Abstract
Cyclic and static loading regimes are commonly used to study tenocyte metabolism in vitro and to improve our understanding of exercise-associated tendon pathologies. The aims of our study were to investigate if cyclic and static stress relaxation affected the mechanical properties of tendon fascicles differently, if this effect was reversible after a recovery period, and if the removal of glycosaminoglycans (GAGs) affected sample recovery. Tendon fascicles were dissected frombovine-foot extensors and subjected to 14% cyclic (1 Hz) or static tensile strain for 30 min. Additional fascicles were incubated overnight in buffer with 0.5 U chondroitinase ABC or in buffer alone prior to the static stress-relaxation regime. To assess the effect of different stress-relaxation regimes, a quasi-static test to failure was carried out, either directly post loading or after a 2 h recovery period, and compared with unloaded control fascicles. Both stress-relaxation regimes led to a significant reduction in fascicle failure stress and strain, but this was more pronounced in the cyclically loaded specimens. Removal of GAGs led to more stress relaxation and greater reductions in failure stress after static loading compared to controls. The reduction in mechanical properties was partially reversible in all samples, given a recovery period of 2 h. This has implications for mechanical testing protocols, as a time delay between fatiguing specimens and characterization of mechanical properties will affect the results. GAGs appear to protect tendon fascicles from fatigue effects, possibly by enabling sample hydration.
Keywords: Bovine tendon, Loading, Mechanical properties, Glycosaminoglycan, Chondroitinase
1. Introduction
Exercise or mechanical loading is an important stimulus, sensed by tenocytes, which subsequently initiate tendon matrix remodelling. A certain amount of loading is required to keep the tendon in a healthy and functional state, while higher loads can lead to tendon adaptation to exercise and anincrease in performance [1]. However, overloading has been associated with the initiation of tendinopathies [2]. It has yet to be established how much loading can be tolerated by the tendon, optimizing adaptation without resulting in the development of tendinopathies. Animal studies aiming to experimentally induce Achilles tendinopathy with intense loading generally fail to initiate pathological changes, pointing towards a high loading tolerance in the Achilles tendon [3–5]. Characterizing the effects of specific loading conditions on the mechanical properties of tendon will help us to understand how the tissue responds to loading.
Cyclic and static stress-relaxation loading regimes are commonly used to study tenocyte metabolism and to investigate mechanotransduction behaviour in vitro [6–9]. As such, it is important to characterize tendon under these loading conditions, to establish how the tissue may respond to, and recover from, both static and cyclic stress relaxation tests. To improve understanding of the tendon tissue response to stress relaxation, two experiments were conducted. The aim of the first experiment was to investigate if cyclic and static stress relaxation protocols affect the mechanical properties of tendon fascicles differently and if any of the mechanical changes were reversible with a recovery period. We hypothesized that cyclic loading would be more damaging, as represented by a reduction in failure properties, and that recovery would be limited in both loading environments, but particularly with cyclic loading.
The concept of a mechanostat set point suggests that tendon cells sense and generate internal forces to maintain tissue homeostasis, such as constant cytoskeletal tension in response to changing loads [10]. However, passive processes (non-cell-mediated) might also contribute to the retensioning of the tendon after stress relaxation. To investigate passive recovery processes, in the absence of any living cells, the experiment was conducted with freeze–thawed tendon tissue.
Passive viscoelastic processes are likely to incorporate water movement. Indeed, tissue hydration has been shown to influence the mechanical properties of tendon fascicles [11], whilst tendons lose water when loaded under tension [12]. Glycosaminoclycans (GAGs), which are hydrophilic in nature, seemed a likely candidate to influence this process, encouraging sample rehydration and thereby influencing the mechanical properties of the tissue. The precise role of GAGs in tendon tissue and their contribution to the mechanical behaviour of tendons is still debated. It has been suggested that GAGs are involved in lateral force transmission between neighbouring fibrils, forming cross-links between collagen molecules,and contributing to the mechanical strength of the tissue [13].However, recent studies could not confirm this hypothetical mechanism; enzymatic removal of GAGs had no effect on the failure properties or viscoelasticity of either human patellar tendon [14]or medial collateral ligament fascicles [15], neither did it influence the elastic behaviour (modulus) of rat tail tendon fascicles [16]. However, one study has shown an elevated GAG content upon static loading of cultured rat tail tendon fascicles, which is also associated with an increase in elastic modulus [17]. This indicates that GAG concentration may influence the mechanical properties of tendon tissue. To the best of our knowledge, the influence of GAG content on the tissue response to stress relaxation and recovery has not yet been investigated.
The aim of the second experiment was therefore to subject GAG-depleted tendon tissue to static stress relaxation, hypothesizing that removal of GAGs would increase the stress-relaxation effect and reduce or completely abolish the recovery effect.
2. Materials and methods
Tendon fascicles were dissected from the bovine foot medial, lateral or common digital extensor tendon (n = 8). These three tendons are localized next to each other on the anterior face of the front foot, perform the same function and have the same mechanical properties [18]. The lateral digital extensor tendon extends the lateral digit (digit IV), the medial digital extensor tendon extends the medial digit (digit III), and the common digital extensor tendon splits in two ends and extends both digits. Previous studies have indicated no differences in their mechanical properties [18]. All tendons were obtained from eight young, healthy animals (male steers between 18 and 36 months of age) from a local abattoir and frozen at −20 °C until used. For each fascicle, the diameter was determined along a 1 cm region in the middle of the fascicle, using a laser micrometer (LSM-501, Mitituyo, Japan). The smallest diameter recorded was used to calculate the cross-sectional area (CSA), assuming a circular shape.
