Abstract
Objective:
To investigate and compare the amount of fluoride release of conventional, resin modified and nanofilled resin modified glass ionomer cements.
Materials and Methods:
Tablets of glass-ionomer cements were immersed in deionized water and incubated at 37°C. After 1, 2, 7, 15 and 30 days, fluoride ion was measured under normal atmospheric conditions by fluoride ion selective electrode. Buffer (TISAB II) was used to decomplex the fluoride ion and to provide a constant background ionic strength and to maintain the pH of water between 5.0 and 5.5 as the fluoride electrode is sensitive to changes in pH. Statistical evaluation was carried out by one way ANOVA (Analysis of Variance) using SPSS 11.0. The significance level was set at p< 0.05.
Results:
The release of fluoride was highest on day 1 and there was a sudden fall on day 2 in all three groups. Initially fluoride release from conven-tional glass-ionomer cement was highest compared to the other two glass-ionomer cements, but the amount drastically reduced over the period. Although the amount of fluoride release was less than both the resin modified and nanofilled resin modified glass-ionomer cement, the release was sustained consistently for 30 days
Conclusion:
The cumulative fluoride release of nanofilled resin modified glass ionomer cement was very less compared to the conventional and resin modified glass ionomer cements and Nanofilled resin modified glass ionomer cement released less but steady fluoride as compared to other resin modified glass ionomer cements.
Keywords: Resin- Modified Glass Ionomer, Nano-filled Glass Ionomer, Fluoride
INTRODUCTION
The oral cavity is constantly exposed to demineralization and remineralization. Demineralization results when the pH falls due to release of acids produced by the action of plaque bacteria in the presence of dietary carbohydrates. Remineralization is seen conversely, when the pH rises with the deposition of calcium and phosphate ions1. Continuous demineralization results in the loss of tooth structure. Therefore, the best strategy for caries management is focus on the methods of improving the remineralizing process. Various materials are available that exclusively deliver remineralizing agents. Incorporating fluoride, a remineralizing agent in a restorative material, definitely reduces the occurrence of secondary caries. Fluoride released from restorative materials has an effective zone of about 1 mm from the margin of glass ionomer restorations [2]. Release of fluoride from restorative materials also maintains the fluoride level in the oral fluid. After placement of glass-ionomer restorations, salivary fluoride concentration is approximately 0.3 ppm immediately after and remains up to 0.04 ppm after 1 year [3].
The content of fluoride in glass ionomer restorative materials is about 10 to 23% [4] and a direct relationship was detected between the presence of fluoride in the cement and the amount released [5]. Development in the field of glass-ionomer cement has led to the introduction of nanoionomer which is a nanofilled resin-modified glass-ionomer cement. The present study compared fluoride release among nanoionomer, conventional and resin-modified glass-ionomer cements.
MATERIALS AND METHODS
The present study was performed on conventional glass ionomer (GC Corporation Tokyo, Japan), resin-modified glass-ionomer and nanoionomer (Ketac™ N100).
Conventional glass ionomer cements (GlC) were first introduced in 1972 by Wilson and Kent. They are derived from aqueous polyalkenoic acid such as polyacrylic acid and a glass component that is usually a fluoroaluminosilicate. They are dispensed as powder and liquid which are mixed together to initiate an acid-base reaction. Resin modified glass ionomer cements are conventional glass ionomer cements with the addition of HEMA. (Hydroxyethylmethacrylate)
They undergo both an acid-base ionomer reaction as well as curing by photo-initiation and self-cure of methacrylate carbon double bonds. Ketac™ N100 is a two-part paste system that makes the dispensing and mixing easier and faster. “Paste A is resin-based and contains fluoroaluminosilicate glass, silane-treated silica and zirconia silica nanofillers, methacrylate and dimethacrylate resins, and photoinitiators. Paste B is water-based and contains polyalkenoic acid copolymer, silane-treated zirconia silica nanoclusters, silane-treated silica nanofiller, and hydroxymethylmethacrylate (HEMA). Ketac™ Nano Primer contains water, HEMA, polyalkenoic acid copolymer, and photoinitiators” [6].
