We are constantly reflecting on whether and how our efforts are transformational as we work to achieve the ambitious goals articulated for the University of Florida’s Clinical and Translational Science Institute (UF CTSI). Being the only Clinical and Translational Science Award (CTSA) recipient in the fourth most populous state, the UF CTSI must aim to support research and improve health care for 19 million Floridians, 6% of the US population.
The strong commitment of our university leadership and a team‐science approach has enabled us to move swiftly in the context of a very large comprehensive public research university. The UF CTSI involves two major health systems, agricultural extension offices in all 67 counties in Florida, and numerous partners throughout the state. All of the UF’s 16 colleges participate in the CTSI, which supports 140 faculty and staff, 10 programs, 13 clinical research units, 5 core labs, and 6 educational programs.
As we enter year 3 of our grant, the UF CTSI is launching a Personalized Medicine Program—a feat made possible by the transformational changes championed by the National Institutes of Health (NIH), its National Center for Research Resources (NCRR), and the CTSA program. We share this as one of many examples unfolding across the country in which CTSA institutions are adding new capacity for translational research, which in turn is leading to new approaches for how we translate scientific discoveries into medical practice—a need around which the NIH is vigorously seeking to catalyze new breakthroughs. 1
Personalized Medicine Program
In the summer of 2011, the UF CTSI launched its Personalized Medicine Program with funding from two federal grants. The NCRR awarded a 1‐year CTSA administrative supplement of $499,920 to create the program at UF and replicate it at Stanford University. In addition, the National Institute of General Medical Sciences, as the lead institute for the NIH Pharmacogenomics Research Network, awarded UF a 4‐year grant of $351,600 in support of a collaboration with five other institutions to collectively gather data on the experience of launching personalized medicine programs.
The UF CTSI’s Personalized Medicine Program will develop a process for determining which genetic and pharmacogenetic findings are clinically actionable, and it will create a system that uses genetic information to create alerts in a patient’s electronic medical record to inform treatment decisions. The program’s first target will be clopidogrel, a cardiovascular drug that carries a black‐box warning on its label to alert clinicians of differences in treatment‐related outcomes based on genotype. Figure 1 illustrates the flow and coordination of information that will be required for the Personalized Medicine Program to function. The program creates a transformational capability for improving care and reducing costs by minimizing adverse reactions and avoiding ineffective therapies—goals that are in direct alignment with the CTSA program’s mission to speed scientific discoveries to patients. In addition, the program creates new opportunities for translational research.
Figure 1.
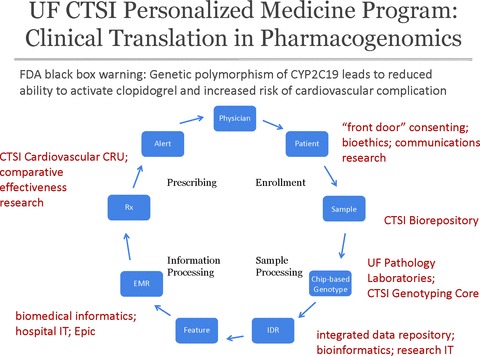
Information flow for the University of Florida personalized medicine pilot application detecting polymorphism of CYP2C19 resulting in creation of alerts in the electronic medical record for prescriptions of clopidogrel.
Patient enrollment:
The Personalized Medicine Program will initially seek to enroll patients undergoing cardiac catheterization, because undergoing balloon angioplasty and stent placement means the patients will be prescribed clopidogrel for at least 1 year. At clinic registration, the program will collect a genetic sample from enrolled patients and will use preemptive genotyping to inform treatment decisions.
Beyond the pilot phase of patient enrollment, the Personalized Medicine Program will be greatly aided by a CTSI‐sponsored project underway to implement a “front door” consent process at UF&Shands, the UF Academic Health Center. Through this process, every eligible inpatient and outpatient will have the opportunity to consent to allow the university to store excess tissue from their health care visit in the CTSI Biorepository, where the tissue and personal health information attached to that tissue will be made available for research in a secure manner that protects a patient’s privacy. In addition, patients will be able to consent to be recontacted if they are found to be candidates for future research studies. This rather simple yet transformational concept has only been realized because of the collaborative opportunities developed by the UF CTSI. The project is led by the chair of the UF Gainesville Health Science Center Institutional Review Board (IRB) and involves several CTSI programs (Bioethics, Communications Research, Regulatory Knowledge and Research Support) and the UF College of Medicine Research Administration and Compliance Office, UF Privacy Office, UF&Shands Compliance and Legal, UF&Shands IT and others. The “front door” consent process will create a fundamental new capability for translational research at UF and allow the patients to take an active role in the discovery process.
Sample processing:
An innovative aspect of the Personalized Medicine Program is that it proposes not to focus on single genetic tests but rather to perform genotyping on a broad, chip‐based panel to allow for the possibility that such data might one day be used across the span of a patient’s clinical care at UF&Shands. The CTSI Biorepository, CTSI Genotyping Core, and CTSI‐sponsored integrated data repository will each play key roles in the processing, storage, analysis, and utilization of the genetic information.
