Abstract
Diets rich in broccoli (Brassica oleracea var italica) have been associated with maintenance of cardiovascular health and reduction in risk of cancer. These health benefits have been attributed to glucoraphanin that specifically accumulates in broccoli. The development of broccoli with enhanced concentrations of glucoraphanin may deliver greater health benefits.
Three high-glucoraphanin F1 broccoli hybrids were developed in independent programmes through genome introgression from the wild species Brassica villosa. Glucoraphanin and other metabolites were quantified in experimental field trials. Global SNP analyses quantified the differential extent of B. villosa introgression
The high-glucoraphanin broccoli hybrids contained 2.5–3 times the glucoraphanin content of standard hybrids due to enhanced sulphate assimilation and modifications in sulphur partitioning between sulphur-containing metabolites. All of the high-glucoraphanin hybrids possessed an introgressed B. villosa segment which contained a B. villosa Myb28 allele. Myb28 expression was increased in all of the high-glucoraphanin hybrids. Two high-glucoraphanin hybrids have been commercialised as Beneforté® broccoli.
The study illustrates the translation of research on glucosinolate genetics from Arabidopsis to broccoli, the use of wild Brassica species to develop cultivars with potential consumer benefits, and the development of cultivars with contrasting concentrations of glucoraphanin for use in blinded human intervention studies.
Keywords: Beneforté® broccoli, Brassica villosa, glucoraphanin, glucosinolates, Myb28, S-methylcysteine sulphoxide, sulforaphane, sulphate assimilation
Introduction
Epidemiological studies have associated diets rich in cruciferous vegetables such as heading broccoli or calabrese (Brassica oleracea L. var italica Plenck) with reduced incidence of myocardial infarction (Cornelis et al., 2007), cardiovascular related mortality (Zhang et al., 2011) and reduced incidence or progression of various cancers, including lung, bowel, kidney, breast and prostate (Seow et al., 2002; Hsu et al., 2007; Kirsh et al., 2007; Lam et al., 2010; Bosetti et al., 2012). Significant levels of protection are most frequently observed in people that consume several portions per week, which is typical of traditional diets in parts of Asia, but is atypical of western diets (Davis et al., 1993; Seow et al., 1998). Cell and animal studies have provided evidence that degradation products of glucosinolates (sulphur-containing glycosides that specifically accumulate within these vegetables, Fig. 1) can mediate these health benefits (Juge et al., 2007). Despite the evidence from both epidemiological and model systems, there have been relatively few dietary intervention studies in humans to provide experimental evidence for the health-promoting activity of cruciferous vegetables, and the potential involvement of glucosinolates in mediating these effects. To facilitate these studies, we have sought to develop broccoli F1 hybrids that have enhanced concentrations of 4-methylsulphinylbutyl glucosinolate, commonly known as glucoraphanin. This glucosinolate is converted to the isothiocyanate sulforaphane, either due to the action of plant thioglycosidases (myrosinases) upon tissue disruption or, if cooking has denatured myrosinases, due to the action of bacterial enzymes within the gastro-intestinal tract. Sulforaphane has been shown in many cell and animal studies to have potentially health-promoting activities (Juge et al., 2007).
Fig. 1.
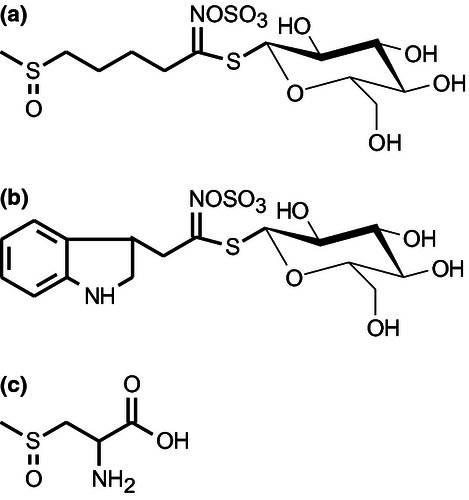
(a) 4-Methylsulphinylbutyl glucosinolate (glucoraphanin) derived from methionine and the precursor of isothiocyanate sulforaphane. (b) 3-Indolylmethyl glucosinolate derived from tryptophan. Tryptophan-derived glucosinolates do not form stable isothiocyanates upon hydrolysis. (c) S-Methylcysteine sulphoxide which is thermally degraded to several volatile sulphur-containing compounds upon cooking which are largely responsible for the ‘sulphurous’ odour of cooked Brassica vegetables.
We previously described the enhanced concentration of glucoraphanin in hybrids between heading broccoli and the wild species B. villosa compared to either parent (Faulkner et al., 1998). A subsequent study reported the mapping of QTLs in segregating backcross populations derived from these F1 hybrids, the identification of a major QTL on linkage group 2 that determined the concentrations of methionine-derived glucosinolates, and the development of the breeding line 428-11-69 (Mithen et al., 2003). In this paper, we describe the use of 428-11-69 to develop three high-glucoraphanin broccoli hybrids (including two Beneforté hybrids) in three independent breeding programmes, and demonstrate the robustness of the high-glucoraphanin phenotype through an extensive series of experimental field studies.
Glucosinolates are sulphur-rich compounds, and cruciferous vegetables such as broccoli require sufficient sulphate supply to ensure yield and quality. Despite the agronomic importance of sulphur, previous studies have not defined the proportion of sulphur within glucosinolates and the other major sulphur-containing metabolites – cysteine and methionine amino acids, glutathione, sulphate (which accumulates in vacuoles) and S-methyl cysteine sulphoxide (SMCSO) (Figs 1, 2). The latter metabolite is thermally degraded upon cooking to produce several volatile S-containing compounds which are the major contributors to the flavour of cooked Brassica vegetables, and may cause ‘sulphurous’ off-flavours (Stoewsand, 1995). However, as with glucosinolate degradation products, SMCSO has also been associated with health-promoting activities (Komatsu et al., 1998; Xiao & Parkin, 2002; Helen et al., 2003). In addition, and also in a similar manner to glucosinolates, high concentrations of SMCSO in fodder Brassica can reduce palatability to livestock and cause toxicity (Stoewsand, 1995). Thus, we quantify sulphur partitioning into glucosinolates, SMCSO and other major S-containing metabolites and explore whether the high-glucoraphanin trait is due to re-partitioning of sulphur between these S pools, or is due to additional sulphur assimilation. Through comparative mapping we identify a common introgressed segment coincident with our previously described QTL that regulates glucosinolate accumulation in each of the three F1 hybrids. Through comparative genomics, we identified the presence of a B. villosa allele of the Myb28 transcription factor, an important regulator of sulphate assimilation and methionine-derived glucosinolate biosynthesis (Gigolashvili et al., 2007; Sonderby et al., 2007), that had been transferred into each of the high-glucoraphanin hybrids within this introgressed segment, and describe its expression in field grown broccoli.
Fig. 2.
