Abstract
Objective:
The T helper 17 (Th17) cells have been suggested to have osteoclast activating effects while T helper 2 (Th2) cells are considered to have an osteoprotective role in periodontitis.
This study was to compare the markers of Th17 cells (RORC2 and IL-17 genes) with that of Th2 cells (IL-4 and GATA-3 genes) between healthy and periodontitis tissues.
Materials and Methods:
The samples were obtained from patients with periodontitis and healthy tissues. The mRNA expression levels of IL-17, RORC2, IL-4 and GATA-3 were measured in both groups using quantitative RT-PCR. The results were compared using SPSS 11.0. Correlation coefficient was analyzed by Spearman’s rho test. Mann-Whitney was used to measure the difference between IL-17 and IL-4 as well as RORC2 and GATA-3.
Results:
In periodontal lesions, the expression levels of all markers were significantly higher than the healthy tissue (p≤ 0.001). The results showed a significant increase in the number of markers of Th17 (RORC2 and IL-17 genes) compared to markers of Th2 (GATA-3, IL-4) in patients with periodontitis compared to controls (p≤0.002). A positive correlation between IL-17 and RORC2 (p≤0.05) and between IL-4 and GATA-3 (p≤0.001) was found.
Conclusion:
The results show that expression levels of IL-4, GATA-3, IL-17 and RORC2 increase significantly in periodontal lesions compared with the controls. In periodontal lesions, IL-17 levels are significantly greater than IL-4, which plays a protective role against alveolar bone loss.
Keywords: TH17 cell, TH2 cell, IL-17F protein, GATA3 factors, Periodontal Disease
INTRODUCTION
Periodontitis is an infectious inflammatory disease characterized by infiltration of considerable numbers of lymphocytes into periodontal lesions which leads to secretion of different cytokines and eventual destruction of the periodontal tissue as well as alveolar bone loss [1, 2]. Some forms of periodontal disease including gingivitis remain stable for years. The supporting tissue of the teeth and bone remain intact and inflammation is limited to gingival tissue. Other forms of periodontal disease progress to periodontitis with consequent loss of periodontal attachment, bone and finally tooth loss--a process that is inevitable even with treatment [3]. In recent years, several surveys have been conducted to identify the cause of this progress. Although periodontal bacteria are the main causative agents in periodontitis, host immune response is responsible for progression into severe forms of this disease [2, 4, 5]. T-cells play an important role in the immune system. T-helpers originate from naïve cells that differentiate to Thelper 1 (Th1) in the presence of antigen presenting cells and IL-12, and to T helper 2 (Th2) under the influence of IL-4. These cells are involved in the cellular and humoral immunity, respectively [6]. Past studies have shown that Th1 are the dominant cells in gingivitis, while Th2 cells are the dominant in periodontitis. This transition from efficient cellular immunity to impaired humoral immunity is called the Th1/Th2 paradigm [7, 8]. More recently, contradictory findings about this paradigm have emerged in the literature. Some studies demonstrate high Th1 activity in periodontitis compared to Th2; other studies show an equal presence of both these T-cell groups in advanced periodontitis lesions. These discrepancies made the understanding of the pathogenesis of periodontitis nearly impossible until a third group of T-cells (that were CD4+) were recognized that had an established role in inflammatory and immune disorders. Their main interleukin is IL-17, accordingly they are known as “T helper-17” (Th17). Discovery of these cells has brought about a radical revision in the Th1/Th2 paradigm [1, 9–12].
There are solid evidences that agree with the importance of IL-17 in inflammation and autoimmunity [1, 12]. Moreover, a remarkable increase in IL-17 has been reported in periodontitis tissues of animal models [13]. Although IL-17 has a vital role in protecting the body from extracellular gram negative and fungal pathogens, Th17 cells are considered to have harmful roles for developing autoimmune and inflammatory responses [13]. Increased expression of IL-17 is evident in human autoimmune diseases such as multiple sclerosis, rheumatoid arthritis and psoriasis [14].
