 | 73±14 | 75±15 | 75±14 | 74 ±15 |
| Rate pressure product rest | 9863 ± 2390 | 9997±2581 | 9719±2171 | 9921±2289 | 9851±2418 |
| Rate pressure product stress | 17531 ± 4283 | 17225±4559 | 17860±3964 | 17625±4386 | 17512±4276 |
Abbreviations: HR, heart rate
Values expressed as mean ± SD
p < .05 Any Events yes vs no
Of the 140 patients whose WMSI decreased or stayed unchanged from low dose to peak dobutamine/atropine infusion, 63 (45%) experienced at least one cardiac event (including 19 [14%] with a hard event: 8 MIs and 11 cardiac deaths). Of the 60 patients with an increase in WMSI from low dose to peak stress, 43 (72%) experienced at least one cardiac event during the 5 years of follow-up (including 16 [27%] with a hard event).
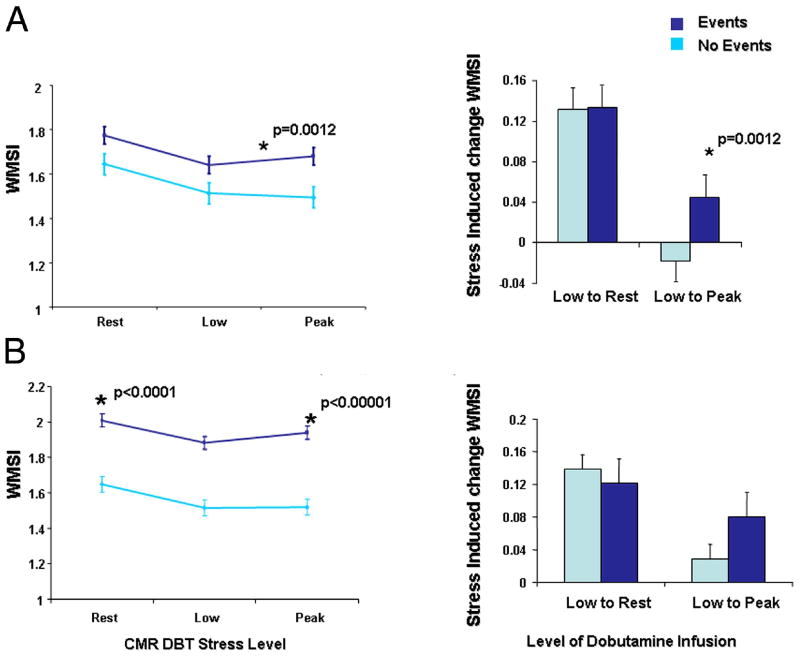
As shown in Figures 1A and B, compared to those without a cardiac event, participants experiencing a cardiac event exhibited a higher WMSI from low dose to peak pharmacologic stress, conversely, a decrease in WMSI from rest to low dose dobutamine infusion, occurred in participants without cardiac events. Participants experiencing a hard event exhibited a higher WMSI at rest and peak stress compared to those without an event (p< 0.0001 and p<0.00001 respectively, as shown in Figure 1B). As shown in Figure 2, an increase in WMSI induced by the infusion of dobutamine was associated with a reduced event-free survival for any event (p=0.0016) and approached significance for hard events (p=0.0503).
Figure 1. Predictive Value of Stress Induced Change in WMSI for Hard and Any Events.
Panel A (top) demonstrates the WMSI (y-axis) during the 3 phases of dobutamine infusion (rest, low dose infusion, and after peak stress). Within each stage, the mean ± the standard error of the estimate is shown. As noted, for any events, the difference in WMSI from low dose to peak stress was higher in those experiencing any events (Panel A). As shown in Panel B, the resting and peak WMSI was higher in patients experiencing myocardial infarction and cardiac death.
Figure 2. Event Free Survival after Dobutamine Stress.
