Abstract
Maintenance of bodily homeostasis requires concerted interactions between the neuroendocrine and the autonomic nervous systems, which generate adaptive neurohumoral outflows in response to a variety of sensory inputs. Moreover, an exacerbated neurohumoral activation is recognized to be a critical component in numerous disease conditions, including hypertension, heart failure, stress, and the metabolic syndrome. Thus, the study of neurohumoral regulation in the brain is of critical physiological and pathological relevance. Most of the work in the field over the last decades has been centered on elucidating neuronal mechanisms and pathways involved in neurohumoral control. More recently however, it has become increasingly clear that non-neuronal cell types, particularly astrocytes and microglial cells, actively participate in information processing in areas of the brain involved in neuroendocrine and autonomic control. Thus, in this work, we review recent advances in our understanding of neuro-glial interactions within the hypothalamic supraoptic and paraventricular nuclei, and their impact on neurohumoral integration in these nuclei. Major topics reviewed include anatomical and functional properties of the neuro-glial microenvironment, neuron-to-astrocyte signaling, gliotransmitters, and astrocytes regulation of signaling molecules in the extracellular space. We aimed in this review to highlight the importance of neuro-glial bidirectional interactions in information processing within major hypothalamic networks involved in neurohumoral integration.
Keywords: paraventricular, supraoptic, astrocytes, microglia, sympathetic, neuroendocrine, oxytocin, vasopressin, nitric oxide, glutamate, GABA
1- Introduction
Maintenance of bodily homeostasis requires concerted interactions between the neuroendocrine and the autonomic nervous systems. By controlling these two major information processing systems, specific neuronal networks within the central nervous system (CNS) generate adaptive neurohumoral responses, which are necessary for proper cardiovascular, fluid and energy balance system regulation. Neurohumoral activation is not only important within the context of adaptive physiological responses, but it is now also recognized to be a critical pathophysiological component in numerous disease conditions, including hypertension, heart failure, stress, and the metabolic syndrome (Brook et al., 2000; Cohn et al., 1981; Felder et al., 2001; Haywood et al., 1985; Middlekauff et al., 1998; Perez-Tilve et al., 2006). In the case of heart failure, for example, a compelling correlation between neurohumoral activation, morbidity and mortality in heart failure patients has been established (Cohn et al., 1984). Thus, elucidating precise anatomical pathways and cellular mechanisms underlying the generation of neurohumoral outflows by the brain is of critical physiological and clinical relevance.
Within the central neuronal circuitry involved in autonomic and neuroendocrine regulation, the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei stand as pivotal centers involved in the generation of coordinated neurohumoral responses (Cohn et al., 1984) (Guyenet, 2006; Swanson et al., 1980a; Swanson et al., 1983). Neurons in the PVN and SON are activated in response to a variety of afferent stimuli triggered by disturbances in the internal milieu, including dehydration, changes in blood pressure and blood volume, among others (Badoer et al., 1997; Krukoff et al., 1997; Li et al., 1994; Lovick et al., 1988; McKinley et al., 1994; Randolph et al., 1998; Schiltz et al., 1997; Stocker et al., 2006). These afferent stimuli elicit in turn robust autonomic and neuroendocrine responses coordinated by tese nuclei, which include increases in sympathetic outflow to the kidney and vasopressin release (Stocker et al., 2005; Stocker et al., 2004; Stricker et al., 2002).
The PVN is a highly heterogeneous nucleus, containing functionally diverse groups of neurons (Swanson et al., 1983). These include: a) magnocellular neurosecretory cell (MNCs), which synthesize the neuropeptides vasopressin (VP) and oxytocin (OT). These neurons project to the posterior pituitary, from where VP and OT are released in response to a variety of stimuli, including hyperosmolarity and lactation; b) parvocellular neuroendocrine neurons, which project to the median eminence and secrete hypophysiotropic hormones, including corticotropin-releasing factor, thyrotropin-releasing hormone, and somatostatin; and c) parvocellular preautonomic neurons, which send long descending projections to autonomic centers in the brainstem and spinal cord, and participate in autonomic control (Saper et al., 1976; Swanson et al., 1980b; van den Pol, 1982). The SON on the other hand, is a “purely” neurosecretory center, containing only OT and VP MNCs.
Neurohumoral outflow from these centers is directly dependent on the degree and pattern of neuronal activity of both neurosecretory and preautonomic neurons (Akine et al., 2003; Allen, 2002; Cazalis et al., 1985; Poulain et al., 1982; Stocker et al., 2004). Thus, elucidating precise mechanisms that regulate firing activity in these centers has been a major focus of work in this field. Over the last two decades, we have gain significant insights on intrinsic membrane properties and synaptic physiology of magnocellular and preautonomic PVN/SON neurons, and we refer the readers to excellent reviews and articles on this topic (Akine et al., 2003; Armstrong et al., 1998; Bourque, 2008; Brown, 2004; Chen et al., 2009; Iremonger et al., 2008; Luther et al., 2000; Sonner et al., 2007; Stern, 2001). More recently however, it has become increasingly clear that non-neuronal cell types, particularly astrocytes and microglial cells, actively participate in information processing in the brain (Araque et al., 2010; Perea et al., 2009; Volterra et al., 2005). Thus, the focus of the present review will be on recent advances in our understanding of neuro-glial interactions within the hypothalamic SON/PVN, with particular emphasis on astrocytes, and to a lesser extent microglial cells, and their impact on neurohumoral integration by these nuclei.