Some studies show moderate to high levels of IL-17 in periodontal lesions which is the main cytokine secreted by Th17. This cytokine can affect the bone loss process both locally and systematically. Some studies reported that Th17 cells have a key role in osteoclast formation in the bone marrow cell culture environment which suggests the bone destructive role of these cells. On the other hand these studies demonstrated an inhibitory effect for IL-4 in bone destruction mentioning it as a protective interleukin against bone loss [15, 16]. However, it should be considered that other cells such as natural killer (NK) cells and neutrophils can secrete IL-17 as well. Hence, it is difficult to say that increasing levels of IL-17 alone are equal to more infiltration of Th17 cells into the tissue [17]. Regarding Th17 cell development, retinoic acid-related orphan nuclear receptor-C2 (RORC2) is the specific and key transcription factor that orchestrates Th17 cell differentiation. Over-expression of RORC2 induces IL-17 production by these cells [18–26].
Th2 cells are known as the main leaders of humoral immune responses, especially against parasitic infections. They are also known as the cells which prevent and attenuate inflammatory responses of Th1 cells. It has been suggested that Th2 cells have a protective role in the immune balance of periodontitis as well as protecting effects against bone loss by inhibiting osteoclast activity [15, 16]. It has been established that the transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression making this gene an excellent marker for the Th2 cell assessment [27]. In this study, we compared the expression levels of main characteristic factors of both Th17 and Th2 between periodontitis and healthy tissue.
MATERIALS AND METHODS
Patients and biopsies
In this case-control study, 27 patients with generalized moderate to severe chronic periodontitis were chosen as the case group. They were randomly selected from the Dental School of Isfahan University of Medical Sciences.
The control group included 26 orthodontic patients with normal gingival tissue. The study was completely clarified for subjects and informed consent was signed by them. The patients were selected on the basis of loss of attachment equal or greater than 3 mm in clinical examination and radiographic findings indicating bone loss. Patients with systemic disorders with affected immune systems were excluded. After primary periodontal treatment by oral hygiene instruction (OHI) and scaling-root planning (SRP), the interdental papilla was used from areas with positive bleeding on probing (BOP) for sampling during the flap surgery. Samples were obtained one month after SRP.
Gingival tissues were obtained by making reverse bevel incisions from the interproximal papilla that contained complete sulcular epithelium, junctional epithelium and connective tissue. Healthy tissue samples were obtained from extracted tooth sites.
The samples were immediately placed in autoclaved DNase free microtubes containing RNAlater solution.
RNA isolation and real-time quantitative PCR
The tissue was excised finely into small pieces using a needle, then placed in RNAlater solution and kept in the refrigerator. Total RNA was extracted using RNeasy mini kit (Qiagen, Milan, Italy) and cDNA was synthesized by QuantiTect reverse transcription kit (Qiagen Milan, Italy) as instructed by the manufacturer. The resulting transcripts were then quantified by real time quantitative PCR on a Rotor-Gene 6000 real time DNA amplification system (Corbett Life Science, Sydney, Australia) with QuantiFast SYBR Green PCR kit (Qiagen, Valencia, CA, USA). Pre-designed primers (QuantiTectPrimer Assay; Qiagen, Valencia, CA, USA) specific for RORC2, IL-17, GATA-3 and IL-4 genes were used.
For each sample, transcript quantity was normalized to the amount of beta-actin (ACTB) expression. First, the PCR reaction was carried out at 95°C for 5 minutes followed by 40 cycles of denature time at 95°C for 10 seconds and a combined anneling/extention step at 60°C for 30 seconds. The results were then analyzed using 2−ΔΔct method.
Statistical Analysis
Clinical and experimental data were analyzed using SPSS 11.0 for Windows (SPSS Inc., Chicago, Illinois, USA). The difference among the mRNA expression of the analyzed transcription factors and cytokines were determined analyzing the ΔCt data and using Mann-Whitney U test.