Two experiments were conducted: the first experiment compared cyclic and static stress-relaxation protocols and the effect of 2 h recovery, while the effect of GAG depletion on static stress relaxation and recovery was investigated in the second experiment. Both experiments were performed with tendons from the same animals. For the control groups (Con; Con_B; Con_Ch) four fascicles per animal were used, while two fascicles per animal per condition were used in the stress-relaxation groups (SR; SRC; SR_2 h; SRC_2 h; SR_Ch; SR_Ch_2 h). See Table 1 for a schematic representation of the groups. For statistical analysis the mean value per animal per condition was used.
Table 1.
Experimental groups and testing conditions.
| Group name | Loading condition | Recovery period before tested to failure | Overnight incubation | Fascicles tested per animal (n = 8) |
|---|---|---|---|---|
| Experiment 1: cyclic and static stress-relaxation comparison | ||||
| Con | No loading | no | no | 4 |
| SR | Static | no | no | 2 |
| SRC | Cyclic | no | no | 2 |
| SR2h | Static | 2 h | no | 2 |
| SRC2h | Cyclic | 2 h | no | 2 |
| Experiment 2: effect of GAG depletion on stress relaxation and recovery | ||||
| Con_B | No loading | no | buffer alone | 4 |
| Con_Ch | No loading | no | buffer with Ch | 4 |
| SR_Ch | Static | No | buffer with Ch | 2 |
| SR_Ch_2 h | Static | 2 h | buffer with Ch | 2 |
Ch = chondroitinase.
2.1. First experiment: Cyclic and static stress-relaxation comparison
Fascicles were secured in individual custom-made stainless steel loading chambers with a grip to grip distance of 10 mm as described previously [8]. Each chamber was filled with Dulbeco’s modified Eagle’s medium (DMEM) supplemented with 50 U ml–1 penicillin and 0.05 mg ml−1 streptomycin. The fascicles were either directly tested to failure (Con) or first subjected to stress-relaxation tests. For the stress-relaxation test, the fascicles were strained for 30 min, either statically (SR), or cyclically at a frequency of 1 Hz (SRC) to 14% strain superimposed on a 2%strain off-set. For the static stress-relaxation test the ramp speed was 0.5 m ms−1, resulting in a rise-time of 3.2 s. 14% strain was chosen, as previous experiments had shown that this leads to a significant reduction in the failure properties of fascicles after 30 min of cyclic stress relaxation [8]. 14% strain corresponded to 60% of the strain at failure in these 10 mm long samples. In short specimens the failure strain is affected by gripping effects, so this would be equivalent to 6% strain when gripping effects are removed [19]. Fascicles were loaded to failure directly after the stress-relaxation test. Another two groups were subjected to identical stress-relaxation protocols, but were then given a recovery period of 2 h prior to quasi-static testing to failure (SR_2 h and SRC_2 h).
During the stress-relaxation test, peak stress was monitored at 1 Hz using a BOSE loading frame with a 225 N load cell (BOSE Corporation, Eden Prairie, MN. USA). For the failure tests the fascicles remained in the loading chambers and each chamber was individually secured in a materials testing machine (Bionix100, MTS, 50 N load cell). Fascicles were then loaded to failure at a rate of 1 m ms−1 at room temperature. Force and deformation were both continuously recorded at 50 Hz and engineering stress and strain calculated using the initial CSA and length of the sample. From the resulting data, the point at which a 0.1 N load was detected was located, and defined as the test start point. The original sample length was corrected accordingly.
2.2. Second experiment: effect of GAG depletion on stress relaxation and recovery
Fascicles were subjected to overnight incubation (15–16 h) in buffer alone (Con_B)or buffer with 0.5UchondroitinaseABC (catalog no 2905, Sigma–Aldrich, St Louis, MO, USA) (Con_Ch; SR_Ch; SR_Ch_2 h) at 37 °C.The buffer solution was 50 mM Tris, 60 mM sodium acetate, in 0.02% bovine serum albumin at pH 8, while chondroitinase ABC enzymatically cleaves GAG chains from their proteoglycan core protein [20]. The fascicles were then secured in loading chambers filled with DMEM, and either tested to failure directly (Con_B and Con_Ch), or tested after being subjected to 30 min static stress relaxation with (SR_Ch_2 h) or without (SR_Ch) 2 h of recovery (Table 1). Subsequent to the failure test, fascicles were harvested and the GAG content determined bya spectrophotometric GAG assay.
2.3. GAG assay
The GAG assay was performed as described previously [21]. Briefly, fascicles were lyophilized overnight and fascicle dry weight was recorded. Samples were digested overnight in 0.4 U ml–1 papain (Sigma–Aldrich, Poole, UK) at 60 °C. The digest supernatant was mixed with 1,9-dimethylmethylene blue (DMB) and the GAG content determined by spectrophotometry at a wavelength of 595 nm, using a standard curve of chondroitin-4-sulfate derived from bovine cartilage.
2.4. Statistical analyses
We used SPSS 16.0 for Windows including a Bonferroni-type correction to account for multiple comparisons. A one-way ANOVA was used to compare failure properties of control fascicles with those subjected to stress relaxation or buffer (Con to SR, SRC, SR_2 h and SRC_2 h; Con_Ch to SR_Ch and SR_Ch_2 h; Con_B to Con_Ch). Two-way ANOVAs were used to analyse the effect of the loading condition (comparing fascicles subjected to static or cyclic stress relaxation), the effect of recovery (comparing stress relaxed fascicles tested directly or after 2 h of recovery) and the effect of GAG removal (comparing fascicles with or without chondroitinase incubation). Differences in force development during the stress relaxation test were compared using a one-way ANOVA. For all statistical tests, significance was established at P ⩽ 0.05.
3. Results
3.1. GAG content
After chondroitinase ABC digestion the GAG content of the fascicles was reduced to 23 ± 8% of that in intact tissue, containing 0.34 ± 0.11 μg sulfated GAG/mg tendon tissue dry weight compared to 1.52 ± 0.30 μg sulfated GAG/mg tendon tissue dry weight in the control group.