2- Bidirectional neuro-glia communication in the brain
While for many years astrocytes were regarded as passive supportive cells, the use of Ca2+ imaging technology (Tsien, 1980; Tsien, 1981) unraveled their contribution in numerous processes including: development (Rakic, 2003), synaptic transmission (Perea et al., 2010; Volterra et al., 2005), modulation of neuronal networks (Araque et al., 2010; Giaume et al., 2010) and the control of cerebral blood flow (Attwell et al., 2010; Filosa et al., 2006; Mulligan et al., 2004; Takano et al., 2006; Zonta et al., 2003), to name a few. The finding that astrocytes express a plethora of receptors and respond to numerous neurotransmitters gave name to the tripartite synapse model (Araque et al., 1999), which defines the ability of astrocytes not only to listen to neurons, but also to talk back. Astrocytes are equipped with the machinery to release gliotransmitters (Parpura et al., 2010; Parpura et al., 1994) (e.g. glutamate, D-serine, ATP), and also express specialized transporters, which aid in the modulation of synaptic activity by altering the milieu at the synaptic/peri-synaptic space. Moreover, astrocytes play an important role in the buffering of extracellular glutamate and K+ (Walz, 2000). Anatomically, a single astrocyte can enwrap thousands of synapses and create a physical barrier allowing direct interaction with neurons as well as neural networks (Giaume et al., 2010). Although controversial (Agulhon et al., 2008), a role for astrocytes in synaptic plasticity has been demonstrated, including both long term potentiation (LTP) and long term depression (LTD) (Henneberger et al.; Panatier et al., 2006a; Pascual et al., 2005; Perea et al., 2007; Zhang et al., 2008). For example, astrocytes in the SON, via the release of the aminoacid D-serine (see more below) modulates the efficacy of NMDA-mediated long-term synaptic plasticity in MNNs) (Panatier et al., 2006b).
Importantly, numerous developments of our current understanding of neuro-glial interactions in the brain emerged from pioneering work in hypothalamic centers involved in neurosecretory and autonomic control, including the SON and PVN. This has been the topic of excellent previous reviews (Hatton, 2004; Oliet et al., 2008b; Tasker et al., 2012; Theodosis et al., 2008), and an account of some of the most salient and recent developments in this area are summarized and discussed here.
3- Unique anatomical characteristics of the neuro-glial microenvironment in the SON and PVN
When considering functional interactions among neurons and glial cells in the SON/PVN, it is important to take into consideration several distinctive aspects of these centers. For example, detailed anatomical studies obtained from the magnocellular neurosecretory system in the SON/PVN revealed a highly “compact” neuro-glial microenvironment, in which the surface membrane of principal neurons are largely surrounded by astrocyte processes. Thus, under normal physiological conditions, astrocytes act as a physical and chemical barrier, limiting neuron-neuron interactions, as well as the diffusion of neurotransmitters in the extracellular space. Remarkably however, during conditions known to stimulate SON/PVN neuronal activity, such as dehydration and lactation, in the case of VP and OT neurons, respectively, the neuro-glial microenvironment undergoes a dramatic structural remodeling (Hatton, 2004; Oliet et al., 2008a; Piet et al., 2004; Tasker et al., 2012; Theodosis et al., 1989; Theodosis et al., 1999). This plasticity involves rapid and reversible glial retraction from between neighbouring neurons, unwrapping their synaptic contacts as well. This activity-dependent structural remodelling results in increased neuron-to-neuron juxtapositions, accumulation of extracellular K+, and build up of neurotransmitter levels in the extracellular space (see more below), all of which facilitate homeostatic responses in order to properly cope with these physiological challenges. A caveat to be taken into consideration, however, is that most of these studies relate to the magnocellular neurosecretory system. Thus, whether a similar neuro-glial organization and plasticity can be extrapolated to other neuronal types in the PVN, particularly preautonomic neurons, is at present unknown. Nonetheless, the SON/PVN have already proven to be a unique experimental model to investigate neuro-glial interactions in the brain.
4- Neuron-to-Astrocyte signaling
In addition to classical neurotransmitters, such as glutamate and GABA (Decavel et al., 1990; van den Pol et al., 1990), an abundance of functionally-relevant neuropeptides, including VP, OT, a-MSH, NPY, AGRP, dynorphin, galanin and endothelins, among others (Hokfelt et al., 2000; Landgraf et al., 2004; Ludwig et al., 2006) are pivotal signalling molecules within hypothalamic neuronal networks.
Here, we summarize recent studies supporting communication from neurons to astrocytes in the SON and PVN, via classical neurotransmitters as well as neuropeptides.