Correlation coefficient was obtained analyzing the RQ data and using Spearman’s rho test. A statistically significant difference was considered when p-value≤ 0.05.
RESULT
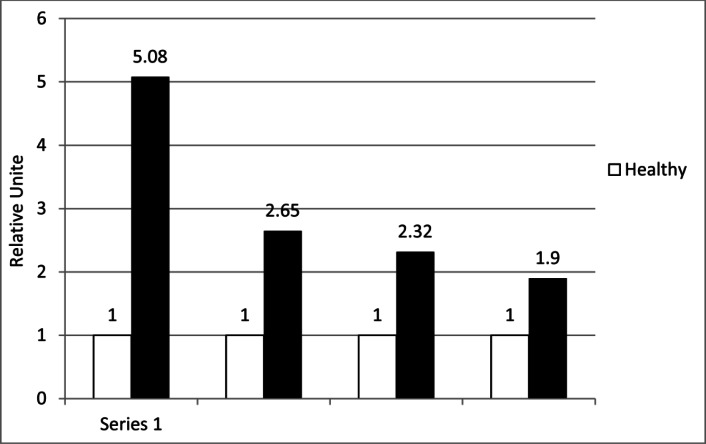
A total of 53 participants (27 cases and 26 control patients) with a mean age of 40.1± 8.6 completed the study. The expression of IL-17, RORC2, IL-4, and GATA-3 was detectable in all case and control samples. Different levels of these markers were detectable in tissue samples. However, the mean mRNA level of IL-17 in the case group was approximately 5.08 fold greater than that of the control group demonstrating a significant difference (p≤0.001)(Table 1). The mean mRNA level of IL-4 in the case group was about 2.56 fold greater than that of the normal control samples (p≤0.001) (Fig 1). The difference between the increase of these two markers in periodontal lesion was significant (p≤ 0.002) (Fig 1). IL-17 was the highest over-expressed cytokine detected within periodontal lesion within RQ of 5.08 (Fig 1). The expression of RORC2 was 2.35 fold higher in the periodontitis group compared to the control samples (p≤ 0.005) (Fig 1). The expression of GATA-3 was 1.90 fold higher in the case group compared to the control group, but the difference between the increase of these two markers in periodontal lesion was not significant (p≤0.42) (Fig 1).
Table 1.
Comparison Between the Expression Levels of IL-17, RORC2, IL-4 and GATA-3 in the Periodontitis and Control Group
| Case Group | Control Group | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | (min, max) | Mean | SD | (min, max) | P-Value | |
| IL-17 | 32.18 | 1.88 | (29.51,35.62) | 4.86 | 3.36 | (0.34,14.32) | <0.001 |
| RORC2 | 36.14 | 1.87 | (32.08,39.02) | 1.84 | 1.21 | (0.19,5.83) | <0.001 |
| IL-4 | 36.74 | 2.63 | (33.73,45.00) | 2.65 | 1.90 | (0.02,7.36) | <0.001 |
| GATA-3 | 29.67 | 1.77 | (25.53,33.43) | 1.83 | 1.57 | (0.51,6.22) | <0.001 |
Fig 1.