3.2. Failure tests
Representative stress–strain curves are shown in Fig. 1. As shown in Fig. 2, the control samples (Con), which were subjected to a failure test directly after dissection, failed at a peak stress of 95.7 ± 20.6 MPa and a strain at failure of 23.1 ± 4.5%, while the modulus was 639 ± 127 MPa. Both cyclic (SRC) and static (SR) stress-relaxation protocols led to a significant decrease in fascicle stress and strain. However, directly comparing cyclic and static loading conditions highlighted a significantly greater reduction in failure strains in samples after cyclic stress relaxation. In addition, there was also a significant effect of recovery in both static and cyclically loaded samples. All samples failed at higher stress and strain values after 2 h recovery, compared to testing directly after stress relaxation (Fig. 2).
Fig. 1.
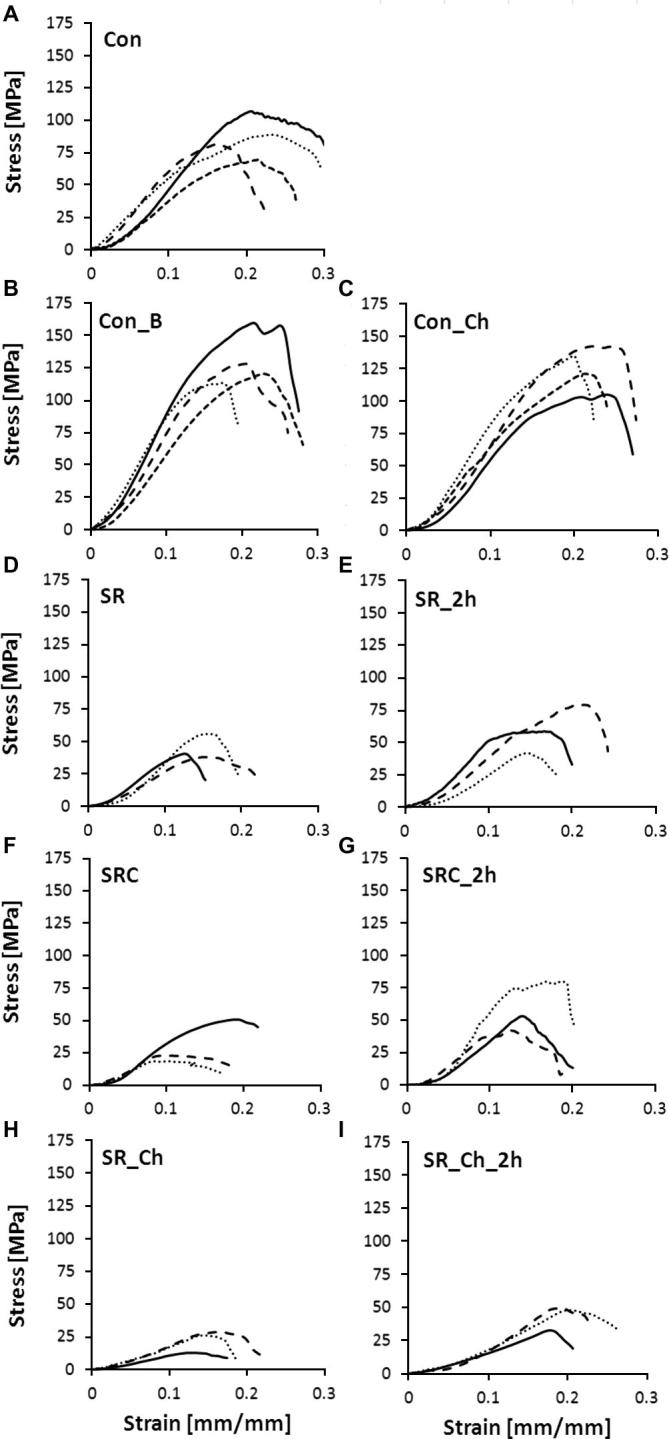
Representative stress–strain curves for (A) Con, (B) Con_B, (C) Con_Ch, (D) SR, (E) SR_2 h, (F) SRC, (G) SRC_2 h, (H) SR_Ch and (I) SR_Ch_2 h. The solid, dotted and small-dotted lines represent different specimens.
Fig. 2.
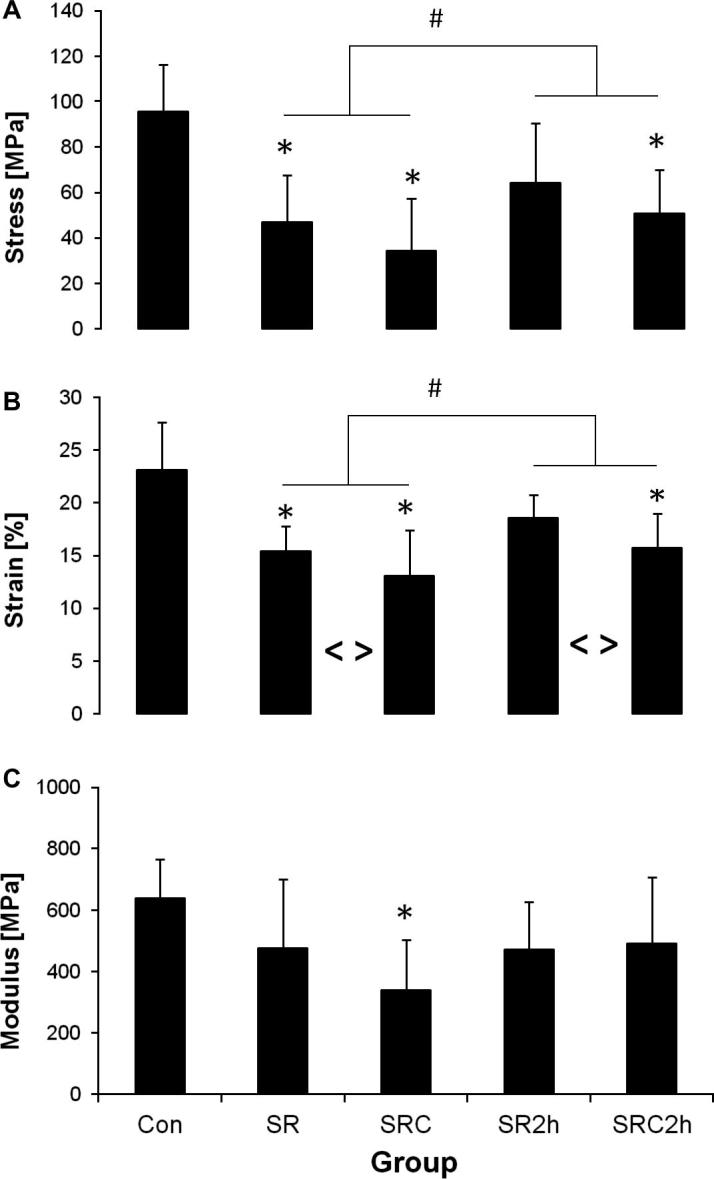
Mechanical properties after stress relaxation as determined by failure test: (A) failure stress, (B) strain at failure and (C) modulus. # = significant recovery effect (two-way ANOVA). <> = significant cyclic vs.static straining effect (two-way ANOVA). ∗significantly different to control (one-way ANOVA). Mean ± SD. n = 8.