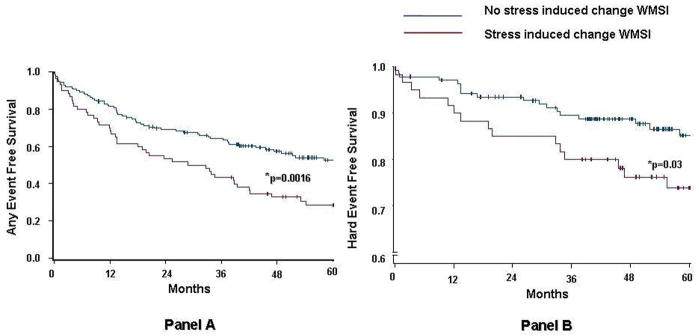
Kaplan-Meier event-free survival curves in patients with resting LV dysfunction, who did or did not experience an increase in WMSI during DCMR stress. Compared with patients with no evidence of stress induced increase of WMSI, event-free survival was significantly lower in patients with increased WMSI either for any event (p=0.002, Panel A) or for hard events (p=0.05, Panel B).
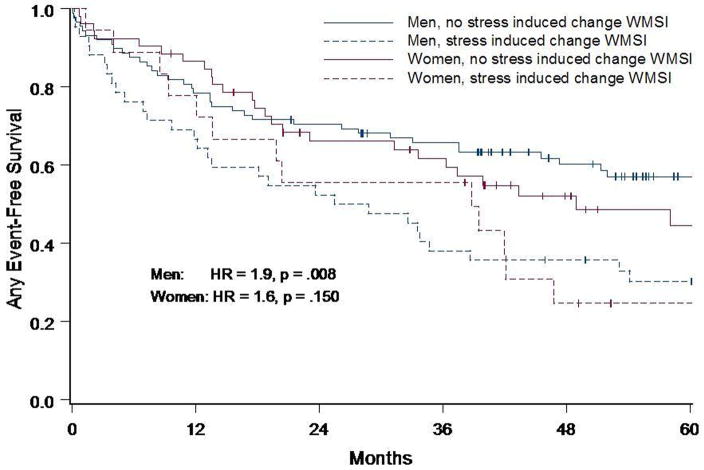
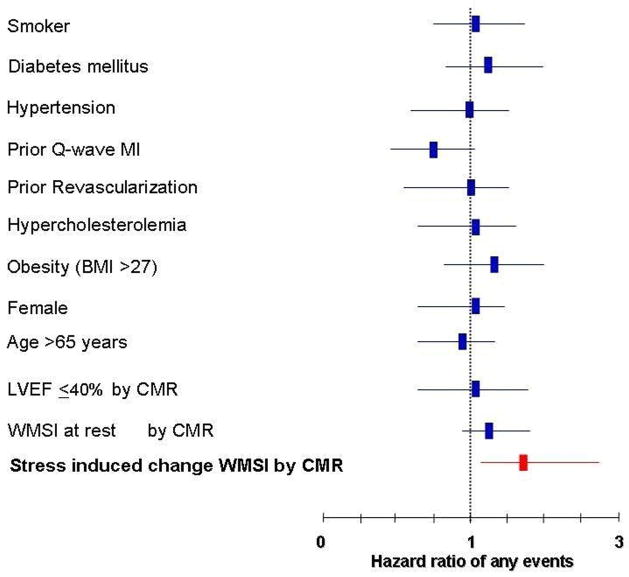
Analysis performed in men and women separately demonstrated a similar hazard ratio (HR) for both genders (Figure 3) of any events, but the p-value for statistical significance was different due to the smaller number of women (men HR=1.93, p=0.008 and women HR=1.64, p=0.1503) studied. These trends were not different from one another (p=0.64). Historical information was assessed to identify risk factors for coronary arteriosclerosis and MI. After accounting for risk factors for coronary arteriosclerosis and MI in a multivariate model (Figure 4), an increase in WMSI from low to peak dobutamine/atropine dose was the only independent predictor of cardiac events (HR=1.7, p=0.008).
Figure 3. Event-free Survival by Gender.
Kaplan-Meier any event-free survival curves in men and women with resting LV dysfunction, who did or did not experience an increase in WMSI during DCMR stress. Both men and women who experienced an increase in WMSI during DCMR had a lower event-free survival at 60 months (p=0.008 for men, and p=0.15 for women) with a similar trend for both gender (p=0.64).
Figure 4. Multivariate Determinants of Cardiac Events.