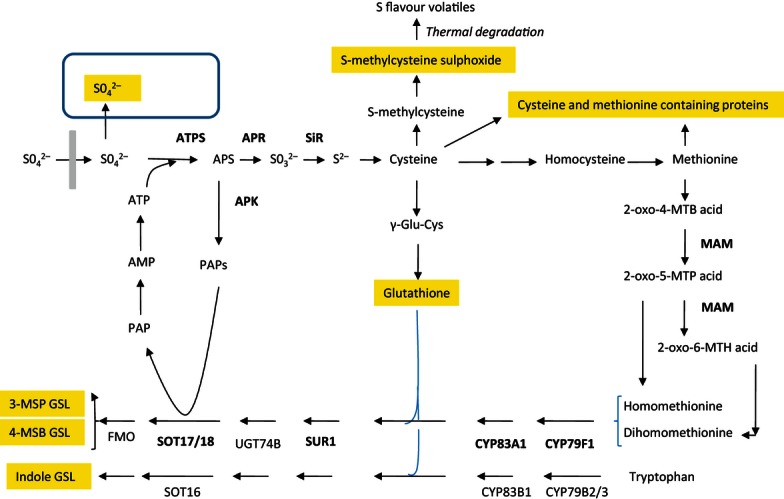
Summary of sulphur metabolism in broccoli. Sulphate, S-methyl cysteine sulphoxide, cysteine and methionine amino acids within proteins, glutathione and methionine-derived and tryptophan-derived glucosinolates are the major sulphur-containing metabolites. Sulphur pools are shown in yellow. ATPS, Adenosine triphosphate-sulphurylase; APR, APS reductase; APK, APS kinase; SiR, sulfite reductase; MAM, methylthioalkylmalate synthase; CYP, cytochrome P-450; SUR1, superroot 1; UGT, S-glycosyltransferase; SOT, Sulfotransferase; FMO, flavin-monooxygenase. Genes in bold have been shown to be upregulated by Myb28 in Arabidopsis (details in text)
Materials and Methods
Development of high-glucoraphanin broccoli F1 hybrids
The broccoli (Brassica oleracea L. var italic Plenck) breeding line 428-11-69 (Mithen et al., 2003), derived from a cross between a double haploid broccoli breeding line and B. villosa Biv. (Fig. 3), was used in breeding programmes in the USA, the Netherlands and the UK. Within each programme a series of 3–5 further backcrosses and inbreeding was undertaken combined with selection for the high-glucosinolate trait, agronomic performance and quality. Breeding lines were not exchanged during the separate programmes. The high-glucoraphanin F1 hybrids from the US and Netherlands programme – identified as 1639 and 1199, respectively – are commercialized as Beneforté® broccoli, whereas an experimental high-glucoraphanin F1 hybrid from the UK programme, identified as HG1, has been used in human intervention studies (Gasper et al., 2005; Traka et al., 2008). Beneforté® is a registered trademark of Seminis Vegetable Seeds, Inc.
Fig. 3.
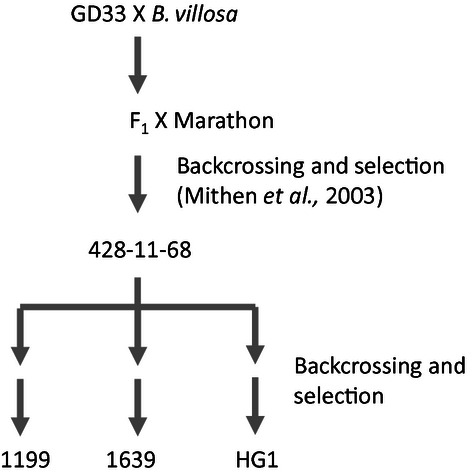
Summary of breeding programmes using the 428-11-69 line which was derived from a cross between broccoli and Brassica villosa, as described previously.
Phenotypic assessment of the high-glucoraphanin trait
The high-glucoraphanin F1 broccoli hybrids HG1, 1199, 1639 and the standard broccoli F1 hybrids Emerald, Belstar, Arcadia, Fiesta and Ironman were grown under normal agronomic conditions in an experimental field plot in Norwich in 2011. Twenty plants of each variety were grown in a randomized design, and six randomly selected heads of each variety were harvested at a stage equivalent to commercial maturity, freeze dried and ground to a fine powder. Fresh florets from each plant were also retained for analyses of SMCSO. Methionine- and tryptophan-derived glucosinolates, total sulphur, sulphate, methionine, cysteine, glutathione and SMCSO were analysed in the florets of each plant, as described below. For large-scale experimental field trials, 1199 was grown along with three standard commercial broccoli varieties – Ironman, Steel and Parthenon – in 31 experimental field trials in Italy, Spain and UK in 2009, 2010 and 2011, and 1639 was grown with the two standard broccoli cultivars Heritage and Marathon in 23 experimental field trials in California, Arizona and Mexico in 2007, 2008 and 2010. Details of trial locations are provided in Supporting Information Tables S1 and S2. At each location, the broccoli was grown under standard agronomic conditions (Anon, 2010). One to three plots of each cultivar were planted, and 1–3 head samples per plot were taken for glucosinolate analyses. For each sample, three randomly selected heads of each variety were harvested at commercial maturity (125 mm–200 mm diameter head), the florets separated (taking c. 60% of total crown weight), pooled, freeze-dried and ground to a fine powder.
Glucosinolate and sulphur metabolite analyses
From large-scale experimental field trials, European samples were analysed for glucosinolates at TNO (http://www.tno.nl) and North American samples were analysed at Covance (http://www.covance.com). Norwich 2011 samples were analysed at IFR. All glucosinolates were analysed by methods based on the determination of glucosinolates in Rapeseed ISO 9167-1 with some modifications as previously described (Saha et al., 2012). Other major sulphur-containing metabolites (methionine, cysteine, glutathione, S-methyl cysteine sulphoxide) were analysed in florets of three plants each of 1199, 1639, HG1, Emerald and Ironman grown in Norwich in 2011. Sulphate and glutathione analysis was performed as described previously (Koprivova et al., 2008; Scheerer et al., 2010). Total sulphur, free and hydrolysed cysteine, and methionine were quantified by Europhins (http://www.europhins.co.uk). SMCSO was determined as previously described with some modifications (Kubec & Dadakova, 2009). Briefly, 2 g fresh or frozen broccoli was steeped overnight in 30 ml acidified cold methanol to allow penetration of methanol into the cellular tissue. The plant material was cut in small pieces and homogenized by using a high-speed PRO 400 tissue homogenizer (Pro Scientific Inc., Oxford, CT, USA). The sample was incubated at 70°C and mixed by vortex for 10 min every 2–3 min. After centrifugation the methanolic fraction was aliquoted into a separate tube. The remaining homogenate was further extracted using 2 × 30 ml of boiling acidified methanol with 10 min incubation. The combined methanolic extracts were concentrated to 2–3 ml under reduced pressure (40°C) and adjusted to 5 ml by addition of 20 mM borate buffer (pH 9.2). The extract was stored at −20°C until derivatization. Dansyl derivatives were prepared by mixing 100 μl of the sample extract with 250 μl of Dns-Cl reagent (10 mM dansyl chloride in acetonitrile) and 0.65 ml of 20 mM borate buffer (pH 9.2). The mixture was briefly shaken, allowed to stand at room temperature for 30 min, centrifuged at 16 200 g for 10 min and analysed by HPLC-DAD/MS method by using the positive polarity mode as described below. Dansyl derivatives were analysed using a Spherisorb ODS2 (250 × 4.6 mm i.d., 5-μm particle size) column (Waters, Milford, MA, USA) connected to a model 1100 HPLC system (Agilent Technologies, Waldbronn, Germany) comprising a binary pump, degasser, cooled autosampler, column oven, diode array and mass spectrometer detectors. Samples were eluted at 0.9 ml min−1 with a gradient of increasing methanol using 50 mM pH 5 ammonium acetate buffer (solvent A) and methanol (solvent B). The gradient started at 30% solution B increasing over 35 min to 40%, then over 60 min to 75% B, and then was maintained for 5 min to 75% B before finally being re-equilibrated to 30% B for 5 min. Dansyl derivatives were monitored at 250 nm, full scan and selecting ion monitoring mode.