Gene expressions in periodontal lesions and healthy tissue
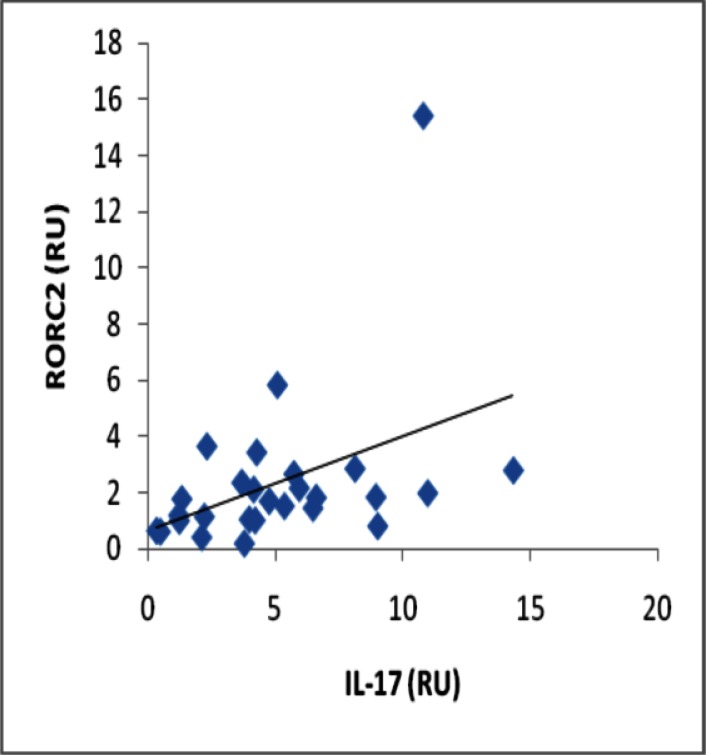
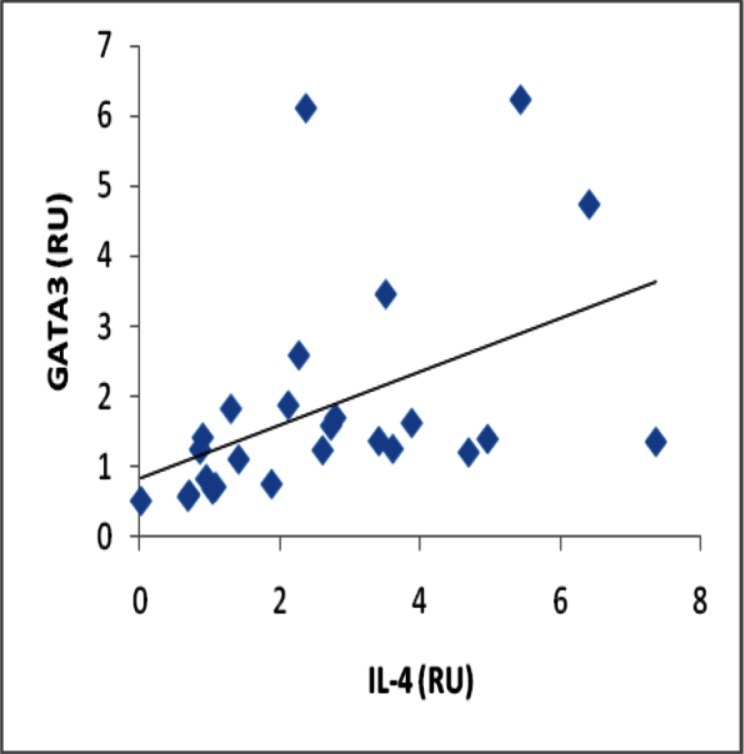
Analyses of correlation between gene expression of IL-17 and RORC2 in periodontal lesion yielded a significant positive correlation (r = 0.508, p≤0.001) (Fig 2), and also a significant positive correlation was observed between gene expression of IL-4 and GATA-3 (r = 0.602, p≤0.001) (Fig 3).
Fig 2.
Correlation between gene expression of IL-17 and RORC2 in the periodontitis affected tissue (p≤0.001)
Fig 3.
Correlation between gene expression of IL-4 and GATA-3 in the periodontitis affected tissue (p≤0.001)
DISCUSSION
This study showed an increased expression of IL-17, RORC2, IL-4 and GATA-3 in periodontal lesions compared with the control sites. The levels of expression of IL-17 are higher than the others. These findings suggest that IL-17 in comparison to IL-4 is more stimulated in periodontal lesions, although additional individuals should be examined to confirm this. Recently, we demonstrated the elevation of this cytokine in the saliva of patients with periodontitis [28].