Multivariate analyses displaying hazard ratios ± 95% confidence intervals (x-axis) for developing MI or cardiac death. This model includes risk factors for coronary arteriosclerosis and myocardial infarction. As shown, stress induced increase of WMSI is an independent predictor of MI and cardiac death after accounting for these variables.
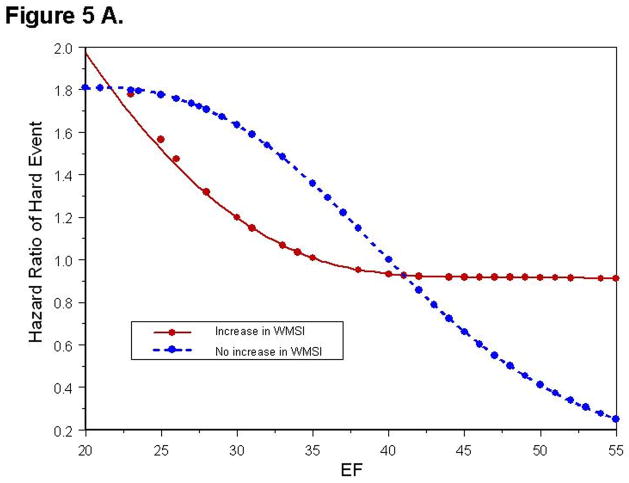
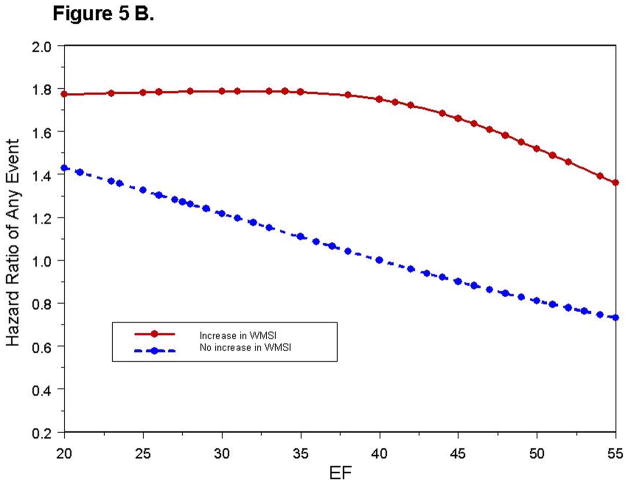
To determine if the risk predicted by an increase in WMSI was consistent for the range of LVEF studied (18% to 55%), we fit nonlinear spline functions to predict the risk of hard and any cardiac events for those with and without an increase in WMSI (Figure 5A, 5B). For individuals with an increase in WMSI and a rest LVEF >40%, the HR for experiencing a hard event was 3.09 (p = 0.017), and for experiencing any event was 2.20 (p = 0.0015). Ninety-one percent of those with an LVEF >40%, but without an increase in WMSI during dobutamine, were hard event free at 5 years. For patients with a LVEF <40%, the relative risk for experiencing a hard event was high with or without an increase in WMSI during dobutamine (HR= 3.64 [68% hard- Event free at 5 years] and 2.84 [70% hard-event free at 5 years]respectively), and was similar to the increased risk of those with a LVEF >40% that experienced an increase in WMSI with stress (HR= 3.09). For a hard event, the HR for subjects that increased their WMSI (Figure 4), or that exhibited a LVEF <40% at rest (HR= 3.01, p=0.0031) were high.
Figure 5. Relationship between Cardiac Events and LVEF.
Hazard ratio of experiencing a hard event (Figure 5A), or any event (Figure 5B) as a function of LVEF. Each point represents an event experienced by a study participant. The hazard values are relative to a patient without a dobutamine induced increase in WMSI and a resting LVEF = 40%.
Overall, an increase in WMSI predicted an increased risk of any events (HR= 1.85, p= 0.002). Figure 5B illustrates that an increase in WMSI was not associated with a significant increase in the risk of any events in subjects with a LVEF <40% (HR= 1.36, p=0.36), but there was a larger increase in risk for subjects with LVEF ≥40% (HR= 2.17, p=0.002).