Manufacturers claim that the presence of nanofillers in this material has improved esthetics, wear resistance and still provides the benefits of fluoride release [7].
The purpose of the present study was to compare the fluoride release among conventional, resin-modified and nanofilled glass ionomer cements.
Sample preparation
Tablets of three glass ionomer cements (10 for each group) were used for the study. They were prepared from 9×2 mm sized cylindrical brass moulds (Fig 1). A thick transparent sheet was first secured on a flat surface. This provided the base for the mould. Glass ionomer cements were then hand mixed according to the manufacturer’s instructions.
Fig 1.
Cylindrical brass moulds
The mixed material was placed into the mould and covered with a mylar strip. A dental floss was incorporated into the tablets during fabrication to allow suspension into the test medium (Fig 2).
Fig 2.
Dental floss incorporated into the tablets
A glass slide was then placed over the mould. Hand pressure was applied to extrude excess material and to ensure uniform and void free specimen discs.Conventional glass ionomer cement was set in the mould. The other two glass ionomer cements were light cured for 20 seconds from the top.
Then the glass slide was removed and cured from below for 20 secs. The set discs were gently pushed out of the moulds.Each disc specimen was immersed in airtight polyethylene bottle containing 20ml of deionized water and incubated at 37°C and stored for 24 hours.
Determination of fluoride ion release
Fluoride ion measurement was performed at the end of the first, second, seventh, fifteenth and thirtieth day under normal atmospheric conditions by fluoride ion selective electrode (Fig 3) connected to an ion selective electrode meter (ORION 940900) after calibration with 2.5, 5 and 10 part per million of standard fluoride solution.
Fig 3.
Fluoride ion selective electrode connected to an ion selective electrode meter
At the end of the first day, each specimen was removed from the bottle, washed with 1ml of deionized water, dried with absorbent paper and then restored in a new 20 ml of fresh deionized water and reincubated. 20 ml of the previous solution from the plastic bottle and 1ml used for washing the disc was mixed with an equal amount of total ionic strength adjustment buffer (TISAB II).
This was then transferred to a plastic vial into which a magnetic stirrer was placed. The electrode was dipped into the solution and fluoride concentration was recorded in parts per million. (TISAB II) is used to decomplex the fluoride ion, to provide a constant background ionic strength and to hold the pH of water between 5.0 and 5.5 as the fluoride electrode is sensitive to changes in pH [4].
TISAB II is prepared by adding 4 gms of CDTA (cyclohexylenedinitrilo tetra acetate) to 57 ml of glacial acetic acid and 58 gms of sodium chloride and dissolving this in 500 ml of double distilled water. The pH of the solution is adjusted between 5.3 and 5.5 by slowly adding 130 ml of 6N sodium hydroxide.
Each constituent plays an important role in controlling the ionic strength and pH of the analytic solution that may otherwise cause error and inaccuracy.
Similar steps were followed for the 2nd, 7th, 15th and 30th day and the electrode was recalibrated with 2.5, 5 and 10 ppm of standard fluoride solution for each day.
Statistical analysis
Statistical evaluation was carried out using one way ANOVA (Analysis of Variance) using SPSS 11.0 for Windows (SPSS Inc., Ill, Chicago, USA) at the level of significance of p< 0.05.
RESULT
In this study, we compared fluoride release among three glass ionomer cements. The means and standard deviations of fluoride release (in ppm) of each group on different days are demonstrated in Table 1 and Table 2 gives the intergroup comparison of fluoride release.
Table 1.