Noradrenaline and glutamate
In an elegant study, Gordon et al (2005) showed that noradrenaline binds on α1-adrenoceptors on PVN astrocytes to stimulate ATP release (Gordon et al., 2005), which then acting on neighbouring MNCs, leads to the Ca2+-dependent insertion of AMPA receptors, increasing neuronal sensitivity to synaptically-released glutamate. In another study, the same group showed that activation of mGLUR1,5 receptors by synaptically released glutamate was also capable of evoking ATP release from PVN astrocytes (Gordon et al., 2009). Along these lines, Espallergues et al. showed that NE and ATP acted synergistically to increase [Ca2+]i on SON astrocytes. However, the functional consequences of the evoked changes in astrocytes [Ca2+]i were not further assessed (Espallergues et al., 2007).
Results from these experiments provide noteworthy insights into the potential contribution of neuro-glial communication to the generation of integrative neurohumoral homeostatic responses by the PVN. Catecholaminergic afferent inputs originating from A1 caudal medulla neurons and from A2 neurons in the nucleus of the tractus solitarious (Cunningham et al., 2004; Renaud et al., 1991), provide critical visceroceptive afferent inputs carrying information about blood volume and pressure to the SON and PVN nuclei. On the other hand, glutamatergic inputs arising from circumventricular organs provide osmosensitive inputs to these nuclei (Oldfield et al., 1991; Richard et al., 1995; Sladek et al., 1998). Thus, distinct sensory modalities are conveyed to the SON/PVN via neuroanatomically and neurochemically separate neuronal pathways. Within this context, the studies summarized above suggest that SON and PVN astrocytes may act as “bridges” between catecholaminergic and glutamatergic afferent inputs, contributing therefore to the integration of distinct afferent input modalities, a necessary feature for the generation of concerted homeostatic responses.
Endothelins
In a recent study, we focused on a family of peptides abundantly present in the hypothalamus, the endothelins (ETs) (Filosa et al., 2012). ETs are composed of three isoforms, ET-1, ET-2 and ET-3, and their biological actions are mediated by two well characterized G-protein-coupled receptors: ETA, which exhibits higher affinity for ET-1 than for ET-2 and ET-3, and ETB, which displays similar affinity for all three isoforms (Davenport, 2002; Watts, 2010; Yanagisawa et al., 1988). While ETs are ubiquitously produced by endothelial cells, neurons (including MNCs), as well as astrocytes, also synthesize ETs (Ehrenreich et al., 1991; Nakamura et al., 1993; Yanagisawa et al., 1988). A large body of work supports an important role for ETs in the regulation of the SON/PVN neurosecretory and autonomic systems (Rossi, 2004; Rossi et al., 2006; Rossi et al., 2001; Rossi et al., 1997a; Rossi et al., 1997b). For example, activation of ETB receptors stimulated both somatodendritic, as well as neurohypophyseal VP release (Rossi, 2004). Nonetheless, the precise mechanisms by which ETs influence neuronal activity in the hypothalamus were not explored in detailed. We found that ETB receptor activation in SON astrocytes induced mobilization of [Ca2+]i, which in turn, via activation of glutamate and nitric oxide signaling pathways, evoked excitatory and inhibitory effects on neuronal firing activity, respectively (see Figure 1) (Filosa et al., 2012). Thus, our studies support the view that ETs participate as signaling molecules mediating neuroglial communication in the SON/PVN. It is important to mention however that in a similar study, Zampronio et al. (Zampronio et al., 2010) did not observe ETs-mediated changes in astrocytes Ca2+, despite the fact that ETs efficiently affected the firing activity of OT and VP neurons.
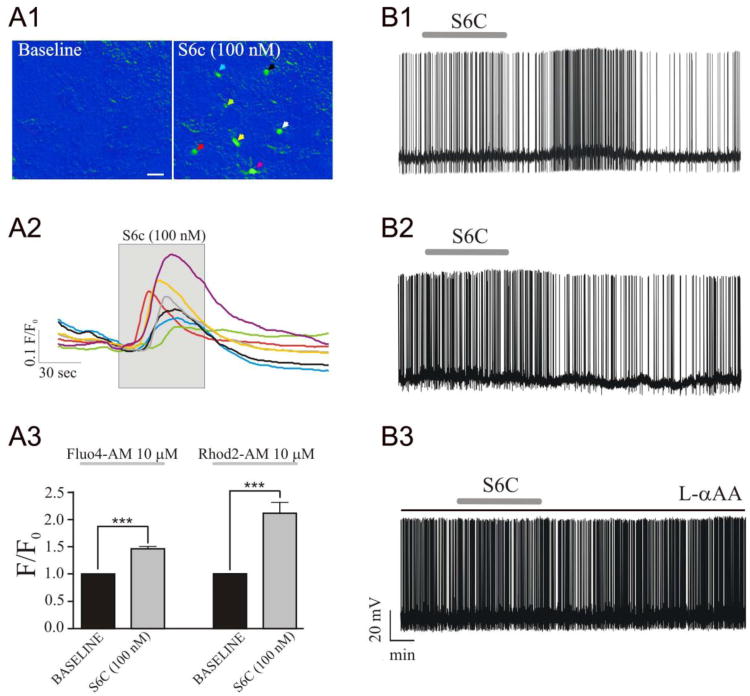
Figure 1. Endothelin B (ETB) receptor activation in the SON increases intracellular Ca2+ levels in astrocytes, and evokes a delayed change in SON firing activity.