Single nucleotide polymorphism (SNP) analyses
Total DNA was isolated from young true leaves from Ironman, 1199, 1639, HG1 and B. villosa, using the DNeasy Plant Maxi kit (Qiagen Inc.). The DNA was genotyped by KBioScience, Cambridge, UK, using Brassica oleracea specific KASPar markers. The assay principal is described online (http://www.kbioscience.co.uk/reagents/KASP_manual.pdf). In total 1577 SNPs were assayed, each marker at least 10 kb apart; 1150 SNP markers were successfully assayed across all four samples and were used for further analysis. Genotype data were analysed using the R environment (R Development Core Team, 2007). In total, we identified 673 SNPs that were polymorphic between B. villosa and Ironman in at least one allele. To detect the SNP markers that were indicative of a B. villosa introgression within the high-glucosinolate cultivars, we identified SNP markers that were homozygous or heterozygous for the B. villosa alleles and were different to the Ironman alleles. The SNP markers were aligned to an unpublished high-density SNP map constructed using data from the AGDH population (P. G. Walley, personal communication). The map includes the previously mapped public markers that have been mapped in the AGDH population (Sebastian et al., 2000). The public markers facilitated the formation of syntenic links between the previously published QTL data (Mithen et al., 2003) and the new SNP data. The RFLP marker pO119 on chromosome 2 is tightly linked to the QTL for concentrations of methionine-derived glucosinolates, this marker is tightly linked to a group of SNPs on chromosome 2 that delineate the B. villosa introgression present in 1199, 1639, HG1 but absent in Ironman.
Myb28 sequencing
Primers were designed against the Myb28 (Bra029311) sequence identified at the BRAD Brassica database (Cheng et al., 2011) using Primer3 v0.4.0 (Rozen & Skaletsky, 2000) and purchased from MWG UK:
Myb28 Forward 5′-TCACGAACATGGAGAAGGTG-3′,
Myb28 Reverse 5′-TGAGCTTGACCGGGAGTATC-3′.
DNA isolated as described above was used. Reactions were performed in 20 μl volumes containing 1X Green GoTaq® Reaction Buffer (Promega), 2.5 mM MgCl2, 0.2 mM dNTPs, 0.2 μM primers, 0.5 units GoTaq® DNA polymerase and 15–50 ng template DNA. Amplification was carried out using 35 cycles of 95°C for 30 s, 53°C for 1 min and 72°C for 1 min with a final extension at 72°C for 5 min. Ampilicons were gel purified using the QIAquick Gel Extraction Kit (Qiagen) and sequenced (TGAC, Norwich, UK).
Real-Time RT-PCR of Myb28 and actin
The Myb28 sequence was identified as described above. A brassica actin sequence was identified using the AT3G18780.2 CDS sequence of Arabidopsis thaliana to BLAST the BRAD Brassica database (Cheng et al., 2011). Both assays were designed using ABI PRISM Primer Express v2 (Applied Biosystems). Primers and TaqMan probes with 5′-FAM and 3′-TAMRA modifications were purchased from MWG UK:
Myb28 For 5′-CTCTTCCTCTTTCCTCGGGTTT-3′,
Myb28 Rev 5′- TGCAACTCAAGGAACCTCTCTGA-3′,
Myb28 probe 5′-AACCCGGTTTCCGAGATCACCACAC-3′;
Actin For 5′- GCAGACCGTATGAGCAAAGAGA-3′,
Actin Rev 5′- GGGAGGTGCAACGACCTTAA3′,
Actin probe 5′- CACAGCACTTGCACCAAGCAGCATG-3′.
Total RNA from all the broccoli cultivars was extracted using a phenol-chloroform-isoamyl alcohol mixture (25 : 24 : 1) and a LiCl precipitation. cDNA was synthesized from 1 μg total RNA with QuantiTect Reverse Transcription Kit (Qiagen), which includes a DNAse step to remove possible DNA contamination. Expression of Myb28 and actin mRNA levels was determined by real time RT-PCR using the ABI Prism Step One Plus Sequence Detection System (Applied Biosystems). The real time RT-PCR reactions were carried out in 20 μl volumes using microamp optical 96-well plates. The reactions contained Taqman® RNA-TO-CT 1-Step master mix reagent kit (Applied Biosystems), 20 ng total RNA, 0.25 U μl−1 Multiscribe™ and optimized concentrations of primers and probes. RT-PCR conditions used were: one cycle of 48°C for 30 min, one cycle of 95°C for 10 min followed by 40 cycles at 95°C for 15 s and one cycle at 60°C for 1 min. The data for Myb28 were analysed using a standard curve generated by a serial dilution of total RNA from one Ironman plant. Actin was used as an invariant endogenous control to verify equal RNA loading.
Nutrient analysis
Protein, total dietary fibre, β-carotene, folic acid and Vitamin C were analysed in florets of 1199 and 1639 from experimental field trials in Europe and North America by Eurofins (http://www.eurofins.co.uk) and Covance (http://www.covance.com), respectively, using standard AOAC methods (http://www.aoac.org). Additionally, Vitamin E was analysed in florets of 1639 and calcium, iron, sodium and total sugar content was analysed in florets of 1199.
Statistical analysis
Concentrations of sulphur-containing and other metabolites, and Myb28 gene expression were compared between cultivars by ANOVA.
Results
Glucosinolate expression
The methionine-derived glucosinolates, glucoraphanin (4-methylsulphinylbutyl glucosinolate) and glucoiberin (3-methylsulphinylpropyl glucosinolate) were significantly higher in florets of HG1, 1199 and 1639 than standard broccoli cultivars (Fig. 4a, Table 1). There were no significant differences in tryptophan-derived glucosinolates (Fig. 4b, Table 1). Glucoraphanin was consistently higher in 1199 compared to standard broccoli cultivars in 31 experimental field trials conducted in UK, Spain and Italy (Fig. 5a), and also in 1639 compared to standard broccoli cultivars in 23 experimental field trials undertaken in California, Arizona and Mexico (Fig. 5b). High-glucoraphanin broccoli harvested from these trials had equivalent yield and floret quality to standard cultivars.
Fig. 4.
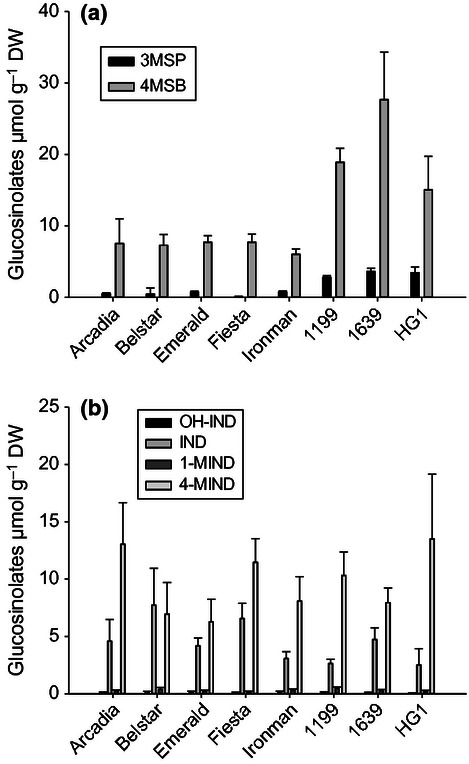
(a) Content (mean ± SD) of the methionine-derived glucosinolates, 3-methylsulphinylpropyl (3MSP) glucosinolate (glucoiberin) and 4-methylsulphinylbutyl (4MSP) glucosinolate (glucoraphanin), in florets of broccoli (Brassica oleracea var italica) F1 hybrids grown in Norwich in 2011. Concentrations of 3MSP and 4MSB are significantly higher in HG1, 1639 and 1199 compared to the other broccoli cultivars (P < 0.001). (b) Content (mean ± SD) of tryptophan-derived glucosinolates, OH-indole (OHIND), indole (IND), 1-methoxyindole glucosinolate (1-MIND) and 4-methoxyindole (4-MIND) glucosinolate
Table 1.