The mean failure properties of fascicles from the second experiment are shown in Fig. 3. The control samples (Con_B), which were subjected to a failure test directly after overnight incubation in buffer, failed at a peak stress of 144.2 ± 20.4 MPa and a strain at failure of 22.0 ± 1.9%, while the modulus was 932 ± 136 MPa. Subjecting tendon fascicles to overnight incubation with chondroitinase ABC to remove the GAGs from the tissue (Con_Ch) did not result in any significant changes in failure properties compared to the controls that had been subjected to the same overnight incubation in buffer solution without chondroitinase (Con_B).
Fig. 3.
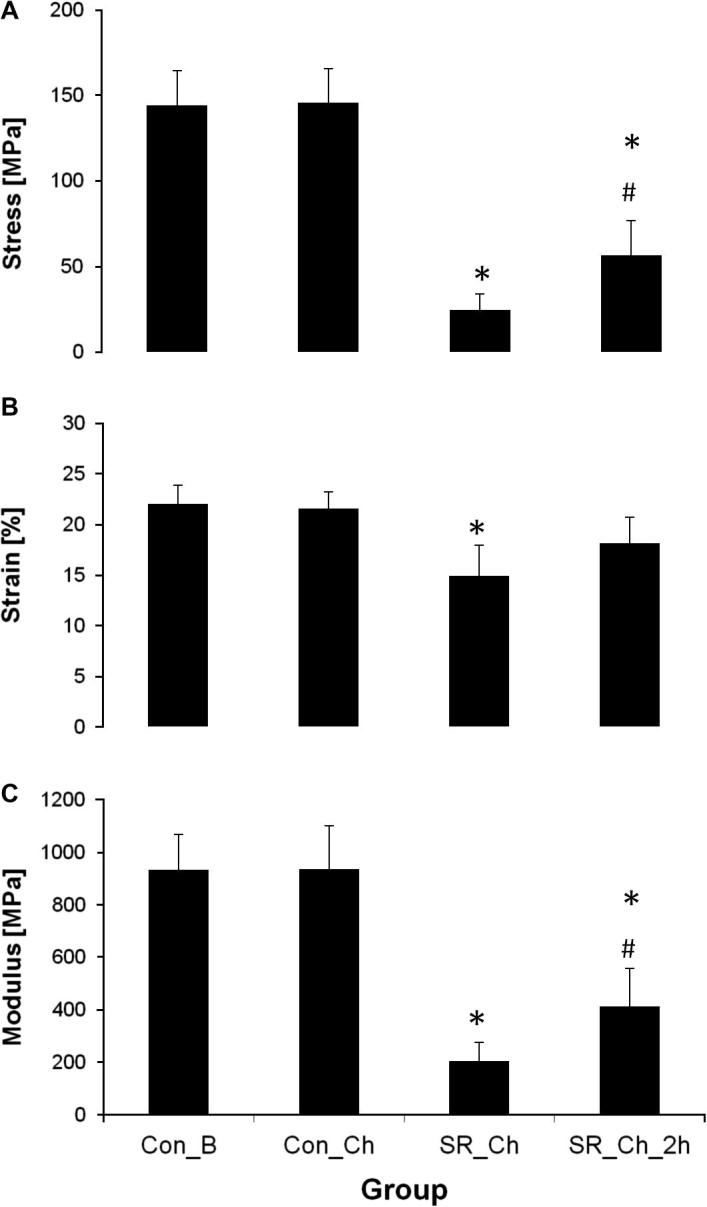
Mechanical properties after stress relaxation as determined by failure test of fascicles subjected to overnight incubation in buffer (Con_B) or buffer with chondroitinase ABC (Con_Ch; SR_Ch; SR_Ch_2 h): (A) failure stress, (B) strain at failure and (C) modulus. ∗ = significantly different to Con_Ch; # = significantly different to SR_Ch (one-way ANOVA). Mean ± SD. n = 7.
However, static stress relaxation of fascicles subjected to chondroitinase (SR_Ch) led to significant reductions in failure stress, strain and modulus compared to the respective control. This was very pronounced when considering failure stress, where an 83% reduction was seen. However, this reduction was partly recoverable, with stress and modulus values being significantly higher with 2 h of recovery (SR_Ch_2 h), to the extent that the failure strain in SR_Ch_2 h was not significantly different from the control (Con_Ch) (Fig. 3).