A1, Representative pseudocolor images of transient Ca2+ changes in Fluo-4 loaded astrocytes (arrows) induced by the ETB receptor agonist sarafotoxin (S6c). A2, traces of Δ Ca2+ (ΔF/F0) corresponding to the astrocytes shown in A1. A3, Summary data of ΔF/F0 in response to S6c in astrocytes loaded with Fluo4-AM or Rhod2-AM. B, Bath application of Sarafotoxin 6c (S6c, 100 nM) induced either excitatory (B1) or inhibitory (B2) responses in SON neurons. Pre-incubation of hypothalamic slices in the presence of the gliotoxin L-αAA (250 μM) prevented S6c effects on SON firing activity (B3). ***P< 0.001. Scale bar= 10 μm. Modified from (Filosa et al., 2012).
Oxytocin and vasopressin
In addition to being released from neurohypophyseal terminals in the posterior pituitary, the neuropeptides OT and VP are also released from the somata and dendrites of MNCs within the SON and PVN (Ludwig et al., 2006). Somatodendritic release of these neuropeptides plays a critical role in modulating neurosecretory outflow from the SON and PVN, serving as powerful autocrine/paracrine signals by which neurosecretory neurons autoregulate their own firing activity (Gouzenes et al., 1998; Ludwig et al., 1997). Still, the question as to whether they also participate as important signaling molecules targeting local astrocytes, has been explored to a much lesser extent. For example, OT is essential for the induction of activity-dependent neuroglial remodeling in the SON (Langle et al., 2003; Theodosis et al., 1986). Moreover, OT receptors are expressed in hypothalamic cultured astrocytes (Di Scala-Guenot et al., 1992). However, it is still unknown whether OT-dependent neuroglial remodeling involves direct actions of OT on astrocytes. Thus, future studies evaluating the actions of these two major SON/PVN neuropeptides on astroglial function are warranted.
5- Astrocyte-to-neuron signaling (Gliotransmitters)
In addition to being capable of sensing neurotransmitters released by neighbouring neurons, there is now compelling evidence that astrocytes can also release a variety of neuroactive substances, termed gliotransmitters. These include ATP, adenosine, D-serine, glutamate and TNF-α, among others (Gordon et al., 2005; Gordon et al., 2009; Halassa et al., 2009; Kang et al., 1998; Panatier et al., 2006b; Perea et al., 2010; Stellwagen et al., 2006; Tritsch et al., 2007), now recognized to play an important role in information processing within neurosecretory and autonomic hypothalamic networks. As mentioned already above, ATP is one such functionally relevant gliotransmitters (Gordon et al., 2005). Here we summarize recent work providing evidence in support of D-serine, taurine and nitric oxide as gliotransmitters influencing synaptic physiology and neuronal activity in the SON/PVN.
D-serine
D-serine is an amino acid exclusively released from astrocytes, which serves as an endogenous co-agonist of NMDA receptors (NMDARs) (Mothet et al., 2000). In an elegant study, Panatier et. al. demonstrated that D-serine is produced by SON astrocytes, which when released to the surrounding environment, enhanced the amplitude of NMDA-mediated EPSCs and contributed to long-term synaptic potentiation in non-identified MNCs (Panatier et al., 2006b). Importantly, when the astrocytic coverage of MNCs was reduced in lactating rats, NMDAR activity was impaired due to a deficiency in D-serine (Panatier et al., 2006b). These results support D-serine as a pivotal gliotransmitter influencing excitatory synaptic function in the SON, and highlight the impact that dynamic changes in the local neuro-glial microenvironment have on information processing.
Taurine
Next to glutamate, taurine is the second most abundant amino acid in the brain (Palkovits et al., 1986). Taurine is mainly known for its involvement in cell volume regulation, being one of the major inorganic cellular osmolytes used to compensate for changes in extracellular osmolarity. Taurine is concentrated intracellularly, and can be released following cell swelling in response to a hypo-osmotic stimulus (Martin et al., 1990; Pasantes-Morales et al., 1990). Taurine is abundantly found in the hypothalamus, where it concentrated in glial cells (Decavel et al., 1995; Palkovits et al., 1986). Importantly, taurine release from SON astrocytes is stimulated with high sensitivity by a local hypo-osmotic stimulus (<5% decrease in osmolarity), and is inhibited by a hyperosmotic stimulus (Deleuze et al., 1998; Hussy et al., 1997). Additionally, taurine is an agonist of extrasynaptically located glycinergic receptors, evoking a robust inhibitory action of SON MNCs (Hussy et al., 1997). Thus, taurine release by astrocytes contributes to inhibition of neurosecretory OT and VP neurons in response to a hypo-osmotic stimulus, playing an important role in the regulation of adaptive neurohumoral responses by the magnocellular neuroendocrine system (Hussy et al., 2000). An important implication of these studies is that modulation of gliotransmitter release from SON/PVN astrocytes is not only dependent on signals arising from neurons (e.g., glutamate, norepinephrine), but can also be directly influenced by functionally-relevant systemic stimuli, such as a change in plasma osmolarity. In other words, and as proposed by Hussy et al., (Hussy et al., 2000), astrocytes may act as direct osmosensors, contributing in turn to osmotic-mediated neurohumoral responses. This notion is further supported by a recent study showing that a systemic hyperosmotic stimulus lead to FOS expression in SON astrocytes, which preceded FOS expression in neurons. Moreover, the hyperosmotic-mediated FOS expression in neurons was prevented by the glial metabolic blocker fluorocitrate (Yuan et al., 2010). Taken together, these results support a major role for astrocytes as mediators of osmotically-driven neurohumoral responses.