Means and Standard Deviations of Fluoride Release (in ppm) by Tested Glass Ionomer Cements
| Day | Conventional Glass Ionomer | Resin modified Glass Ionomer | Nanoionomer |
|---|---|---|---|
| 1 | 3.38 ± 0.42 | 2.88 ±0.49 | 1.76 ±0.23 |
| 2 | 0.88 ± 0.18 | 0.77 ±0.19 | 0.65 ±0.07 |
| 7 | 2.51± 0.37 | 2.06±0.18 | 1.74 ±0.29 |
| 15 | 1.42±0.16 | 2.02 ±0.41 | 1.27±0.14 |
| 30 | 1.85±0.1 | 2.19±0.15 | 1.36±0.15 |
Level of Significance: <0.05
Table 2.
Intergroup Comparison of Fluoride Release
| Group B (Resin Modified GIC) | Group C (Nanofilled Resin Modified GIC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1st Day | 2nd Day | 7th Day | 15th Day | 30th Day | 1st Day | 2nd Day | 7th Day | 15th Day | 30th Day | |
| Group A (Conventional GIC) | p=0.024 | p=0.247 | p=0.005 | p=0000. | p=0.005 | p=0.000 | p=0.007 | p=0.000 | p=0.370 | p=0.000 |
| Group B | p=0.000 | p=0.220 | p=0.048 | p=0.000 | p=0.000 | |||||
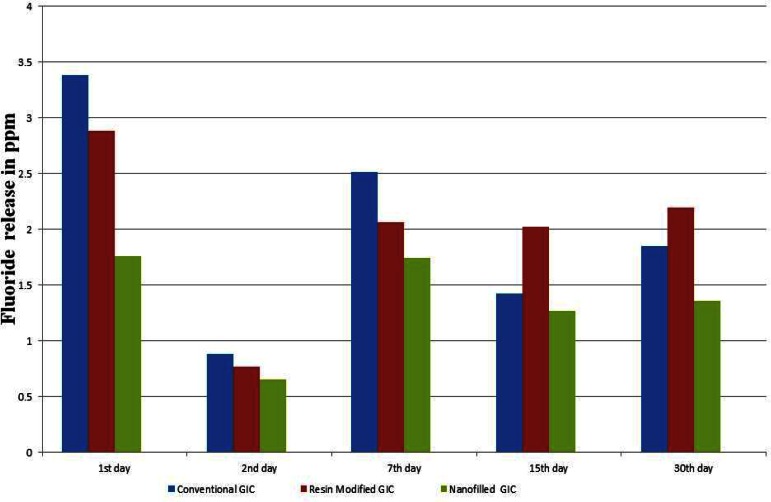
Fluoride release was highest on day 1 and there was a sudden fall on day 2 in all three groups. Fluoride release later increased up to day seven following which there was a gradual decrease (Fig 4).
Fig 4.
Fluoride release from three glass ionomer cements on different days
On the first day, fluoride release from conventional glass ionomer cement was highest compared to the other two glass ionomer cements (p< 0.05), but the amount of release of fluoride drastically reduced over the period. On the contrary, the resin modified and nanoionomer materials released less amounts on the first day, but they continued to release fluoride consistently for 30 days.For evaluation of the cumulative fluoride release, both the conventional and resin modified glass ionomer cements released significantly higher fluoride than the nanoionomer cement (p< 0.05).
DISCUSSION
Fluoride release from a GIC is a complex process and the released amount depends on various factors. Intrinsic factors that may affect fluoride release include formulation, solubility or porosity of the material [8].
The lower the pH, the higher the fluoride release [10].
The higher the environmental temperature, the greater the fluoride release11. Other factors such as an improper powder liquid ratio, improper mixing and improper curing also affects fluoride release [12–14].
El Mallakh and Sarkar NK [9] found that fluoride release of glass ionomer cement was higher in distilled water as compared to artificial saliva. pH of the media also affects the fluoride release.
All glass ionomer cements evaluated in this study released measurable amounts of fluoride which was highest on the first day after which the release reached to a low steady level. This is a normal feature of glass ionomer cements and is called as “Burst Effect” [15–17].