The existence of different T-lymphocyte population leads to different immune responses during the course of an infection. IL-17 is a pro-inflammatory cytokine that induces other cytokines and tissue-degrading enzymes, including IL-6 and matrix metalloproteinases. This cytokine is also involved in osteoclastogenesis by inducing RANKL expression on osteoblasts [29]. In the present study, we found significantly higher levels of IL-17A expression in periodontal lesions than in the control sites, consistent with previous reports [11, 12]. Moreover, in the present study, we also demonstrated that mRNA expression level of IL-17 was higher than IL-4 at the sites with attachment loss and bone destruction. Several studies implicated an increased level of B cells and plasma cells in periodontitis and a T cell mediated response in the gingivitis lesion. On the basis of traditional Th1/Th2 model conversion of gingivitis to periodontitis involved a switch from T cells to B cells and plasma cells. Some studies have shown an increased expression of Th1 cytokines compared to Th2 cytokines in the periodontitis lesion, [30, 31]. Other studies have shown equal expression of both Th1 and Th2 cytokines in advanced periodontitis [9, 32, 33]. Recently, a new subset of CD4+ T cells which has been named Th17 based on secretion of proinflammatory cytokine IL-17 has been discovered. Some studies indicate that Th17 cytokines participate in periodontitis and chronic periapical lesion and may have a role in immunoregulation of periodontal disease [10–12, 34]. The key transcription factors driving Th17 differentiation are of the orphan nuclear receptor ROR (retinoic acid receptor) [21,35]. Immunohistochemical analysis showed the presence of IL-17 in periodontitis lesion, suggesting a potential role in pathogenesis [10,11, 36]. IL-17 regulates matrix metalloproteinases and inflammatory cytokines in gingival fibroblasts [37], and P. gingivalis can stimulate IL-17 production from T-cells in vitro [38]. Prostaglandin E2 (PGE2), which has been associated with periodontal tissue destruction enhances IL-23 expression and hence may contribute to Th17 development [39, 40].
In this study, the gene expression of interleukins and their transcription factors were obtained by qRT-PCR. As a number of cells other than Th17 are also reported to secret IL-17; using this method to assess RORC2 strengthens the probability of Th17 cells being the main source of IL-17 in our study. There were significant correlations between IL-17 and RORC2 as well as between IL-4 and GATA-3. However, there was no significant difference between RORC2 and GATA-3 in the healthy compared to periodontal tissue.
In 2008, Yamazaki et al. showed that the IL-17 level in periodontitis affected tissue was significantly higher than that of healthy tissue [4]. Accordingly, in 2009, Dutzan et al. indicated that the IL-17 level is remarkably higher in active periodontitis affected tissue compared to its non-active form. They reported the correlation coefficient of 0.96 between IL-17 and RORC2, and the significant correlation coefficient of 0.602 between IL-4 and GATA-3[16]. The findings of all the above studies agree with our results, yet from these studies only the latter was conducted on human subjects.
However, Takahashi et al. showed that the level of the IL-17 protein is moderate in the cultured cell-free environment of periodontitis affected tissue [11]. This different finding could be due to using different methods, as we used qRT-PCR to measure the expression of genes, whereas in the mentioned study, the IL-17 protein was measured by western blot method. In the current paper, the expression level of IL-4 increased significantly in periodontitis compared to healthy tissue which is predictable as many studies consider IL-4 to have protecting effects against bone loss by inhibiting the osteoclast activity[1, 41].
According to Pradeep and Toroko in 2008, the IL-4 concentration in gingival cervicular fluid is decreased significantly in the periodontitis affected areas and suggests that IL-4 has a protecting role for alveolar bone [41]. In 2008, Gaffen showed that mice that were unable to produce Th2 cytokines had more destruction due to P. gingivalis [1].
Sato et al. showed that Th-17 cells are important subsets of CD4+T-cells which have a key role in osteoclast formation in T-cell and bone marrow cell cultured environments, but demonstrate an inhibitory effect for IL-4 [15].
In our study, the relation between IL-4 and GATA-3 was also significant. This relationship seems to be predictable since the Th2 cells are known as the main source of IL-4.