We also performed analyses to determine if the location and distribution of myocardial segments responsible for the increase in WMSI was associated with an adverse cardiac prognosis. In these analyses, segments were defined as adjacent or remote depending on whether segments with worsening contraction during dobutamine were contiguous or separate from one another in 3-dimensional space. All but one participant with ≥ 5 myocardial segments with worsening wall motion during dobutamine exhibited at least 1 myocardial segment that was remote to the others. In participants with 2 to 4 segments developing an inducible wall motion abnormality during testing, those participants with segments located remote to one another experienced a worse cardiac prognosis compared to those with segments located adjacent to one another (p=0.04).
The results in this study address the prognostic significance of the change in LV wall motion score averaged among the 17 segments assessed (WMSI). In a prior study (4), in individuals with an average LVEF of 58%, we demonstrated that a change in LV wall motion score in any segment was associated with an adverse cardiac prognosis (HR of 7.9, p <0.001). In the current study of participants with an average LVEF of 44%, 53% of subjects exhibited a change in wall motion score in a single segment, but this single segment change did not predict an increase in the risk of hard events (HR=1.20, p=0.59) or any events (HR=1.17, p=0.43).
Discussion
Previously, our group reported the utility of DCMR-induced LV WMA for identifying those at risk for future cardiac events, including MI and cardiac death (4). A limitation of the prior work was that there were too few patients with a resting LVEF <40% for determining the utility of dobutamine induced WMA to predict those at risk of future MI and cardiac death. The current study addresses this limitation by enrolling 200 individuals with satisfactory examinations that exhibited a resting LVEF ranging from 18% to 55%. No participants were lost to follow-up, and all participants’ events were confirmed through review of their medical records.
In the current study, in patients with a LVEF <55%, resting and stress induced changes in LV WMSI during DCMR were observed in participants with adverse cardiac events including MI and cardiac death (Figures 1 and 2). In addition, the utility of the results for predicting adverse events were similar in men and women (Figure 3), and DCMR stress induced changes in WMSI predicted future cardiac events independent of conventional risk factors for coronary arteriosclerosis or MI (Figure 4).
Importantly however, the utility of an increment in WMSI during dobutamine for the purpose of forecasting cardiac events depended on the resting LVEF. We found that in patients with a LVEF >40%, an increase in WMSI during dobutamine forecasted a high future risk of cardiac events that was similar to the risk experienced by individuals with a LVEF <40% with or without an increase in WMSI during DCMR. Subjects with a LVEF >40%, but <55%, that did not have an increase in WMSI, incurred a small risk of a hard event (91% were hard event free at 5 years). However, those with a LVEF <40% with or without an increase in WMSI experienced a high risk of incurring a hard event within the next five years (only 68% [with an increase in WMSI] and 70% [without], were hard event free at 5 years). After accounting for a resting LVEF of <40%, an increase in WMSI during dobutamine did not independently predict future MI and cardiac death. Likewise, however, if the WMSI increased during dobutamine, an LVEF <40% did not predict future hard events.
As shown in Figure 5B, there was a trend toward the utility of a dobutamine induced change in WMSI for predicting any adverse event across the range of LVEF studied. Most (67%) of the “any” events in our population included admission to the hospital with USA or CHF (often termed “soft” events). Because hard and soft events were correlated (and thus a hard event would preclude observation of a later soft event), we were unable to use the data from this study to determine if a change in WMSI during dobutamine could serve as an independent predictor of admission to the hospital with USA or CHF.
An increase in WMSI in remote or non-contiguous LV wall segments was associated with a higher incidence of cardiac events compared to those individuals experiencing inducible ischemia in adjacent or contiguous LV segments. Inadequate blood flow remote to regions of ischemia or infarction has been shown to be related to adverse LV remodeling (11,12), and the presence of flow limiting stenoses in multiple epicardial coronary arterial segments (often termed, “multi-vessel coronary artery disease”) (13,14). The results of this study indicate that it is important for those interpreting DCMR exams to recognize the presence of remote segmental ischemia during image interpretation because its presence during DCMR confers an adverse prognosis in patients with a reduced resting LVEF.