Perrin et al. [18] reported that the greatest release of fluoride from glass ionomer occurred on the first day followed by a sharp decrease on the second day, and gradually diminished over 3 weeks to a low-level, long-term release.
The burst effect is probably associated with the release of fluoride which is loosely bound in the glass ionomer cement and originates from the initial acid-base reaction between the glass and polyalkenoic acid [19]. The later gradual release results from a balance between erosive leaching of glass particles in the bulk of the cement and diffusion of the leached fluoride through the cement matrix.
The burst effect of fluoride release is important for remineralization as well as for the reduction of viability of bacteria that may have been left in the inner carious dentine [20].
According to De Araujo FB et al. [21] and Diaz Arnold et al. [22] resin modified glass ionomer cements release less fluoride initially compared to the conventional glass ionomer cement. In the present study, cumulative fluoride release from conventional and resin modified glass ionomer cements were almost similar. This was in agreement with the study by Mitra SB [23].
In accordance with the present study Paschoal et al .[24] also observed that nanoparticulated glass ionomer cement releases less but steady release of fluoride as compared to other resin modified glass ionomer cements. Fluoride reduces the translucency of the material and thus the manufacturers may have compromised on the initial amount of fluoride added to improve the esthetic property of the material. This may be the reason for low release of fluoride by the nanoionomer cement.
The minimum amount of fluoride that is necessary for preventing or arresting a carious lesion has not been well established [25]. Thus it is difficult to conclude whether the lesser amount of fluoride released by nanoionomer may be adequate or not as an anticaries agent. A long term clinical trial would be a step further in establishing the anticaries effect of nanoionomer.
CONCLUSION
The cumulative fluoride release of nanofilled resin modified glass ionomer cement was very less compared to the conventional and resin modified glass ionomer cements which were almost similar to each other.
Nanofilled resin modified glass ionomer cement released less but steady fluoride as compared to other resin modified glass ionomer cements.
REFERENCES
- 1.Margeas RC. Remineralization with a unique delivery system. Inside Dentistry. 2006;2(4):863. [Google Scholar]
- 2.Tantbirojn D, Douglas WH, Versluis A. Inhibitive effect of resin-modified glass ionomer cement on remote artificial caries. Caries Res. 1997;31(4):275–80. doi: 10.1159/000262411. [DOI] [PubMed] [Google Scholar]
- 3.Hatibovic-Kofman S, Koch G. Fluoride release from glass ionomer cement in vivo and in vitro. Swed Dent J. 1991;15(6):253–8. [PubMed] [Google Scholar]
- 4.Markovic DLj, Petrovic BB, Peric TO. Fluoride content and recharge ability of five glassionomer dental materials. BMC Oral Health. 2008 Jul;8:21. doi: 10.1186/1472-6831-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francci C, Deaton TG, Arnold RR, Swift EJ, Perdigao J, Bawden JW, et al. Fluoride release from restorative materials and its effects on dentin demineralization. J Dent Res. 1999;78:1647–54. doi: 10.1177/00220345990780101001. [DOI] [PubMed] [Google Scholar]
- 6.Croll TP, Berg J. Nano-ionomer restorative cement: Observations after 2 years of use. Inside Dent. 2009 Jan;5(1):60–7. [Google Scholar]
- 7.http://solutions.3m.com/wps/portal/3M/en_US/3M-ESPE-NA/dentalprofessionals/products/espe-catalog/~/Ketac-Nano-Light-Curing-Glass-Ionomer-Restorative