Comparing the untreated control fascicles from the second experiment to the first (Con to Con_B), failure stresses were considerably higher in the second experiment. Additional experiments, directly comparing fresh to buffer-incubated samples, have demonstrated that this was not due to the incubation in buffer (data not shown) but should be most likely attributed to sample variation (see also Sec. 4.1). As a result of these findings, we have not attempted to compare specimens from the first and second experiment directly, but have assessed the effect of each treatment or test as a percentage change with respect to the appropriate control condition. Subsequently, when comparing the percentage reduction in fascicle failure stress after subjection to static stress relaxation, the removal of GAGs led to a more pronounced reduction in failure stress compared to intact fascicles. However, even with depleted GAG content in these samples, the recovery effect after 2 h was similar to that seen in intact fascicles (Fig. 4).
Fig. 4.
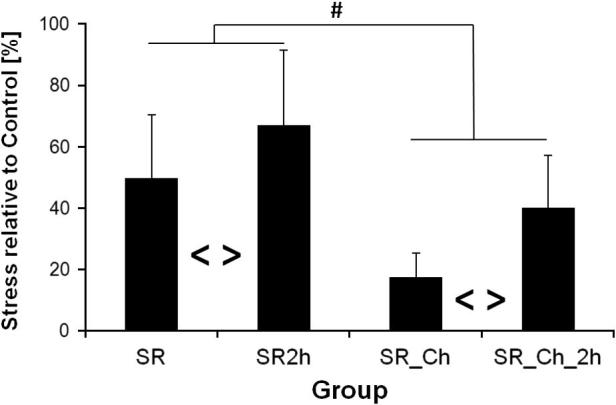
Reduction in failure stress relative to respective control (Con and Con_Ch). # = significant effect of chondroitinase (two-way ANOVA). <> = significant recovery effect (two-way ANOVA). Mean ± SD.
Sample length at the 0.1 N tare load was also compared in samples before and after the stress-relaxation test. Compared to the control group (10.19 ± 0.05 mm) sample length increased significantly with static (SR 10.79 ± 0.36 mm) and cyclic stress relaxation (SRC11.08 ± 0.27 mm). After the recovery, a significant increase in sample length was still evident in the cyclic stress-relaxation group (SRC_2 h 11.13 ± 0.52 mm) but not in the static stress relaxation group (SR_2 h 10.66 ± 0.24 mm). There were no differences in length amongst any of the chondroitinase treated groups (Con_Ch:10.29 ± 0.13 mm; SR_Ch: 10.32 ± 0.23 mm; SR_Ch_2 h: 10.47 ± 0.29 mm) nor between these and the buffer-incubated control group (Con_B10.34 ± 0.21 mm).
3.3. Stress-relaxation behaviour
The percentage stress relaxation was significantly greater in the cyclically strained specimens compared to those held under static strain at all time points from 5 s onwards. However, this increase originated solely from an increased relaxation rate during the first 30 s of the test, after which the difference between statically and cyclically loaded specimens remained constant, at a value of approximately 13%. When the fascicles were subjected to static stress relaxation after chondroitinase incubation (SR_Ch), the percentage stress relaxation was significantly greater than seen in untreated samples (SR) at all time points from 300 s onwards. In addition, it was notable that the stress-relaxation response of GAG-depleted specimens followed a different profile, with a steady increase in relaxation throughout the test period (Fig. 5). Fitting second-order exponential equations to these stress-relaxation curves highlighted the differences in the τ1 and τ2 time constants in chondroitinase-treated samples relative to both untreated groups (Table 2).
Fig. 5.
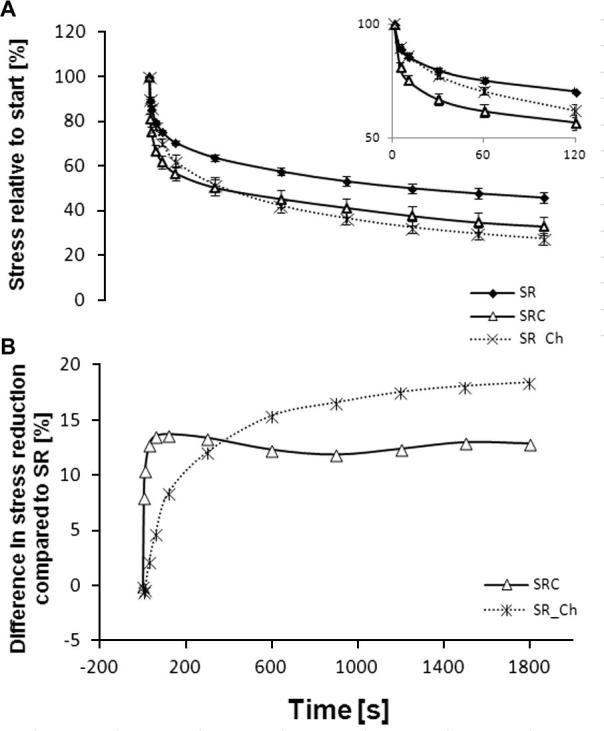
Stress development during the 30 min stress-relaxation test. (A) Stress drop relative to the starting stress of 100% (mean ± SE). The inset shows the first 60 s. Compared to the SR group the stress reduction in the SRC group is significantly greater at all time points from the 5th second onwards, and in the SR_Ch group from 300 s onwards (one-way ANOVA). (B) In order to assess differences in the stress-relaxation behaviour of the three test groups, the percentage difference in SR behaviour between the SRC and SC_Ch group, relative to the SR group, is plotted. The difference between SR and SRC is constant from 30s onwards, while the difference between SR and SR_Ch is established later and increases with time, as visualized by the different shape of the curve.
Table 2.