The significant relation between IL-17 and RORC2 is in accordance with the findings of Gaffen et al. and Bettelli et al., where Th17 cells were introduced as the main producers of IL-17 [1, 14]. Although the significant relationship between IL-17 and RORγt (The transcription factor for IL-17 in mice) has been suggested, our study and the study conducted by Dutzan support this relationship in human subjects. In our study, there was no significant difference between RORC2 and GATA-3 in the affected tissues, indicating that the changes in these two genes, which represent different cell lines, are independent.
CONCLUSION
The current study showed an increased expression of IL-17, RORC2, IL-4 and GATA-3 in periodontal lesions compared with control sites. The levels of expression of IL17 are higher than the other measured factors. Moreover, our study revealed a significant increase in IL-17 levels compared to IL-4 in periodontitis tissue. The domination of IL-17 with known inflammatory and alveolar bone destructive effects can lead to more inflammation and higher alveolar bone destruction in periodontal tissues to the extent that IL-4 fails to compensate by its protective effects.
This research is another step toward understanding the unknown immunopathogenesis of periodontitis.
REFERENCES
- 1.Gaffen SL, Hajishengallis G. A New Inflammatory Cytokine on the Block: Re-Thinking Periodontal Disease and the TH1/TH2 Paradigm in the Context of TH17 Cells and IL-17. J Dent Res. 2008 Sep;87(9):817–28. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000 Aug;2(10):1181–92. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- 3.Lindhe J, Lang N, Karring T. Clinical Periodontology and Implant Dentistry. 5th ed. Hong Kong: Blackwell Munksgaard; 2008. [Google Scholar]
- 4.Yamazaki K, Nakjima T, Aoyagi T, Hara K. Immunohistological analysis of memory T lymphocytes and activated B lymphocytes in tissues with periodontal disease. J Periodontal Res. 1993 Sep;28:324–34. doi: 10.1111/j.1600-0765.1993.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 5.Seymour GJ, Gemmell E, Reinhardt RA, Eastcott J, Taubman MA. Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodontal Res. 1993 Nov;28:478–86. doi: 10.1111/j.1600-0765.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 6.Abul-Abbas K, Litchman AH. Basic immunology functions and disorders of the immune system. 2nd ed. 2004. [Google Scholar]
- 7.Gemmell E, Yamazaki K, Seymour GJ. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit Rev Oral Bio Med. 2002;13(1):17–34. doi: 10.1177/154411130201300104. [DOI] [PubMed] [Google Scholar]
- 8.Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32(Suppl 6):87–107. doi: 10.1111/j.1600-051X.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 9.Berglundh T, Liljenberg B, Lindhe J. Some cytokine profiles of T-helper cells in lesions of advanced periodontitis. J Clin Periodontol. 2002 Aug;29(8):705–9. doi: 10.1034/j.1600-051x.2002.290807.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004 Jan;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. The potential role of interleukin-17 in the immunopathology of periodontal disease. J Clin Periodontol. 2005 Apr;32:369–374. doi: 10.1111/j.1600-051X.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 12.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, et al. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005 Apr;32:383–9. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 13.Berglundh T, Liljenberg B, Lindhe J. Some cytokine profiles of T-helper cells in lesions of advanced periodontitis. J Clin Periodontol. 2002 Aug;29(8):705–9. doi: 10.1034/j.1600-051x.2002.290807.x. [DOI] [PubMed] [Google Scholar]
- 14.Bettelli E, Kurn T, Kuchroo V. Th17: The third member of the effector T cell Trilogy. Curr Opin Immunol. 2007 Dec;19(6):652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato K, Takaynagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol. 2006 Jul;18(4):419–26. doi: 10.1097/01.bor.0000231912.24740.a5. [DOI] [PubMed] [Google Scholar]