Content of the major sulphur-containing metabolites in florets of five broccoli F1 hybrids
| Total sulphur | Methionine | Cysteine | S-methyl cysteine sulphoxide | Sulphate | Glutathione | Tryptophan-derived glucosinolates | Methionine-derived glucosinolates | |
|---|---|---|---|---|---|---|---|---|
| Ironman | 245 ± 4.9a | 33.4 ± 1.06a | 28.9 ± 1.84a | 53.0 ± 3.30a | 89.8 ± 5.27a | 6.9 ± 0.79a | 10.7 ± 2.95a | 6.3 ± 0.55a |
| Emerald | 282 ± 28.1a | 33.5 ± 1.36a | 28.7 ± 0.96a | 53.8 ± 5.26a | 102.2 ± 8.70a | 6.9 ± 0.53a | 11.4 ± 1.88a | 8.8 ± 0.91a |
| HG1 | 345 ± 14.1b | 38.7 ± 0.54b | 30.5 ± 1.83a | 38.2 ± 2.00b | 98.4 ± 19.9a | 6.9 ± 0.41a | 11.4 ± 0.36a | 22.9 ± 1.05b |
| 1199 | 332 ± 20.4b | 36.9 ± 2.35a,b | 30.6 ± 1.69a | 44.5 ± 1.56b | 100.7 ± 20.6a | 6.9 ± 0.71a | 13.4 ± 2.17a | 21.7 ± 1.31b |
| 1639 | 394 ± 17.7c | 39.4 ± 2.89b | 32.3 ± 2.48a | 53.7 ± 1.96a | 112.5 ± 13.10a | 5.6 ± 0.82a | 13.7 ± 1.83a | 30.1 ± 5.58c |
Data are expressed in μmol g−1 DW as mean ± SD of six plants. Within columns data followed by the same letter are not significantly different (P < 0.05).
Fig. 5.
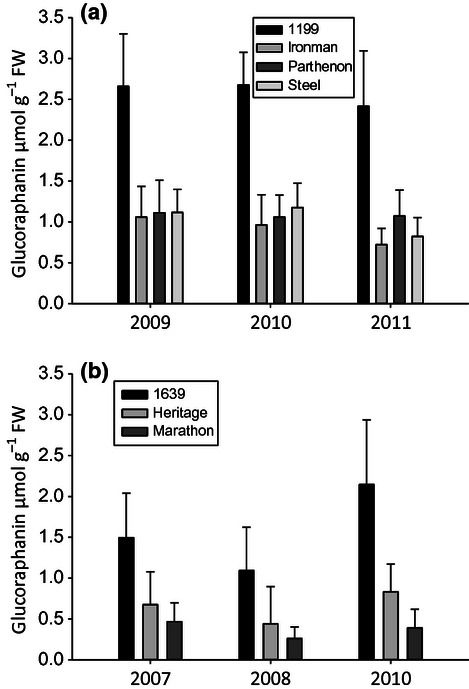
(a) Summary (mean ± SD) of glucoraphanin content of florets of broccoli 1199, Ironman, Parthenon and Steel in 31 experimental field trials undertaken in Spain, Italy and UK. The content of 1199 is significantly higher than other cultivars (P < 0.001). (b) Summary of glucoraphanin content (mean ± SD) of florets of 1639, Heritage and Marathon in 23 experimental field trials undertaken in California, Arizona and Mexico. The content of 1639 is significantly higher than other cultivars (P < 0.001).
Sulphur assimilation and partitioning
The hybrids 1199, 1639 and HG1 had significantly higher amounts of total sulphur compared to Ironman and Emerald. 1639 also had significantly higher concentrations compared to HG1 and 1199 (Table 1, Fig. 6). Concentrations of other major S-containing metabolites are summarized in Table 1. There is a higher absolute amount of methionine in HG1 and 1639, and a lower absolute amount of S-methyl cysteine sulphoxide in HG1 and 1199. As methionine- and tryptophan-derived glucosinolates have three and two sulphur atoms per molecule, respectively (Fig. 1), a greater insight into sulphur partitioning comes from considering the relative proportion of total sulphur within each of the major sulphur-containing metabolites (Table 2 and Fig. 6). Thus, the enhanced concentrations of methionine-derived glucosinolates in HG1, 1199 and 1639 arises through, first, an increase in the total sulphur, through enhanced sulphate assimilation and subsequent reduction, and, second, an increase in the percentage of sulphur allocated to methionine-derived glucosinolates, and a decrease in that allocated to SMCSO (Table 2). In 1199 and HG1 this results in an absolute reduction in SMCSO, whereas in 1639 there is no reduction in absolute amount due to the greater total amount of sulphur in this hybrid.
Fig. 6.
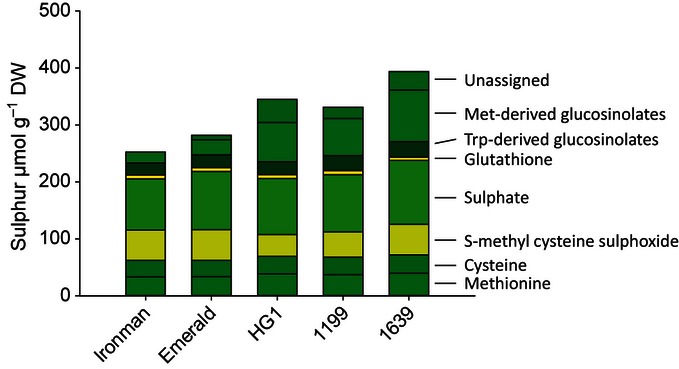
Sulphur content and sulphur partitioning between major sulphur-containing metabolites in broccoli Ironman, Emerald, 1199, HG1 and 1639. Details are provided in Tables 1 and 2.
Table 2.
Percentage of sulphur within the major sulphur-containing metabolites in florets of five broccoli F1 hybrids
| % Total sulphur | ||||||||
|---|---|---|---|---|---|---|---|---|
| Methionine | Cysteine | S-methyl cysteine sulphoxide | Sulphate | Glutathione | Tryptophan-derived glucosinolates | Methionine-derived glucosinolates | Unaccounted | |
| Ironman | 13.6 ± 0.32a | 11.8 ± 0.78a | 19.2 ± 2.53a | 36.5 ± 1.43a | 2.8 ± 0.27a | 8.7 ± 2.40a | 7.7 ± 0.75a | −2.9 ± 2.73a |
| Emerald | 11.9 ± 0.94b | 10.2 ± 0.47a,b | 21.6 ± 1.77a | 36.3 ± 0.76a,b | 2.5 ± 0.30a,b | 8.1 ± 0.69a | 9.5 ± 1.12a | 2.3 ± 6.86a,b |
| HG1 | 11.2 ± 0.32b | 8.8 ± 0.40b,c | 11.1 ± 1.03b | 28.4 ± 1.44b | 2.0 ± 0.12b | 6.6 ± 0.07a | 19.9 ± 1.73b | 11.9 ± 1.22b |
| 1199 | 11.2 ± 0.73b | 9.2 ± 0.46b,c | 13.5 ± 1.20b | 30.2 ± 4.51a,b | 2.1 ± 0.18b | 8.1 ± 1.04a | 19.7 ± 1.28b | 6.1 ± 2.33a,b |
| 1639 | 10.0 ± 0.73b | 8.2 ± 0.49c | 13.6 ± 0.91b | 28.5 ± 2.80b | 1.4 ± 0.16c | 7.0 ± 0.82a | 23.0 ± 4.71b | 8.2 ± 4.90a,b |
Data are expressed as mean ± SD. Within columns data followed by the same letter are not significantly different (P < 0.05).