Values for the second-order exponential equation (y = A1∗exp(−x/t1) + A2∗exp(−x/t2) + y0) fitted to curves representing stress relaxation behaviour.
| Group name | A1 | A2 | τ1 | τ2 | y0 |
|---|---|---|---|---|---|
| SR | 0.23 ± 0.02 | 0.33 ± 0.02 | 8.1 ± 1.8 | 552.5 ± 93.6 | 0.46 ± 0.02 |
| SRC | 0.39 ± 0.03 | 0.33 ± 0.02 | 6.6 ± 1.3 | 571.6 ± 139.5 | 0.33 ± 0.02 |
| SR_Ch | 0.46 ± 0.02 | 0.26 ± 0.03 | 522.2 ± 91.3 | 17.9 ± 5.2 | 0.27 ± 0.02 |
4. Discussion
We have shown that static and cyclic stress-relaxation regimes affect the mechanical properties of the tendon fascicles differently, with cyclic loading leading to greater levels of stress relaxation, and a larger reduction in failure properties, in agreement with our hypothesis. However, contrary to our hypothesis, even when loading to relatively high strain levels (60% of the failure strain), the reduction in mechanical properties of stress-relaxed fascicles was partly reversible in all samples, given a recovery period of 2 h. Our data demonstrate that the removal of GAGs did not abolish or even reduce this recovery effect as hypothesized. However, with GAG depletion, the amount of fascicle stress relaxation increased and the failure stress subsequently decreased, implicating a protective role of GAGs towards limiting viscoelastic behaviour and thus preventing tissue fatigue. As GAG depletion and cyclic loading of samples have both been reported to reduce tissue hydration, it might be speculated that the increased reduction in failure properties of these sample groups post stress relaxation might be directly related to sample dehydration. However, the different shapes of the stress-relaxation curves of GAG-depleted and cyclically loaded samples suggest different mechanisms. Indeed, GAGs may have a direct role in relaxation mechanics that is not related to water movement, potentially as a result of intermolecular bridges.
The stress-relaxation behaviour of soft tissues, such as ligaments and tendons, when subjected to a constant strain has been well described [22]. This effect has been shown to be influenced by a number of intrinsic variables such as tendon composition and also extrinsic variables such as temperature and strain rate. Lower temperatures resulted in the human supraspinatus tendon being less viscous and more elastic when subjected to a static stress-relaxation test [23]. The rate of relaxation is also a function of strain, with the relaxation rate of tendons increasing with increased levels of strain [24]. Functionally distinct tendons also exhibit different stress-relaxation behaviour, possibly due to their different structures and compositions, e.g. the porcine common digital extensor tendon (positional) showed a significantly greater degree of relaxation than the superficial digital flexor tendon (load-bearing) [25]; similarly bovine digital extensor fascicles were less fatigue resistant than deep digital flexor fascicles, with significantly increased stress relaxation [26]. This seems a reasonable response considering the functional role and the need for elastic energy return in more highly loaded tendons.
Whilst the stress relaxation behaviour of tendons has been extensively studied, comparatively little is known about the recovery from stress-relaxation testing. The limited evidence available indicates that after stress relaxation, recovery rate is slower than relaxation rate; in the primate patellar tendon, recovery from a 600 s long static stress relaxation test (2.5% strain) was not complete after 1800 s [27], while porcine digital flexor tendon had fully recovered within 1000 s, following a strain of 6% applied for 100 s [24]. In our study the recovery period was four times the length of the testing time and still did not result in complete recovery. However, considering that the applied strain was much higher than in previous studies and intended to cause permanent structural changes, the extent of recovery was surprisingly large.
Although a number of influencing factors have been identified, the origins of relaxation behaviour are poorly understood. Water movement and matrix reorganization have both been implicated as potential mechanisms. From a structural perspective, stress relaxation at low strain levels occurs predominantly through sliding between collagen fibres, while at high strain levels fibril reorganization and relaxation might play a major role, with interfibrillar linkages eventually failing and leading to subsequent excessive fibril sliding [25]. However, no change in collagen fibre realignment was found during a 600 s long static stress-relaxation test with rat supraspinatus tendon [28]. In terms of fluid interactions within the material, several studies suggest the involvement of water movement in the stress response of tendon tissue. Indeed, an intradiffusion of water and solvent molecules from the water-rich, extrafibrillar proteoglycan matrix into the porous collagen fibril has been proposed, based on the finding that the time-dependent stress response in tendon tissue is associated with fibrillar expansion at the nanoscale [29]. At the tissue level, both static and cyclic tensile loads have been shown to result in a significant reduction in tendon water content [12].Tensile loading has also been suggested to result in water extrusion from the tendon core to the rim [30]. A change in water distribution or content would undoubtedly have an effect on the mechanical behaviour of the tendon. For example, changing the state of hydration by immersing human patellar tendon in hypotonic solution resulted in a greater tendon stiffness (20%) compared to tendons immersed in a hypertonic solution [31].
The current data suggest an involvement of water movement in stress-relaxation behaviour. Cyclic loading facilitated a more pronounced relaxation than static, while the evidence of sample recovery indicated that the reduction in fascicle mechanics was not permanent. The removal of GAGs had no effect on the immediate failure properties of tendon fascicles, when not subjected to fatigue loading, which is in agreement with the literature [14]. However, GAG depletion led to more stress relaxation and a greater reduction in subsequent failure stress (Fig. 4), suggesting a protective role of GAGs towards limiting viscoelasticity and fatigue effects, possibly by maintaining sample hydration. Indeed, the continual reduction in force during the 30 min stress-relaxation test in GAG-depleted specimens (Fig. 5) suggests that fascicle elasticity is more heavily impaired with GAG loss. However, it should be considered that this effect might be influenced by the CSA of the specimens. In our study we tested isolated tendon fascicles with a CSA ranging from 0.1 to 0.15 mm2. This high surface-to-volume ratio compared to whole tendons is likely to facilitate fluid exchange in the tissue, probably resulting in a more pronounced effect of GAG loss on fascicles compared to whole tendons.