- 16.Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-k B ligand, interleukin (IL)-17, IL-10 and transforming growth factor-b during the progression of chronic periodontitis. J Clin Periodontol. 2009 May;36:396–403. doi: 10.1111/j.1600-051X.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 17.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004 Oct;21(4):467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Aranami T, Yamamura T. Th17 Cells and Autoimmune Encephalomyelitis (EAE/MS) Allergol Int. 2008 Jun;57(2):115–120. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- 19.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007 Sep;8(9):942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 20.Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007 Feb 1;178(3):1341–8. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006 Sep 22;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007 Mar;26(3):71–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008 Jan;57(2):121–34. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- 24.Streeck H, Cohen KW, Jolin JS, Brockman MA, Meier A, Power KA, et al. Rapid ex vivo isolation and long-term culture of human Th17 cells. J Immunol Methods. 2008 Apr 20;333(1):115–25. doi: 10.1016/j.jim.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007 Sep;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008 Sep 1;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997 May;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 28.Behfarnia P, Birang R, Andalib AR, Asadi SH. Comparative Evaluation of INFγ, IL4 and IL17 Cytokines in Healthy Gingiva and Moderate to Advance Chronic Periodontitis. Dent Res J. 2010;7(2):45–50. [PMC free article] [PubMed] [Google Scholar]
- 29.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999 May;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000 Aug;79:1548–55. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- 31.Ukai T, Mori Y, Onoyama M, Hara Y. Immunohistological study of interferon-gamma- and interleukin-4-bearing cells in human periodontitis gingiva. Arch Oral Biol. 2001 Oct;46:901–8. doi: 10.1016/s0003-9969(01)00057-7. [DOI] [PubMed] [Google Scholar]
- 32.Fujihashi K, Yamamoto M, Hiroi T, Bamberg TV, McGhee JR, Kiyono H. Selected Th1 and Th2 cytokine mRNA expression by CD4(+) T cells isolated from inflamed human gingival tissues. Clin Exp Immunol. 1996 Mar;103:422–8. doi: 10.1111/j.1365-2249.1996.tb08297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhu A, Michalowicz BS, Mathur A. Detection of local and systemic cytokines in adult periodontitis. J Periodontol. 1996 May;67:515–22. doi: 10.1902/jop.1996.67.5.515. [DOI] [PubMed] [Google Scholar]
- 34.Colić M, Vasilijić S, Gazivoda D, Vucević D, Marjanović M, Lukić A. Interleukin-17 plays a role in exacerbation of inflammation within chronic periapical lesions. Eur J Oral Sci. 2007 Aug;115(4):315–20. doi: 10.1111/j.1600-0722.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008 Jan;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lester SR, Bain JL, Johnson RB, Serio FG. Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J Periodontol. 2007 Aug;78:1545–50. doi: 10.1902/jop.2007.060458. [DOI] [PubMed] [Google Scholar]
- 37.Beklen A, Ainola M, Hukkanen M, Gurgan C, Sorsa T, Konttinen YT. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007 Apr;86:347–51. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- 38.Oda T, Yoshie H, Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol. 2003 Feb;18(1):30–6. doi: 10.1034/j.1399-302x.2003.180105.x. [DOI] [PubMed] [Google Scholar]
- 39.Heasman PA, Benn DK, Kelly PJ, Seymour RA, Aitken D. The use of topical flurbiprofen as an adjunct to non-surgical management of periodontal disease. J Clin Periodontol. 1993 Jul;20(6):457–64. doi: 10.1111/j.1600-051x.1993.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 40.Roberts FA, Houston LS, Lukehart SA, Mancl LA, Persson GR, Page RC. Periodontitis vaccine decreases local prostaglandin E2 levels in a primate model. Infect Immun. 2004 Feb;72(2):1166–8. doi: 10.1128/IAI.72.2.1166-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pradeep AR, Roopa Y, Swati PP. Interleukin-4, a T-helper 2 cell cytokine, is associated with the remission of periodontal disease. J Periodontal Res. 2008 Dec;43:712–6. doi: 10.1111/j.1600-0765.2007.01079.x. [DOI] [PubMed] [Google Scholar]