- 8.DeSchepper EJ, Berr EA, 3rd, Cailleteau JG, Tate WH. A comparative study of fluoride release from glass-ionomer cements. Quintessence Int. 1991 Mar;22(3):215–9. [PubMed] [Google Scholar]
- 9.el Mallakh BF, Sarkar NK. Fluoride release from glass ionomer cements in water and artificial saliva. Dent Mater. 1990 Apr;6(2):188–22. doi: 10.1016/s0109-5641(05)80041-7. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho AS, Cury JA.Fluoride release from some dental materials in different solutions Oper Dent 1999January–Feb24114–9. [PubMed] [Google Scholar]
- 11.Yan Z, Sidhu SK, Mahmoud GA, Carrick TE, McCabe JF.Effects of temperature on the fluoride release and recharging ability of glass ionomers Oper Dent 2007March–Apr322138–43. [DOI] [PubMed] [Google Scholar]
- 12.McKnight-Hanes C, Whitford GM. Fluoride release from three glass ionomer materials and the effect of varnishing with or without finishing. Caries Res. 1992;26(5):345–50. doi: 10.1159/000261466. [DOI] [PubMed] [Google Scholar]
- 13.Miller BH, Komatsu H, Nakajima H, Okabe T. Effect of glass ionomer manipulation on early fluoride release. Am J Dent. 1995 Aug;8(4):182–6. [PubMed] [Google Scholar]
- 14.Ulukapi H, Benderli Y, Soyman M. Determination of fluoride release from light cured glass ionomer and fluoridated composite resin from view point of curing time. J Oral Rehabil. 1996 Mar;23(3):197–201. doi: 10.1111/j.1365-2842.1996.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 15.Suljak JP, Hatibovic-Kofman S. A fluoride release-absorption-release system applied to fluoride-releasing restorative materials. Quintessence Int. 1996 Sep;27(9):635–8. [PubMed] [Google Scholar]
- 16.Weidlich P, Miranda LA, Maltz M, Samuel SM. Fluoride release and uptake from glass ionomer cements and composite resins. Braz Dent J. 2000;11(2):89–96. [PubMed] [Google Scholar]
- 17.Vermeersch G, Leloup G, Vreven J. Fluoride release from glass ionomer cements, compomers and resin composite. J Oral Rehabil. 2001 Jan;28(1):26–32. doi: 10.1046/j.1365-2842.2001.00635.x. [DOI] [PubMed] [Google Scholar]
- 18.Perrin C, Persin M, Sarrazin J. A comparison of fluoride release from four glass ionomer cements. Quintessence Int. 1994 Sep;25(9):603–8. [PubMed] [Google Scholar]
- 19.Verbeeck RM, De Maeyer EA, Marks LA, Moor RJ, De Witte AM, Trimpeneers LM. Fluoride release process of resin modified glass ionomer cements versus polyacid modified composite resins. Biomaterials. 1998 Mar;19(6):509–19. doi: 10.1016/s0142-9612(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 20.Forsten L. Fluoride release and uptake by glass ionomer and related materials and its clinical effect. Biomaterials. 1998 Mar;19(6):503–8. doi: 10.1016/s0142-9612(97)00130-0. [DOI] [PubMed] [Google Scholar]
- 21.de Araujo FB, Garcia-Godoy F, Cury JA, Conceicao EN.Fluoride release from fluoride containing materials Oper Dent 1996September–Oct215185–190. [PubMed] [Google Scholar]
- 22.Diaz-Arnold AM, Wistrom DW, Swift EJ., Jr Topical fluoride and glass ionomer microhardness. Am J Dent. 1995 Jun;8(3):134–6. [PubMed] [Google Scholar]
- 23.Mitra SB. In vitro fluoride release from a light-cured glass-ionomer liner/base. J Dent Res. 1991 Jan;70(1):75–8. doi: 10.1177/00220345910700011301. [DOI] [PubMed] [Google Scholar]
- 24.Paschoal MA, Gurgel CV, Rios D, Magalhaes AC, Buzalaf MA, Machado MA. Fluoride release profile of a nanofilled resin-modified glass ionomer cement. Braz Dent J. 2011;22(4):276–9. doi: 10.1590/s0103-64402011000400002. [DOI] [PubMed] [Google Scholar]
- 25.Mousavinasab SM, Meyers I. Fluoride release and uptake by glass ionomer cements, compomers and giomers. Res J Biol Sci. 2009;4(5):609–16. [PMC free article] [PubMed] [Google Scholar]