Gas molecules: nitric oxide (NO) and carbon monoxide (CO)
The gas molecule nitric oxide (NO) acts as a key signaling molecule within the SON and PVN, playing major roles in neurohumoral regulation. Constitutively produced NO tonically inhibits neurosecretory and preautonomic neuronal activity (Li et al., 2002; Li et al., 2003; Stern, 2004; Stern et al., 2001) restraining in turn sympathohumoral outflow to the circulation (Krukoff, 1999; Zhang et al., 1997). Importantly, blunted NO function within the SON/PVN is linked to neurohumoral activation in heart failure (HF) and diabetes (Zhang et al., 2001; Zheng et al., 2005). It is generally assumed that constitutive NO in the SON/PVN arises from a neuronal (nNOS) source. However, an alternative source of constitutive NO in the brain is the endothelial isoform (eNOS), commonly found to be expressed both in endothelial cells and astrocytes (Doyle et al., 1997; Iwase et al., 2000; Lin et al., 2007; Topel et al., 1998). In a recent study, we demonstrated high expression of eNOS within the SON and PVN, which was largely confined to astrocytes (Biancardi et al., 2011). Moreover, using a combination of fluorescent imaging, patch-clamp electrophysiology, along with in vivo sympathetic nerve recordings, we showed that astroglial eNOS contributes to NO bioavailability, as well as tonic inhibition of presympathetic neuronal activity and sympathoexcitatory outflow from the PVN (Biancardi et al., 2011) (Figure.2). Finally, we found astrocyte eNOS expression and function to be blunted in the PVN of heart failure rats. Taken together, these results support NO as another major gliotransmitter in the SON/PVN, playing an important role in neurohumoral regulation, both in control and disease conditions.
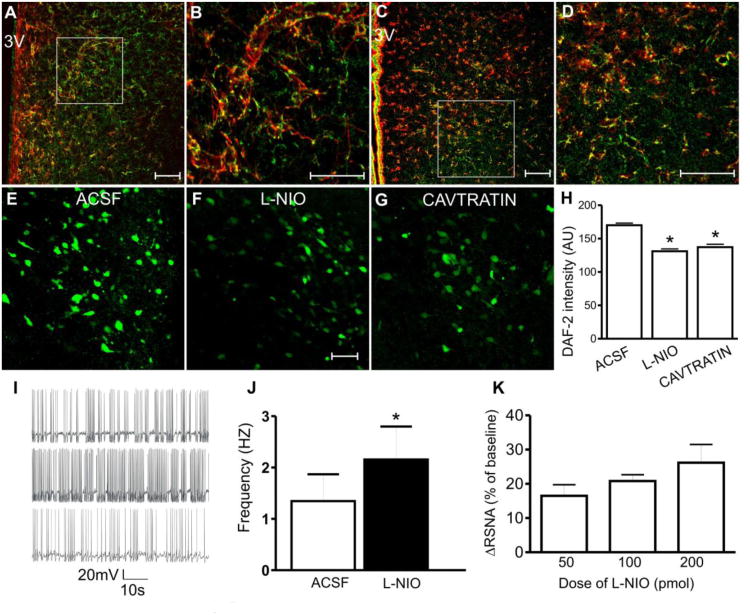
Figure 2. Glial eNOS modulates presympathetic neuronal activity and renal sympathetic nerve outflow in the PVN.
A and B: Immunostaining of eNOS (green) and the glial marker GFAP (red) within the PVN. B: Higher magnification image of the area outlined in A. C and D: eNOS (green) and glial S100b (red) immunoreactivities within the PVN. D: Higher magnification image of the area outlined by the square in C. Note the dense eNOS staining in the PVN, and the colocalization (yellow color: green + red) with both glial markers. E – G: Photomicrographs showing staining for the nitric oxide sensitive dye DAF-2 under (E) basal condition (ACSF), or in the presence of (F) eNOS inhibitor L-NIO (10μmol/L), and (G) eNOS inhibitor Cavtratin (10μmol/L). H: Summary data showing mean DAF-2 intensity in each experimental condition. I: in vitro electrophysiological recordings sowing firing activity of a PVN-RVLM projecting neuron before (top), during (middle) and after (bottom) bath application of L-NIO (10μmol/L). J: Summary data for mean firing frequency in PVN-RVLM neurons. K: Dose-dependent changes in renal sympathetic nerve activity (RSNA) in response to microinjections of L-NIO (50, 100, and 200pmol) into the PVN. Scale bars: 50μm. 3V: third ventricle; *P<0.05 vs *ACSF (H and J). Modified from (Biancardi et al., 2011).