Nutrient analyses
In 10 experimental field trials in California, Arizona and Mexico, no differences among the key nutrients (protein, total dietary fibre, beta carotene, folic acid, Vitamin E and Vitamin C) were observed for 1639 relative to current common broccoli varieties marketed in the U.S. (Table 3). A similar result was obtained for 1199 in Europe (Table 4), with the exception of Vitamin C which was higher in Steel.
Table 3.
Nutrient analysis of the Beneforté 1639 hybrid compared to commercial cultivars
| 1639 | Heritage | Ironman | Marathon | |
|---|---|---|---|---|
| Protein (g 100 g−1) | 3.9 ± 0.49 | 3.92 ± 0.51 | – | 4.23 ± 0.44 |
| Total fibre (g 100 g−1) | 3.25 ± 0.2 | 3.17 ± 0.23 | 3.3 ± 0.17 | 3.17 ± 0.22 |
| β-carotene (mg 100 g−1) | 0.79 ± 0.28 | 0.87 ± 0.31 | 0.66 ± 0.1 | 0.62 ± 0.15 |
| Total folate (μg g−1) | 1.28 ± 0.14 | 1.35 ± 0.16 | 1.45 ± 0.05 | 1.28 ± 0.12 |
| Vitamin C (mg g−1) | 0.94 ± 0.09 | 0.96 ± 0.09 | 0.97 ± 0.02 | 0.99 ± 0.08 |
| Vitamin E (mg 100 g−1) | 1.04 ± 0.25 | 1.03 ± 0.23 | 0.68 ± 0.19 | 1.04 ± 0.2 |
Data are expressed as mean ± SD in FW tissue. No significant differences were found between cultivars (P < 0.05).
Table 4.
Nutrient analysis of the Beneforté 1199 hybrid compared to commercial cultivars
| 1199 | Ironman | Parthenon | Steel | |
|---|---|---|---|---|
| Protein (g 100 g−1) | 4.6 ± 1.6 | 4.1 ± 0.96 | 4.4 ± 1.64 | 4.4 ± 1.44 |
| Total fibre (g 100 g−1) | 4.4 ± 1.49 | 3.9 ± 0.83 | 4.7 ± 1.74 | 4.6 ± 1.71 |
| β-carotene (mg 100 g−1) | 0.1 ± 0.05 | 0.2 ± 0.07 | 0.1 ± 0.05 | 0.1 ± 0.06 |
| Total folate (μg g−1) | 1.0 ± 0.26a,b,c | 1.08 ± 0.24b,c | 0.8 ± 0.24a | 1.1 ± 0.14b,c |
| Vitamin C (mg g−1) | 1.2 ± 0.16a | 1.2 ± 0.17a | 1.2 ± 0.17a | 1.4 ± 0.22b |
| Calcium (Ca) (mg 100 g−1) | 71.5 ± 47.43 | 57.6 ± 24.38 | 67.5 ± 40.71 | 72.8 ± 50.13 |
| Iron (Fe) (mg 100 g−1) | 0.7 ± 0.14 | 0.7 ± 0.1 | 0.7 ± 0.21 | 0.7 ± 0.17 |
| Sodium (Na) (mg 100 g−1) | 5.0 ± 3.26 | 4.2 ± 1.82 | 5.6 ± 3.79 | 5.1 ± 3.4 |
| Total sugars (g 100 g−1) | 1.7 ± 0.67 | 2.1 ± 0.36 | 1.7 ± 0.72 | 2.0 ± 0.92 |
Data are expressed as mean (± SD) in fresh weight tissue. No significant differences were found between cultivars except for Vitamin C and total folate, where within rows data followed by the same letter are not significantly different (P < 0.05).
Quantifying the extent of B. villosa introgression – SNP mapping
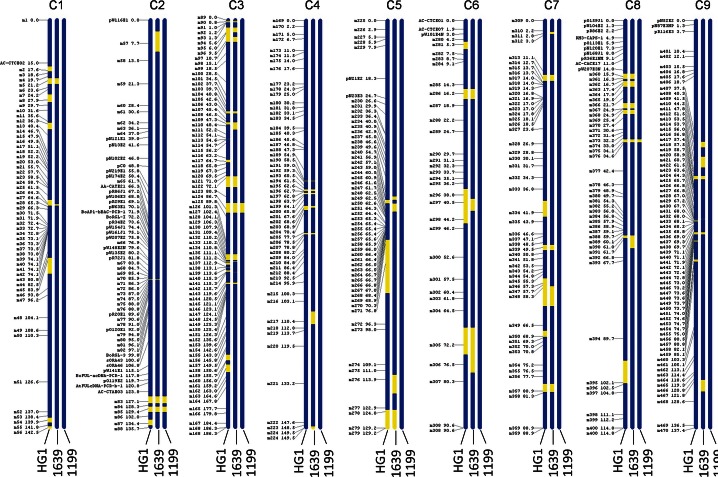
In order to quantify the extent of the distribution of Brassica villosa genome introgression into the genomes of 1199, 1639 and HG1 we undertook global SNP genotyping using B. oleracea specific KASPar assays. 673 SNPS were identified that were polymorphic between Ironman and B. villosa. Of these, 234 B. villosa SNP alleles were present in HG1, 177 in 1639 and eight in 1199 (Figs 7a, 8), indicative of the different extents of introgression of the B. villosa genome in the high-glucoraphanin hybrids.
Fig. 7.
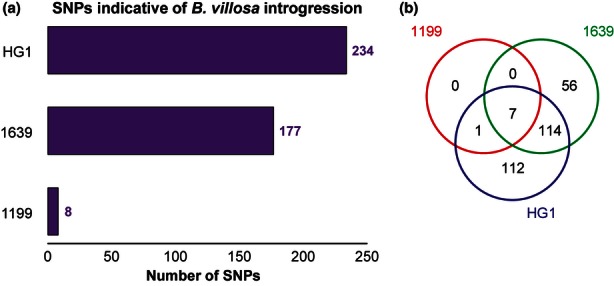
SNP analysis in the 1199, 1639 and HG1 broccoli F1 hybrids. (a) Number of SNPs indicative of introgression from Brassica villosa. (b) Overlap of introgressed SNPs in the three broccoli F1 hybrids.
Fig. 8.
Linkage map illustrating the locations of SNPs that fall within introgressed segments (shown in yellow) of Brassica villosa in the HG1, 1639 and 1199 broccoli F1 hybrids and the broccoli background in blue. Unpublished SNP markers are prefixed ‘m’. RFLP probes are prefixed pN, pO, pR (see Mithen et al., 2003), and pW; AFLP markers are labelled using the primer pairs (see Sebastian et al., 2000). AtFULcDNA, BoAP1-bBAC, BoFUL-acDNA, and pCO are unpublished RFLP markers; RM-3 is an unpublished CAPS marker and sORA43 and 46 are unpublished SSR markers. BoRGL-I is a CAPS marker (Vicente & King, 2001).