Genetic samples acquired for the Personalized Medicine Program will be stored in the CTSI Biorepository, which provides a centralized, large‐scale, and cost‐efficient alternative to individual tissue and data banks. With the informed consent of patients, it utilizes state‐of‐the‐art repository protocols for obtaining and storing tissues, and for ensuring that accurate information about the tissues can be provided to researchers in accordance with approved protocols. In addition to the Personalized Medicine Program’s genetic samples, the CTSI Biorepository will be acquiring hundreds of thousands of additional specimens through the “front door” consent process described previously. In the future, the availability of such tissues will greatly expand the possibilities for personalized medicine, particularly in oncology and organ‐specific diseases.
The UF Pathology Laboratories will conduct genotyping for the Personalized Medicine Program under CLIA/CAP standards, and in the early stages will be assisted by the CTSI Genotyping Core in platform selection and genotype validation. This new core has transformed genotyping at UF by synergizing the services of the university’s Center for Pharmacogenomics and the Genomics Core of the UF Interdisciplinary Center for Biotechnology Research, both of which have unique genotyping technologies that allow for single through genome‐wide SNP analysis.
To manage the resulting data, the Personalized Medicine Program will create a genetic data repository that will be linked with clinical records as one component of the university’s larger integrated data repository. Championed by the UF CTSI as a prerequisite for transforming the university’s ability to expand and accelerate translational research, this sophisticated new resource is integrating multiple sources of data from the university’s clinical and research environments. For the first time, investigators will be able to explore, among other queries, whether viable cohorts exist before developing a protocol. The availability of genetic data as part of the integrated data repository will support additional genetics research and continued discovery of genetic predictors of disease and drug response. Further, the integrated data repository is being built on the NIH‐supported Informatics for Integrating Biology to the Bedside (i2b2) platform, which will allow for the sharing of data and search capabilities with other institutions using i2b2. The integrated data repository is a joint effort at UF led by the CTSI, UF&Shands IT and the IRB.
Information processing:
Once the Personalized Medicine Program’s genetic samples have been processed and the data converted to the nomenclature required for clinical implementation, that information must make its way back to the clinical setting. This requires developing biomedical informatics approaches for warehousing all available genetic information, and then pulling actionable genetic information into electronic medical records to create alerts. The biomedical informatics needs of the Personalized Medicine Program will be addressed with support from the CTSI Biomedical Informatics Program, UF&Shands IT, and others. The UF CTSI is playing an integral role in developing this and other research capabilities within the Epic electronic medical record system recently implemented by UF&Shands.
Prescribing:
With genetic alerts available by the electronic medical record system, physicians in the CTSI Cardiovascular Clinical Research Unit will be able to act on that information in the course of prescribing medications for individual patients. To facilitate this change in medical practice, the Personalized Medicine Program will collaborate with the CTSI Training and Professional Development Program to educate relevant clinician groups on the literature behind the clinical pharmacogenetic alerts, as well as how to interpret and act on the data.
As the program is implemented, researchers in health services research, health outcomes policy, epidemiology and cardiology will participate in comparative effectiveness studies to determine the effectiveness of alerts, changes in prescribing patterns, cost‐effectiveness of sample processing, and outcomes for patients—efficacy, safety, and quality of care.
Future:
The UF CTSI has carefully defined the scope of its Personalized Medicine Program to allow a measured first step into this burgeoning field. We will carefully assess whether the program leads to improved quality and safety of care and a lower total cost of treatment. If successful, we will seek to expand the system for wider translation of pharmacogenetics and genetics findings into clinical practice, as well as export the approach to other health care systems. In addition to replicating the program at Stanford University, the UF CTSI will be in a position to explore the expansion and further testing of its model in partnership with other collaborators, such as the Orlando Health system or Florida State University and its statewide network of physician practices.
The Personalized Medicine Program at the University of Florida represents a transformative initiative in health care and research for the people of Florida. This initiative would not have been possible without the collaborative partnerships and fundamental changes pursued by the UF CTSI during its first 2 years.
Acknowledgments
The UF CTSI is supported in part by the NIH/NCRR Clinical and Translational Science Awards UL1 RR029890, KL2 RR029888 and TL1 RR029889. Additional support is provided by the UF Office of Research and the UF College of Medicine. Fifteen additional colleges provide in‐kind support. The Personalized Medicine Program is additionally supported by an administrative supplement to U01 GM074492.
The University of Florida consists of 16 colleges: Agricultural and Life Sciences; Business Administration; Dentistry; Design, Construction and Planning; Education; Engineering; Fine Arts; Health and Human Performance; Journalism and Communications; Law; Liberal Arts and Sciences; Medicine; Nursing; Pharmacy; Public Health and Health Professions; Veterinary Medicine.
reference
- 1. Collins FS. Reengineering translational science: the time is right. Sci Transl Med. 2011; 3: 90cm17. [DOI] [PMC free article] [PubMed] [Google Scholar]