Women represent roughly 40% of those referred for noninvasive stress testing (15). In this study, women represented 35% of the enrolled participants. As shown in Figure 3, an increase in WMSI in women trended (p=0.10) towards a reduced event-free survival, whereas in men, the increase in WMSI was associated with a significantly (p=0.017) worse event-free survival. Statistically, the event-free survival curves for men and women with increased WMSI were not different (p=0.64). A study with 46% more events in women is needed to have adequate power to detect the statistical significance of the observed effects in women.
When managing patients with a low LVEF, what conclusions from this and other studies should be drawn regarding the use of dobutamine stress testing. First, the rate of MI and cardiac death is high for patients referred for dobutamine stress that exhibit a low LVEF at rest. In this study, the proportion of patients who experienced events over an average 5 years of follow-up without (45%) and with (72%) an increase in WMSI was similar to that reported in previous studies (16,17) of stress echocardiography and radionuclide scintigraphy (5 year event rates of 45% to 75% for those with evidence of inducible ischemia during testing).
Second, rather than changes in single segments, it is the combined change of the average change in LV wall motion scored across the left ventricle (WMSI) that is predictive of events. Interestingly, this finding is similar to the observations in the low dose dobutamine echocardiography “viability” literature in which multiple (≥2), rather than single LV segmental wall changes are needed to forecast recovery of LV wall motion after coronary artery revascularization (18). Of note, the results of this study do not refute prior dobutamine echo and CMR results indicating that dobutamine induced improvements in LV wall motion “termed contractile reserve” are useful for predicting LV wall motion and contractility after successful coronary artery revascularization (19).
Third, and perhaps most importantly, for those with moderate to severe reductions in LVEF (in this study a LVEF < 40%), observed increases in WMSI during dobutamine do not forecast MI and cardiac death to a greater degree than the assessment of resting LVEF. Therefore, high dose intravenous dobutamine may offer little utility for predicting MI and cardiac death when the resting LVEF is <40%. Perhaps other markers, such as abnormalities of MRI perfusion, or delayed enhancement (that identify border zones of infarcts of high arrhythmogenic potential), should be sought to differentiate (or since the cardiac risk is high due to the rest LVEF, further select those at very high risk) those at the greatest risk of MI and cardiac death (20,21) in individuals with a LVEF <40%.
We recognize the following limitations to our study: first, we excluded participants from our final analysis that underwent revascularization within 6 weeks of DCMR. We sought to avoid the artificial introduction of study events due to consequences of interventional procedures performed on the basis of DCMR test results. We recognize, however, that individuals with ischemia not revascularized may reflect a higher risk group. Second, the majority of our subjects were Caucasian; thus the application of the results of this study to individuals of other race and ethnic groups remains to be determined. Third, this particular study incorporated a bi-plane apical measurement of LVEF. Also conventional gradient-echo rather than steady-state free precession was used to gather the cine white blood images. Newer faster steady-state free precession imaging techniques may enable the acquisition of more slices throughout the course of stress testing such that more accurate multi-slice Simpson’s Rule determinations of left ventricular volumes could be assessed throughout the course of a stress test (22). Fourth, we did not implement quantitative wall motion analysis techniques in our patients. Given the recent association of myocardial strain with cardiac events (23), it would be useful to understand the relative value of these techniques to dobutamine stress results in future investigations.
In conclusion, in individuals with mild to moderate reductions in LVEF (40% to 55%), dobutamine induced increases in WMSI forecast MI and cardiac death to a greater extent than an assessment of resting LVEF. In those with a LVEF < 40%, a dobutamine induced increase in WMSI does not predict MI and cardiac death beyond the assessment of resting LVEF.
Acknowledgments
Research supported in part by NCBH Technology Development Fund (B-03-97/98; Hundley), and NIH (RO1HL076438-01A2; Hundley).