We sought to identify whether there were common B. villosa SNP alleles amongst the three high-glucoraphanin hybrids indicative of a B. villosa introgression that could contain alleles required for enhancing glucoraphanin accumulation. We identified seven such SNP markers (Fig. 7b) which mapped to three linkage groups. Markers mapping to linkage group 2 were genetically linked to an allele of the RFLP marker pO119 that had previously been shown to be associated with the major QTL determining methionine-derived glucosinolate accumulation in segregation populations derived from B. villosa (Mithen et al., 2003). Comparative genomic analyses with B. rapa suggested that this region of the genome contained the transcription factor Myb28 (Wang et al., 2011), which has previously been associated with determining methionine-derived glucosinolate concentrations in Arabidopsis thaliana (Sonderby et al., 2007) and B. napus (Harper et al., 2012).
Sequencing Myb28
A 947-bp genomic region of Myb28 that contained parts of all three exons constituting the Myb28 gene (Fig. 9) was amplified from B. villosa (the original donor of the high-glucoraphanin trait), Ironman, HG1, 1199 and 1639 and sequenced. Three SNPs were identified that were indicative of the presence of a B. villosa Myb28 allele in the three high-glucoraphanin hybrids; two located in the intronic region upstream of exon 3 and one at the 5′-end of exon 3 (Fig. 9).
Fig. 9.
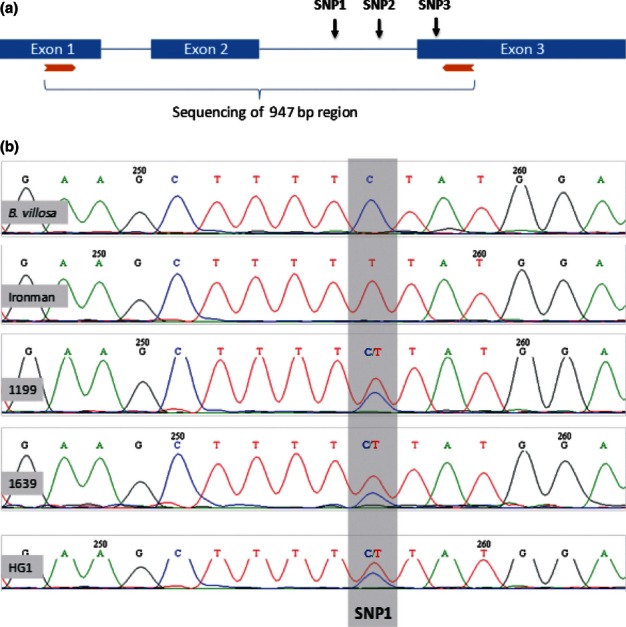
Sequencing the Myb28 transcription factor. (a) Structure of the Myb28 gene showing the 947-bp region that was sequenced and the location of the identified SNPs. (b) Sequence of the intronic SNP1 from Brassica villosa, Ironman, 1199, 1639 and HG1 broccoli cultivars showing the introgression of a B. villosa allele in the three broccoli F1 hybrids, indicated by the presence of cytosine in 1199, 1639 and HG1 (shaded).
Expression of the Myb28 transcription factor in broccoli cultivars
In order to determine whether expression of Myb28 is altered in different broccoli cultivars we measured its level of expression in the leaves and florets of high-glucoraphanin broccoli hybrids and standard broccoli cultivars grown in field plots in Norwich in 2011, and also in the leaves of glasshouse grown B. villosa and the cultivar Lord. The expression of Myb28 in leaves of the high-glucoraphanin hybrids was intermediate between that observed in the standard cultivars and B. villosa (Fig. 10), consistent with these hybrids being heterozygous for a ‘standard broccoli’ Myb28 allele and a B. villosa Myb28 allele. Expression of Myb28 in florets was more variable, with high concentrations observed in HG1, but with concentrations comparable to Ironman in 1199 and 1639 (data not shown).
Fig. 10.
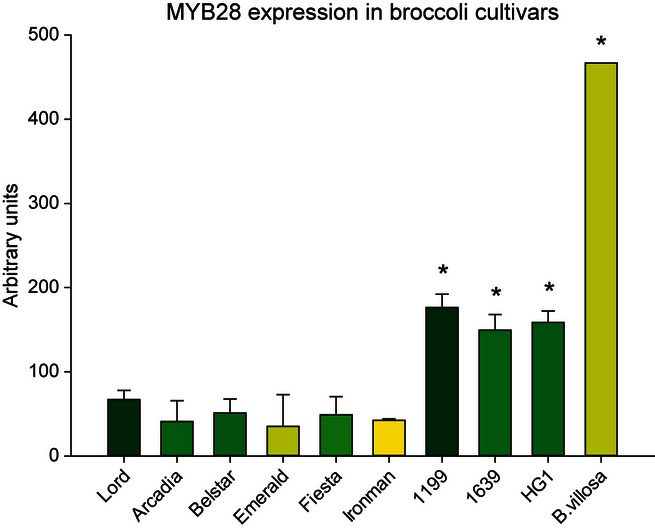
Gene expression of Myb28 in leaves of the standard broccoli cultivars, Lord, Arcadia, Belstar, Emerald, Fiesta, Ironman, the three broccoli F1 hybrids, 1199, 1639 and HG1, and Brassica villosa. Data are expressed as means ± SD (n = 5 for all cultivars except Lord (n = 3) and B. villosa (n = 1)). An asterisk indicates significantly increased expression (P < 0.001) relative to Ironman.
Discussion
Epidemiological studies that correlate diets rich in broccoli with health benefits, and experimental research that associates these health benefits with sulforaphane derived from glucoraphanin, suggest that broccoli cultivars with enhanced concentrations of glucoraphanin may have improved nutritional qualities. Faulkner et al. (1998) reported that a hybrid between broccoli and Brassica villosa accumulated remarkably high concentrations of glucoraphanin in floret, and that aqueous extracts of these florets in which the glucoraphanin was hydrolysed to sulforaphane were potent inducers of quinone reductase in mammalian cell assays. Subsequent breeding programmes led to the development of commercial broccoli F1 hybrids with enhanced glucoraphanin expression in florets derived from this original cross (Fig. 3). An extensive series of field studies demonstrated the robustness of the high-glucoraphanin trait, with a consistent 2.5–3-fold enhancement of glucoraphanin compared to standard broccoli cultivars (Figs 4, 5, Table 1), when grown under similar environmental conditions.
The enhanced concentrations of glucoraphanin could be derived by repartitioning of existing sulphur stores within broccoli, enhanced assimilation of sulphate from the soil, or redistribution of existing glucosinolates to florets from other tissues. We show that the high-glucoraphanin trait is due to two changes in sulphur metabolism. First, the high-glucoraphanin hybrids have significantly higher concentrations of total sulphur (Table 1, Fig. 6) due to enhanced sulphate assimilation, and, secondly, a higher percentage (≈ 20%) of the assimilated sulphur is channelled to methionine-derived glucosinolates than that in standard broccoli cultivars (≈ 8%) (Table 2). This increase in sulphur being channelled to methionine-derived glucosinolates was associated with a decrease of that being channelled to SMCSO (≈ 13% as opposed to 20%), resulting in a significant decrease in SMCSO in HG1 and 1199 compared to standard broccoli cultivars, but not in 1639 due to the higher concentration of total sulphur in this cultivar.