Another biologically active gas is carbon monoxide (CO) (Maines, 1993; Verma et al., 1993). While CO has been the focus of numerous studies in cerebral vascular biology (Motterlini et al., 2002), a growing body of evidence also supports CO as a gaseous neurotransmitter within the CNS (Maines, 1993; Snyder et al., 1998; Verma et al., 1993). CO is generated endogenously by heme oxygenase (HO), an enzyme that cleaves the hemin derived from heme-proteins (Fe-protoporphyrin) such as hemoglobin, myoglobin and cytochromes, resulting in the formation of CO, free iron and biliverdin (Maines, 1993; Verma et al., 1993). There are three isoforms of HO: (a) the inducible HO-1, which can be upregulated by a variety of stimuli including oxidative stress and inflammation (Ewing et al., 1991; Verma et al., 1993); (b), the constitutive isoform HO-2, widely expressed in several tissues, and shown to be regulated by a few selected stimuli, including glucocorticoids (Raju et al., 1997); and (c) HO-3, whose function is yet unknown (Raju et al., 1997). HO-1 is expressed in specific CNS regions, including the hypothalamus (Ewing et al., 1992; Vincent et al., 1994). In fact, a growing body of evidence supports a modulatory role for CO on OT and VP release (Gomes et al., 2010; Mancuso et al., 1999). In a recent study, we reported HO1 to be expressed not only on SON/PVN magnocellular neurons, but also in astrocytes within these nuclei (Reis et al., 2012). This is in agreement with previous studies showing HO-1 presence in astrocytes and their ability to contribute to CO bioavailability in other brain regions (Li et al., 2008; Mancuso, 2004; Schipper et al., 2009). Importantly, we found CO to tonically stimulate SON neuronal activity, having thus opposite effects to NO (Reis et al., 2012). Thus, astrocytes in the SON/PVN are important cellular sources of two opposingly acting gas gliotransmitters, NO and CO. Given their unique biological properties as gas molecules, including their ability to readily diffuse, and to freely cross cell membranes, it is likely that NO/CO may affect relatively distant targets (∼100-200 μm) from its source of production (Santos et al., 2012; Wood et al., 1994), suggesting that astrocytes in the SON/PVN may lack well-defined spatial domains, compared to astrocytes in other brain regions (Bushong et al., 2002) (see more below).
Astrocyte regulation of concentration, diffusion and half-life of signaling molecules
Another major mechanism by which astrocytes influence neuronal activity within the tripartite synapse, is via regulation of the time course and concentration of neurotransmitters in synaptic and perisynaptic areas. This is achieved largely by the activity of selective and powerful neurotransmitter uptake transporters, particularly for the amino acid transmitters glutamate and GABA. These include the astrocytic GLT1 and GLAST glutamate transporter isoforms, which account for most glutamate clearance in the CNS (Rothstein et al., 1996), as well as the GAT3 isoform of the GABA transporter (Minelli et al., 1996; Ribak et al., 1996). According to the activity of these transporters, as well as their physical proximity to the source of their substrates, astrocytes can influence a variety of physiologically relevant processes, including modulation of heterosynaptic interactions between excitatory and inhibitory synaptic inputs. Synaptically-released glutamate can diffuse beyond its release site (i.e., spillover) (Isaacson, 2000), and activate metabotropic mGLUR receptors expressed in GABAergic terminals, resulting in depression of GABA release. This process, known as heterosynaptic depression, takes place in the SON and PVN (Piet et al., 2004; Schrader et al., 1997), and is tightly regulated by astrocytes. The activity of astrocyte GLT1 transporters efficiently “buffer” perisynaptic glutamate levels, limiting their access to presynaptic mGLURs in GABAergic terminals. However, pharmacological block of GLT1, or the physical retraction of astrocytic processes during lactation or dehydration, results in diminished neurotransmitter clearance, build-up in the extracellular space, and enhanced diffusion capacity. This has several consequences, including facilitation of heterosynaptic interactions via activation of presynaptic mGLURs (Di et al., 2004; Oliet et al., 2001; Piet et al., 2004).