It has previously been hypothesized that non-covalent interactions between GAGs, cross-linking neighbouring fibrils, could contribute to the mechanical behaviour of tendon fascicles [14,15]. The current data indicates that the contribution of GAG may specifically focus on preventing excess sample stress relaxation, which would be important for limiting fatigue damage. However, the speed of recovery from stress relaxation experiments was not affected by GAG removal. Although the initial reduction in mechanical properties of GAG-depleted specimens after stress relaxation was greater, the extent of recovery was similar to that seen in intact specimens. As only one time point post loading was investigated, it remains to be established if stress relaxation would continue beyond the 30 min test period, the reduction in mechanical properties post stress relaxation was permanent, or if the recovery of any of the specimens would continue beyond the 2 h time-point. Further studies to investigate this would be a benefit. It is possible that the 23% of remaining GAGs in the GAG-depleted specimens were enough to enable sample rehydration at the same rate as in the intact specimens. Further experiments to assess the contribution of GAGs to the recovery process are warranted, including greater depletion of tendon GAG, selective digestion of specific GAG types and a study of more frequent intervals over an extended time course.
4.1. Limitations of the study
It was found that the failure stress of control specimens in the first and second experiment was different. Although the tendons were harvested from the same animals and the mechanical testing conditions were kept the same, different bovine foot extensor tendons were used for the two experiments. It seems unlikely that exposure to the Tris buffer would have increased the mechanical properties. Indeed, incubation in phosphate-buffered saline (PBS) has previously been shown to decrease mean modulus and peak force in tendon fascicles [11], whilst another study suggests that both PBS and Tris would have no effect on the mechanical properties of tendon fascicles [14]. To investigate this, we have tested the effect of overnight incubation in Tris buffer on the mechanical properties. Fascicles from a single animal were dissected, half tested immediately, the other half incubated overnight in the buffer before testing. Comparing these groups, we found no difference between the failure properties of these two groups of samples. We then repeated this on tendons from a second animal, once again finding no significant differences between the incubated and non-incubated groups (mean ± SD): fresh 86 ± 25 MPa (range 57–136 MPa) vs. overnight incubation 74 ± 29 MPa (range 31–126 MPa).
However, this additional experiment once again demonstrated the large variability commonly found when testing tendon fascicles from different tendons, also evident in Fig. 1, where the wide range in failure stresses between fascicles can be seen in the representative curves for the different test groups. It has been shown previously that even small changes in the anatomical site from which a fascicle is harvested [32] can have an impact on its mechanical properties, indicating that at least some of the variation between our two experiments is likely attributed to the fact that different pieces of tendon were used, in addition to inter-animal variability. To account for the different failure stress, the groups from the first and second experiment were not compared directly, but only the percentage in force reduction after loading was compared (Fig. 4).
4.2. Conclusion and future directions
We have shown that the reduction in mechanical properties of tendon fascicles following stress relaxation was partly reversible with time. Although the mechanism by which this happens is yet to be elucidated, this has implications for mechanical testing protocols. A time delay between the fatiguing of specimens and characterization of the mechanical properties will affect the results. However, the time-dependent manner of this effect needs to be further investigated. While we know that a 2 h recovery period is sufficient to significantly increase stress and strain values in stress-relaxed samples, it remains to be established how quickly a measurable recovery effect occurs and if the failure properties are completely recoverable given sufficient time. It is likely that the time course and scale of the recovery effect are also influenced by the strain level applied during stress relaxation, with low strain levels leading to complete recovery and high strain levels leading to irreversible damage with no or only partial recovery.
Acknowledgements
We would like to acknowledge the contribution of the students Chee-Kiong Goh, Chineye Princess Udeze and Christian Klemt, in assisting with experiments and data analysis. This work was supported by Arthritis Research UK (grant no.18424). G.P.R. is an Arthritis Research UK Senior Research Fellow (grant no.17826).
References
- 1.Arampatzis A., Karamanidis K., Morey-Klapsing G., De Monte G., Stafilidis S. Mechanical properties of the triceps surae tendon and aponeurosis in relation to intensity of sport activity. J Biomech. 2007;40:1946–1952. doi: 10.1016/j.jbiomech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Riley G.P. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports. 2005;15:241–251. doi: 10.1111/j.1600-0838.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 3.Huang T.F., Perry S.M., Soslowsky L.J. The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study. Ann Biomed Eng. 2004;32:336–341. doi: 10.1023/b:abme.0000017537.26426.76. [DOI] [PubMed] [Google Scholar]
- 4.Archambault J.M., Hart D.A., Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect Tissue Res. 2001;42:13–23. doi: 10.3109/03008200109014245. [DOI] [PubMed] [Google Scholar]
- 5.Messner K., Wei Y., Andersson B., Gillquist J., Rasanen T. Rat model of Achilles tendon disorder. A pilot study. Cells Tissues Organs. 1999;165:30–39. doi: 10.1159/000016671. [DOI] [PubMed] [Google Scholar]