In order to elucidate the genetic basis of the high-glucoraphanin trait, we identified 673 SNPs between B. villosa, the donor of the high-glucoraphanin trait, and the cultivar Ironman. Of these we found 234, 177, and eight B. villosa SNPs in HG1, 1639 and 1199, respectively, indicative of different extents of B. villosa introgression in the three high-glucoraphanin hybrids. Seven of these B. villosa SNPs were common to each of the three F1 hybrids, and occurred in three clusters on linkage groups 2, 3 and 8 (Fig. 8). The cluster on linkage group 2 had previously been shown to be genetically linked to the RFLP maker pO119 which itself has been associated with the major QTL determining methionine-derived glucosinolate accumulation in broccoli (Mithen et al., 2003). Comparative genomics with B. rapa suggested the presence of the transcription factor Myb28 in the region of linkage group 2 associated with the introgressed B. villosa SNP alleles. Moreover, the ‘low glucosinolate trait’ in B. napus ssp oleifera (oilseed rape) that is associated with a QTL on linkage group C2 (homologous to B. oleracea linkage group 2) has also been associated with Myb28 (Harper et al., 2012). Thus, we amplified and sequenced Myb28 in B. villosa and the different high-glucoraphanin hybrids and showed that the high-glucoraphanin hybrids possessed one Myb28 allele that was derived from B. villosa, and one allele that was derived from standard broccoli, as would be expected in an F1 hybrid. Furthermore, we showed that the high-glucoraphanin hybrids had consistently higher constitutive expression of Myb28 in leaves compared to the standard broccoli cultivars. This is consistent with leaves being the major source of methionine-derived glucosinolates that are transported into the florets, as has been shown in Arabidopsis (Chen et al., 2001; Brown et al., 2003). In contrast to expression in leaves, the expression of Myb28 in florets was more variable between the high-glucoraphanin hybrids. Studies in Arabidopsis have suggested that reproductive tissues are not major sites of glucosinolate biosynthesis (Redovnikovic et al., 2012).
The Myb28 transcription factor mapped in B. rapa is genetically linked to two putative methylthioalkylmalate (MAM) synthase genes that may be involved in the biosynthesis of methionine-elongated homologues as precursors of glucosinolates (Fig. 2) (Wang et al., 2011). It is conceivable that a novel B.villosa MAM allele may have been introgressed along with the B. villosa Myb28 allele into the high-glucoraphanin broccoli. A novel MAM allele is, however, unlikely to be the cause of the high-glucoraphanin trait as there is no evidence that modification of MAM gene expression can affect sulphur uptake and metabolism, or lead to enhanced concentrations of methionine-derived glucosinolates as opposed to changing the ratio of side chain lengths (Field et al., 2004; Textor et al., 2007).
Thus, both genetic and gene expression studies indicate that the high-glucoraphanin trait is likely to be due to the introgression of a B. villosa Myb28 allele into a standard broccoli genetic background. In particular, the remarkably small extent of the B. villosa genome introgressed in 1199 suggests that this is the only B. villosa allele that is required for the expression of enhanced concentrations of glucoraphanin. The explanation of the high-glucoraphanin trait being determined by a B. villosa Myb28 allele is entirely consistent with the metabolic analyses which demonstrated that enhanced concentrations of glucoraphanin were due to increased sulphate assimilation and its channelling through to glucoraphanin. Studies in Arabidopsis have shown that Myb28 not only upregulates genes within methionine-derived glucosinolate biosynthesis but also other genes associated with sulphate assimilation and the synthesis of cysteine and methionine (Sonderby et al., 2007). Overexpression of Myb28 in Arabidopsis also results in altered sulphur partitioning, as the increased concentrations of Met-derived glucosinolates are accompanied by reduced concentrations of glutathione (Yatusevich et al., 2010). In Brassica napus spp. oleifera (canola), it appears that novel Myb28 alleles for low glucosinolate content (or a Myb28 deletion) have been introduced into oilseed rape (B. napus ssp oleifera) to result in lower quantities of 2-hydroxy-3-butenyl glucosinolate (progoitrin) (Harper et al., 2012), whereas we have introgressed a B. villosa allele of Myb28 into broccoli to result in enhanced concentrations of 4-methylsulphinylbutyl glucosinolate (glucoraphanin), the precursor of the putative anti-cancer compound sulforaphane.
Glucosinolate concentrations in broccoli are reported to fluctuate with environmental and soil conditions (Bjorkman et al., 2011). To demonstrate reproducibility of the high-glucoraphanin phenotype and to understand the impact on yield and quality, extensive experimental field trials were carried out to monitor and validate glucosinolate concentrations in the high-glucoraphanin hybrids in 54 experimental field trials undertaken over 3 years at multiple locations in Arizona, California, Mexico, Spain, Italy and UK. The high-glucoraphanin hybrids including the Beneforté broccoli hybrids were shown to consistently produce 2–3 times the glucoraphanin compared to other leading commercial broccoli varieties while maintaining normal nutrient concentrations (Tables 3, 4), yield and crown quality.
Wild Brassica species have previously been used to enhance disease resistance within oilseed rape (Crouch et al., 1994; Bradburne et al., 1999). In this paper, we describe the use of a wild Brassica species to enhance a trait within broccoli with potential consumer benefit. Through the introgression of a B. villosa Myb28 allele that enhanced sulphate assimilation and specifically channelled the additional sulphur to methionine-derived glucosinolates, we developed commercially viable broccoli F1 hybrids with increased concentrations of glucoraphanin, the precursor of sulforaphane. These hybrids are suitable for blinded human intervention studies to investigate the effects of glucoraphanin on human health in a common broccoli matrix without compromising eating quality or other nutritional elements.
Acknowledgments
We would like to thank Don James for his constructive comments on an earlier version of this manuscript, and Jan Chojecki of PBL for advice and support for the development of high-glucoraphanin broccoli. We would also like to thank the field staff of John Innes Centre for the Norwich field trials. Finally, we acknowledge financial support from the Biotechnology and Biological Sciences Research Council through the Institute Strategic Programme Grant in Food and Health (BB/J004545/1).
Supporting Information
Additional supporting information may be found in the online version of this article.