Astrocytes processes and neurotransmitter transporters also play important roles in regulating the ability of extracellular neurotransmitters to access and activate extrasynaptic receptors. Besides acting on conventional postsynaptic receptors, glutamate and GABA can also activate receptors located extrasynapticaly (Dalby et al., 2003; Le Meur et al., 2007; Sah et al., 1989). Extrasynaptic receptors have been typically considered a nonfunctional pool of receptors, which could be recruited to active synapses ‘on-demand’ (Clark et al., 2002; Harney et al., 2008). However, extrasynaptic receptors are recognized to play important roles in information processing within the CNS, mediating quite a distinct communication modality, when compared to their synaptic counterparts. Synaptic receptors mediate spatially and temporally restricted transfer of information between two neurons (i.e. postsynaptic current; EPSC). On the other hand, given their characteristic low degree of desensitization and high-affinity for their agonists (Monyer et al., 1992; Priestley et al., 1995; Tovar et al., 1999), extrasynaptic receptors mediate a persistent, ‘tonic’ current, assumed to more globally influence neuronal excitability, as well as the overall gain within a network of neurons. We recently demonstrated the presence of functional extraynaptic NMDARs in SON neurosecretory neurons (Fleming et al., 2011). Their activation by ambient, extracellular glutamate levels results in a persistent inward current, which tonically stimulates SON neuronal activity. As with mGLURs, astrocyte GLT1 transporters determine the efficiency of this alternative excitatory modality. Thus, either pharmacological block of GLT1 activity or glial retraction during dehydration, results in an enhanced activation and contribution of eNMDARs to both oxytocin and vasopressin SON firing activity (Fleming et al., 2011). We proposed this phenomenon to contribute to the homeostatic increase in neurosecretory firing activity during dehydration. In addition, blockade of GLT1 activity within the PVN resulted in an enhanced renal sympathetic nerve activity (Dr. Patel, KP, University of Nebraska, unpublished observations). Finally, we recently reported a blunted GLT1 expression and function in SON neurons in rats with ischemic heart failure, a disease characterized by neurohumoral activation (Potapenko et al., 2012). Thus, astrocyte regulation of extrasynaptic NMDAR-mediating excitatory modality is not only an important signaling mechanism influencing physiological homeostatic neurohumoral responses, but may also contribute to exacerbated neurohumoral activities during disease conditions, such as heart failure.
Similar to the case of glutamate, we also found SON and PVN astrocytes to regulate the ability of extracellular GABA to activate extrasynaptic GABAA receptors. For example, we reported that blockade of astroglial GABA transporters in the PVN resulted in a buildup of extracellular GABA and activation of extrasynaptic GABAA receptors in presympathetic PVN neurons, diminishing in turn their ongoing firing activity, as well as sympathoexcitatory outflow to the kidney (Park et al., 2009). Similar results were observed in magnocellular neurosecretory neurons (Park et al., 2006). Taken together, these studies indicate by modulating the degree of extrasynaptic GABAA receptor activation, astrocytes can efficiently influence the activity of both neurosecretory and preautonomic neurons, affecting consequently neurohumoral outflows from these regions (Park et al., 2009; Park et al., 2007; Park et al., 2006).
Summary and Future directions
We aimed in this review to highlight the importance of neuro-glial bidirectional interactions in information processing in the CNS in general, and within the SON and PVN, major hypothalamic networks involved in neurohumoral integration, in particular. As summarized above, and schematically shown in Figure 3, we provided an overview of several mechanisms and signaling pathways by which neurons and astrocytes communicate with each other in these areas, to ultimately influence the integration of peripheral sensory inputs, as well as the generation of neurosecretory and autonomic outflows from these nuclei. These included the ability of astrocytes to “sense” synaptically-released neurotransmitters (e.g., glutamate, norepinephrine) and to actively release gliotransmitters (e.g., D-serine, ATP, nitric oxide). Moreover, astrocytes are actively involved in the clearance of neurotransmitters from the extracellular space, influencing in turn other important signaling modalities (e.g., heterosynaptic interactions and activation of extrasynaptic receptors). Importantly, these cell-cell communication modalities in these nuclei take place within a unique and remarkable plastic neuro-glial microenvironment.
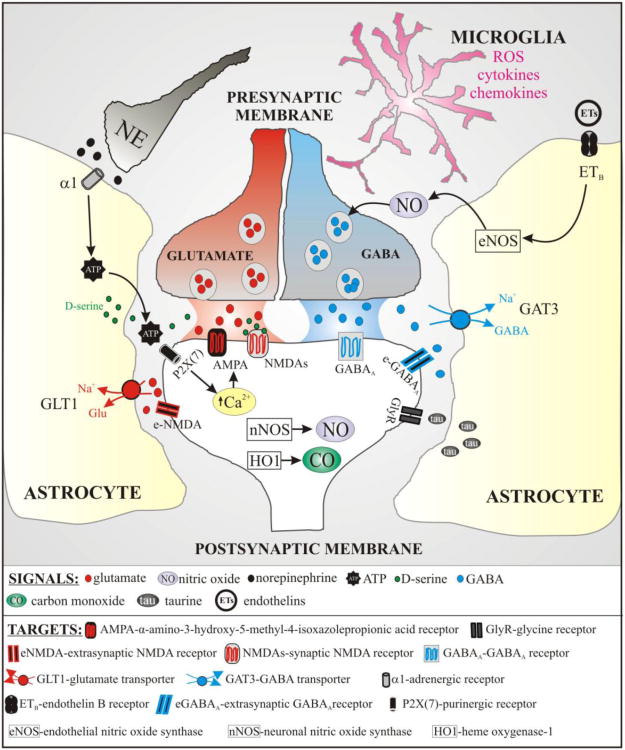
Figure 3. Schematic diagram depicting the various signals and targets at the SON/PVN tripartite synapse.