Abbreviations and acronyms
- CAD
coronary artery disease
- CHF
congestive heart failure
- DCMR
dobutamine cardiovascular magnetic resonance
- HR
hazard ratio
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- MPHRR
maximum predicted heart rate response
- WMA
wall motion abnormalities
- WMSI
wall motion score index
Footnotes
Conflict of Interest: W. Gregory Hundley, MD, and Craig A. Hamilton, PhD, hold a minor financial interest in MRI Cardiac Services, Inc., a company that provides a tool for managing cardiac image display.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;16:763–770. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 2.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100:1697–1702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 3.Paetsch I, Jahnke C, Ferrari VA, et al. Determination of interobserver variability for identifying inducible left ventricular wall motion abnormalities during dobutamine stress magnetic resonance imaging. European Heart Journal. 2006;27:1459–1464. doi: 10.1093/eurheartj/ehi883. [DOI] [PubMed] [Google Scholar]
- 4.Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–2333. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, McGowan J. Heart failure due to ischaemic heart disease: Epidemiology, pathophysiology and progression. J Cardiovasc Pharmacol. 1999;33 (Suppl 3):S17–S29. doi: 10.1097/00005344-199906003-00003. [DOI] [PubMed] [Google Scholar]
- 6.Al-Mohammad A, Mahy IR, Norton MY, et al. Prevalence of hibernating myocardium in patients with severely impaired ischaemic left ventricles. Heart. 1998;80:559–564. doi: 10.1136/hrt.80.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman N, Schechter D, Klein M, Marciano R, Rozenman Y, Chisin R. SPECT attenuation artifacts in normal and overweight persons: Insights from a retrospective comparison of Rb-82 positron emission tomography and TI-201 SPECT myocardial perfusion imaging. Clin Nucl Med. 2000;25:1019–1023. doi: 10.1097/00003072-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Antman E, Bassand JP, Klein W, et al. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction: The Joint European Society of Cardiology/American College of Cardiology Committee. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 9.Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781–1788. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- 10.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi A, Ino T, Adachi H, et al. Left ventricular volume predicts postoperative course in patients with ischemic cardiomyopathy. Ann Thorac Surg. 1998;65:434–438. doi: 10.1016/s0003-4975(97)01155-7. [DOI] [PubMed] [Google Scholar]
- 12.Weisman HF, Bush DE, Mannisi JA, Weisfeldt ML, Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation. 1988;78:186–201. doi: 10.1161/01.cir.78.1.186. [DOI] [PubMed] [Google Scholar]
- 13.Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med. 1994;331:222–227. doi: 10.1056/NEJM199407283310402. [DOI] [PubMed] [Google Scholar]
- 14.Abbate A, Bussani R, Biondi Zoccai GG, et al. Infarct-related artery occlusion, tissue markers of ischaemia, and increased apoptosis in the peri-infarct viable myocardium. Eur Heart J. 2005 doi: 10.1093/eurheartj/ehi419. [DOI] [PubMed] [Google Scholar]
- 15.Mieres JH, Shaw LJ, Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111:682–696. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 16.Elhendy A, O’Leary EL, Xie F, McGrain AC, Anderson JR, Porter TR. Comparative accuracy of real-time myocardial contrast perfusion imaging and wall motion analysis during dobutamine stress echocardiography for the diagnosis of coronary artery disease. J Am Coll Cardiol. 2004;44:2185–2191. doi: 10.1016/j.jacc.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 17.Smart SC, Bhatia A, Hellman R, et al. Dobutamine-atropine stress echocardiography and dipyridamole sestamibi scintigraphy for the detection of coronary artery disease: Limitations and concordance. J Am Coll Cardiol. 2000;36:1265–1273. doi: 10.1016/s0735-1097(00)00825-1. [DOI] [PubMed] [Google Scholar]
- 18.Wellnhofer E, Olariu A, Klein C, et al. Magnetic resonance low-dose dobutamine test is superior to scar quantification for the prediction of functional recovery. Circulation. 2004;109:2171–2174. doi: 10.1161/01.CIR.0000128862.34201.74. [DOI] [PubMed] [Google Scholar]
- 19.Cigarroa CG, deFilippi CR, Brickner ME, Alvarez LG, Wait MA, Grayburn PA. Dobutamine stress echocardiography identifies hibernating myocardium and predicts recovery of left ventricular function after coronary revascularization. Circulation. 1993;88(2):430–436. doi: 10.1161/01.cir.88.2.430. [DOI] [PubMed] [Google Scholar]
- 20.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 23.Edvardsen T, Rosen BD, Pan L, et al. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging--the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2006;151:109–114. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]