- 6.Arnoczky S.P., Tian T., Lavagnino M., Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–333. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 7.Asundi K.R., Rempel D.M. MMP-1, IL-1beta, and COX-2 mRNA expression is modulated by static load in rabbit flexor tendons. Ann Biomed Eng. 2008;36:237–243. doi: 10.1007/s10439-007-9427-2. [DOI] [PubMed] [Google Scholar]
- 8.Legerlotz K., Jones G.C., Screen H.R., Riley G.P. Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes. Scand J Med Sci Sports. 2012 doi: 10.1111/j.1600-0838.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Screen H.R., Shelton J.C., Bader D.L., Lee D.A. Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles. Biochem Biophys Res Commun. 2005;336:424–429. doi: 10.1016/j.bbrc.2005.08.102. [DOI] [PubMed] [Google Scholar]
- 10.Arnoczky S.P., Lavagnino M., Egerbacher M., Caballero O., Gardner K., Shender M.A. Loss of homeostatic strain alters mechanostat “set point” of tendon cells in vitro. Clin Orthop Relat Res. 2008;466:1583–1591. doi: 10.1007/s11999-008-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Screen H.R., Chhaya V.H., Greenwald S.E., Bader D.L., Lee D.A., Shelton J.C. The influence of swelling and matrix degradation on the microstructural integrity of tendon. Acta Biomater. 2006;2:505–513. doi: 10.1016/j.actbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Hannafin J.A., Arnoczky S.P. Effect of cyclic and static tensile loading on water content and solute diffusion in canine flexor tendons: an in vitro study. J Orthop Res. 1994;12:350–356. doi: 10.1002/jor.1100120307. [DOI] [PubMed] [Google Scholar]
- 13.Cribb A.M., Scott J.E. Tendon response to tensile stress: an ultrastructural investigation of collagen:proteoglycan interactions in stressed tendon. J Anat. 1995;187(Pt 2):423–428. [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson R.B., Hassenkam T., Hansen P., Kjaer M., Magnusson S.P. Tensile force transmission in human patellar tendon fascicles is not mediated by glycosaminoglycans. Connect Tissue Res. 2011;52:415–421. doi: 10.3109/03008207.2010.551569. [DOI] [PubMed] [Google Scholar]
- 15.Lujan T.J., Underwood C.J., Henninger H.B., Thompson B.M., Weiss J.A. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res. 2007;25:894–903. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- 16.Fessel G., Snedeker J.G. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009;28:503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Abreu E.L., Leigh D., Derwin K.A. Effect of altered mechanical load conditions on the structure and function of cultured tendon fascicles. J Orthop Res. 2008;26:364–373. doi: 10.1002/jor.20520. [DOI] [PubMed] [Google Scholar]
- 18.Screen HR. The contribution of structural components to tendon mechanics. PhD thesis. London: Queen Mary University of London; 2003.
- 19.Legerlotz K., Riley G.P., Screen H.R. Specimen dimensions influence the measurement of material properties in tendon fascicles. J Biomech. 2010;43:2274–2280. doi: 10.1016/j.jbiomech.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koob T.J. Effects of chondroitinase-ABC on proteoglycans and swelling properties of fibrocartilage in bovine flexor tendon. J Orthop Res. 1989;7:219–227. doi: 10.1002/jor.1100070209. [DOI] [PubMed] [Google Scholar]
- 21.Screen H.R., Shelton J.C., Chhaya V.H., Kayser M.V., Bader D.L., Lee D.A. The influence of noncollagenous matrix components on the micromechanical environment of tendon fascicles. Ann Biomed Eng. 2005;33:1090–1099. doi: 10.1007/s10439-005-5777-9. [DOI] [PubMed] [Google Scholar]
- 22.Rigby B.J., Hirai N., Spikes J.D., Eyring H. The mechanical properties of rat tail tendon. J Gen Physiol. 1959;43:265–283. doi: 10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C.Y., Wang V.M., Flatow E.L., Mow V.C. Temperature-dependent viscoelastic properties of the human supraspinatus tendon. J Biomech. 2009;42:546–549. doi: 10.1016/j.jbiomech.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duenwald S.E., Vanderby R., Jr., Lakes R.S. Viscoelastic relaxation and recovery of tendon. Ann Biomed Eng. 2009;37:1131–1140. doi: 10.1007/s10439-009-9687-0. [DOI] [PubMed] [Google Scholar]
- 25.Screen H.R., Toorani S., Shelton J.C. Microstructural stress relaxation mechanics in functionally different tendons. Med Eng Phys. 2012 doi: 10.1016/j.medengphy.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd J.H., Demirci T., Legerlotz K., Riley G.P., Screen H.R. British Orthopaedic Research Society; London, UK: 2012. A comparison of the creep and stress relaxation behaviour of functionally distinct bovine tendons. p. 28. [Google Scholar]
- 27.Graf B.K., Vanderby R., Jr., Ulm M.J., Rogalski R.P., Thielke R.J. Effect of preconditioning on the viscoelastic response of primate patellar tendon. Arthroscopy. 1994;10:90–96. doi: 10.1016/s0749-8063(05)80298-1. [DOI] [PubMed] [Google Scholar]
- 28.Miller K.S., Edelstein L., Connizzo B.K., Soslowsky L.J. Effect of preconditioning and stress relaxation on local collagen fiber re-alignment: inhomogeneous properties of rat supraspinatus tendon. J Biomech Eng. 2012;134:031007. doi: 10.1115/1.4006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Screen H.R.C., Seto J., Krauss S., Boesecke P., Gupta H.S. Extrafibrillar diffusion and intrafibrillar swelling at the nanoscale are associated with stress relaxation in the soft collagenous matrix tissue of tendons. Soft Matter. 2011;7:11243–11251. [Google Scholar]
- 30.Helmer K.G., Nair G., Cannella M., Grigg P. Water movement in tendon in response to a repeated static tensile load using one-dimensional magnetic resonance imaging. J Biomech Eng. 2006;128:733–741. doi: 10.1115/1.2244573. [DOI] [PubMed] [Google Scholar]
- 31.Haut T.L., Haut R.C. The state of tissue hydration determines the strain-rate-sensitive stiffness of human patellar tendon. J Biomech. 1997;30:79–81. doi: 10.1016/s0021-9290(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 32.Haraldsson B.T., Aagaard P., Krogsgaard M., Alkjaer T., Kjaer M., Magnusson S.P. Region-specific mechanical properties of the human patella tendon. J Appl Physiol. 2005;98:1006–1012. doi: 10.1152/japplphysiol.00482.2004. [DOI] [PubMed] [Google Scholar]