Table S1 Content of glucoraphanin in florets of broccoli of 1199, Ironman, Steel and Parthenon in experimental field trials
Table S2 Content of glucoraphanin in florets of broccoli of 1639, Heritage and Marathon in experimental field trials
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
References
- Anon. Fertiliser manual. Norwich, UK: The Stationery Office; 2010. [Google Scholar]
- Bjorkman M, Klingen I, Birch ANE, Bones AM, Bruce TJA, Johansen TJ, Meadow R, Molmann J, Seljasen R, Smart LE, et al. Phytochemicals of Brassicaceae in plant protection and human health - Influences of climate, environment and agronomic practice. Phytochemistry. 2011;72:538–556. doi: 10.1016/j.phytochem.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Filomeno M, Riso P, Polesel J, Levi F, Talamini R, Montella M, Negri E, Franceschi S, La Vecchia C. Cruciferous vegetables and cancer risk in a network of case-control studies. Annals of Oncology. 2012;23:2198–2203. doi: 10.1093/annonc/mdr604. [DOI] [PubMed] [Google Scholar]
- Bradburne R, Majer D, Magrath R, Werner CP, Lewis B, Mithen R. Winter oilseed rape with high levels of resistance to Pyrenopeziza brassicae derived from wild Brassica species. Plant Pathology. 1999;48:550–558. [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62:471–481. doi: 10.1016/s0031-9422(02)00549-6. [DOI] [PubMed] [Google Scholar]
- Chen S, Petersen BL, Olsen CE, Schulz A, Halkier BA. Long-distance phloem transport of glucosinolates in Arabidopsis. Plant Physiology. 2001;127:194–201. doi: 10.1104/pp.127.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Liu S, Wu J, Fang L, Sun S, Liu B, Li P, Hua W, Wang X. BRAD, the genetics and genomics database for Brassica plants. BMC Plant Biology. 2011;11:136. doi: 10.1186/1471-2229-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis MC, El-Sohemy A, Campos H. GSTT1 genotype modifies the association between cruciferous vegetable intake and the risk of myocardial infarction. American Journal of Clinical Nutrition. 2007;86:752–758. doi: 10.1093/ajcn/86.3.752. [DOI] [PubMed] [Google Scholar]
- Crouch JH, Lewis BG, Mithen RF. The effect of a-genome substitution on the resistance of Brassica napus to Infection by Leptosphaeria maculans. Plant Breeding. 1994;112:265–278. [Google Scholar]
- Davis FG, Fischer ME, VanHorn L, Mermelstein RM, Sylvester JL. Self-reported dietary changes with respect to American Cancer Society nutrition guidelines (1982–1986) Nutrition and Cancer. 1993;20:241–249. doi: 10.1080/01635589309514292. [DOI] [PubMed] [Google Scholar]
- Faulkner K, Mithen R, Williamson G. Selective increase of the potential anticarcinogen 4- methylsulphinylbutyl glucosinolate in broccoli. Carcinogenesis. 1998;19:605–609. doi: 10.1093/carcin/19.4.605. [DOI] [PubMed] [Google Scholar]
- Field B, Cardon G, Traka M, Botterman J, Vancanneyt G, Mithen R. Glucosinolate and amino acid biosynthesis in Arabidopsis. Plant Physiology. 2004;135:828–839. doi: 10.1104/pp.104.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. American Journal of Clinical Nutrition. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- Gigolashvili T, Yatusevich R, Berger B, Muller C, Flugge UI. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant Journal. 2007;51:247–261. doi: 10.1111/j.1365-313X.2007.03133.x. [DOI] [PubMed] [Google Scholar]
- Harper AL, Trick M, Higgins J, Fraser F, Clissold L, Wells R, Hattori C, Werner P, Bancroft I. Associative transcriptomics of traits in the polyploid crop species Brassica napus. Nature Biotechnology. 2012;30:798–802. doi: 10.1038/nbt.2302. [DOI] [PubMed] [Google Scholar]
- Helen A, Krishnakumar K, Vijayammal PL, Augusti KT. A comparative study of antioxidants S-allyl cysteine sulphoxide and vitamin E on the damages induced by nicotine in rats. Pharmacology. 2003;67:113–117. doi: 10.1159/000067796. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Chow WH, Boffetta P, Moore L, Zaridze D, Moukeria A, Janout V, Kollarova H, Bencko V, Navratilova M, et al. Dietary risk factors for kidney cancer in Eastern and Central Europe. American Journal of Epidemiology. 2007;166:62–70. doi: 10.1093/aje/kwm043. [DOI] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cellular and Molecular Life Sciences. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, Hayes RB. Prospective study of fruit and vegetable intake and risk of prostate cancer. Journal of the National Cancer Institute. 2007;99:1200–1209. doi: 10.1093/jnci/djm065. [DOI] [PubMed] [Google Scholar]
- Komatsu W, Miura Y, Yagasaki K. Suppression of hypercholesterolemia in hepatoma-bearing rats by cabbage extract and its component, S-methyl-L-cysteine sulphoxide. Lipids. 1998;33:499–503. doi: 10.1007/s11745-998-0233-7. [DOI] [PubMed] [Google Scholar]
- Koprivova A, North KA, Kopriva S. Complex signaling network in regulation of adenosine 5'-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiology. 2008;146:1408–1420. doi: 10.1104/pp.107.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubec R, Dadakova E. Chromatographic methods for determination of S-substituted cysteine derivatives–a comparative study. Journal of Chromatography A. 2009;1216:6957–6963. doi: 10.1016/j.chroma.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Lam TK, Ruczinski I, Helzlsouer KJ, Shugart YY, Caulfield LE, Alberg AJ. Cruciferous vegetable intake and lung cancer risk: a nested case-control study matched on cigarette smoking. Cancer Epidemiology, Biomarkers & Prevention. 2010;19:2534–2540. doi: 10.1158/1055-9965.EPI-10-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithen R, Faulkner K, Magrath R, Rose P, Williamson G, Marquez J. Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theoretical and Applied Genetics. 2003;106:727–734. doi: 10.1007/s00122-002-1123-x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- Redovnikovic IR, Textor S, Lisnic B, Gershenzon J. Expression pattern of the glucosinolate side chain biosynthetic genes MAM1 and MAM3 of Arabidopsis thaliana in different organs and developmental stages. Plant Physiology and Biochemistry. 2012;53:77–83. doi: 10.1016/j.plaphy.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols: methods in molecular biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Saha S, Hollands W, Teucher B, Needs PW, Narbad A, Ortori CA, Barrett DA, Rossiter JT, Mithen RF, Kroon PA. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Molecular Nutrition & Food Research. 2012;56:1906–1916. doi: 10.1002/mnfr.201200225. [DOI] [PubMed] [Google Scholar]
- Scheerer U, Haensch R, Mendel RR, Kopriva S, Rennenberg H, Herschbach C. Sulphur flux through the sulphate assimilation pathway is differently controlled by adenosine 5'-phosphosulphate reductase under stress and in transgenic poplar plants overexpressing gamma-ECS, SO, or APR. Journal of Experimental Botany. 2010;61:609–622. doi: 10.1093/jxb/erp327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian RL, Howell EC, King GJ, Marshall DF, Kearsey MJ. An integrated AFLP and RFLP Brassica oleracea linkage map from two morphologically distinct doubled-haploid mapping populations. Theoretical and Applied Genetics. 2000;100:75–81. [Google Scholar]
- Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiology, Biomarkers & Prevention. 1998;7:775–781. [PubMed] [Google Scholar]
- Seow A, Yuan JM, Sun CL, Van den Berg D, Lee HP, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23:2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- Sonderby IE, Hansen BG, Bjarnholt N, Ticconi C, Halkier BA, Kliebenstein DJ. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS ONE. 2007;2:e1322. doi: 10.1371/journal.pone.0001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoewsand GS. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables–a review. Food and Chemical Toxicology. 1995;33:537–543. doi: 10.1016/0278-6915(95)00017-v. [DOI] [PubMed] [Google Scholar]
- Textor S, de Kraker JW, Hause B, Gershenzon J, Tokuhisa JG. MAM3 catalyzes the formation of all aliphatic glucosinolate chain lengths in Arabidopsis. Plant Physiology. 2007;144:60–71. doi: 10.1104/pp.106.091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M, Gasper AV, Melchini A, Bacon JR, Needs PW, Frost V, Chantry A, Jones AM, Ortori CA, Barrett DA, et al. Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS ONE. 2008;3:e2568. doi: 10.1371/journal.pone.0002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente JG, King GJ. Characterisation of disease resistance gene-like sequences in Brassica oleracea L. Theoretical and Applied Genetics. 2001;102:555–563. [Google Scholar]
- Wang H, Wu J, Sun S, Liu B, Cheng F, Sun R, Wang X. Glucosinolate biosynthetic genes in Brassica rapa. Gene. 2011;487:135–142. doi: 10.1016/j.gene.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Xiao H, Parkin KL. Antioxidant functions of selected allium thiosulfinates and S-alk(en)yl-L-cysteine sulphoxides. Journal of Agriculture and Food Chemistry. 2002;50:2488–2493. doi: 10.1021/jf011137r. [DOI] [PubMed] [Google Scholar]
- Yatusevich R, Mugford SG, Matthewman C, Gigolashvili T, Frerigmann H, Delaney S, Koprivova A, Flugge UI, Kopriva S. Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant Journal. 2010;62:1–11. doi: 10.1111/j.1365-313X.2009.04118.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shu XO, Xiang YB, Yang G, Li H, Gao J, Cai H, Gao YT, Zheng W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. American Journal of Clinical Nutrition. 2011;94:240–246. doi: 10.3945/ajcn.110.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.