Despite all the recent advances highlighted in this and other topical reviews (Bains et al., 2007; Hatton, 2004; Oliet et al., 2004; Tasker et al., 2012), there are still essential gaps and unanswered questions in this area of research, which clearly will motivate additional work in the near future. For example, astrocytes have the ability to produce and release a plethora of neuroactive substances, which in some cases have opposing actions on the targeted SON/PVN neurons. Thus, a future challenge will be to elucidate more precisely how gliotransmitter release is precisely regulated.
The majority of the work reported so far on neuro-glial interactions in the SON/PVN has been centered on magnocellular neurosecretory neurons. However, given the high degree of neuronal heterogeneity within the PVN, and the importance of preautonomic neurons in neurohumoral integration, it will be critical to define whether the same principles reported in the magnocellular neurosecretory system applies to the other major neuronal systems in the PVN.
In the cortex and the hippocampus, brain regions that display a well-defined and highly organized laminar cytoarchitecture, astrocytes are also highly organized, comprising segregated spatial domains. Each domain represent a volume of neuropil under the control of a single astrocyte, and contains ∼150,000 synapses (Bushong et al., 2002; Halassa et al., 2007; Ogata et al., 2002; Volterra et al., 2005). The PVN, on the other hand, displays a less-defined, and heterogeneously organized architecture. Thus, whether astrocytes in the PVN (and SON) also display organized and segregated domains, and whether an astrocytic volume domain is restricted to a specific neuronal type, is still unknown.
Finally, while most studies in the neuro-glial communication field have focused on astrocytes, microglial cells constitute another major glial cell type in the brain. Microglia cells, the resident immune cells of the brain (Saijo et al., 2011) contribute to a number of CNS homeostatic processes, including surveillance of their surroundings (Nimmerjahn et al., 2005), brain development and maturation (Dalmau et al., 1998; Fiske et al., 2000; Perry et al., 1985; Tremblay et al., 2011b; Wake et al., 2009), and synaptic remodeling (Tremblay et al., 2011a), among others. Moreover, microglia are the first responders to injury and pathogen infection in the brain, turning into an activated, pro-inflammatory state (Saijo et al., 2011). Active microglia release a variety of pro-inflammatory and also neuroactive substances, including cytokines, chemokines, reactive oxygen species, and nitric oxide, among others (Saijo et al., 2011), leading to gliosis, exacerbated neuronal activity, and ultimately, neuronal cell death. Importantly, there is a growing body of evidence supporting that cardiovascular-related signals, in particular angiotensin II, can lead to microglial activation (Joglar et al., 2009; Rodriguez-Pallares et al., 2008; Shi et al., 2010; Villar-Cheda et al., 2012), contributing in turn to exacerbated neurohumoral activation in disease conditions, such as heart failure, hypertension and diabetes (Francis et al., 2004; Luo et al., 2002; Rana et al., 2010; Shi et al., 2010). Thus, future studies are warranted to start elucidating communication modalities among microglial cells, neurons and astrocytes within CNS areas involved in autonomic and neuroendocrine regulation.
Acknowledgments
This work was supported National Heart, Lung, and Blood Institute Grants R01HL085767 and R21HL113801 to Stern JE and a R01HL089067 to Filosa JA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akine A, Montanaro M, Allen AM. Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Auton Neurosci. 2003;108:17–21. doi: 10.1016/j.autneu.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- Araque A, Navarrete M. Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Stern JE. Phenotypic and state-dependent expression of the electrical properties of oxytocin and vasopressin neurones. Prog Brain Res. 1998;119:97–109. doi: 10.1016/s0079-6123(08)61564-2. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoer E, McKinlay D, Trigg L, McGrath BP. Distribution of activated neurons in the rabbit brain following a volume load. Neuroscience. 1997;81:1065–1077. doi: 10.1016/s0306-4522(97)00232-7. [DOI] [PubMed] [Google Scholar]
- Bains JS, Oliet SH. Glia: they make your memories stick! Trends Neurosci. 2007;30:417–424. doi: 10.1016/j.tins.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Biancardi VC, Son SJ, Sonner PM, Zheng H, Patel KP, Stern JE. Contribution of central nervous system endothelial nitric oxide synthase to neurohumoral activation in heart failure rats. Hypertension. 2011;58:454–463. doi: 10.1161/HYPERTENSIONAHA.111.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Brook RD, Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens. 2000;13:112S–122S. doi: 10.1016/s0895-7061(00)00228-4. [DOI] [PubMed] [Google Scholar]
- Brown CH. Rhythmogenesis in vasopressin cells. J Neuroendocrinol. 2004;16:727–739. doi: 10.1111/j.1365-2826.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Toney GM. Excitability of paraventricular nucleus neurones that project to the rostral ventrolateral medulla is regulated by small-conductance Ca2+-activated K+ channels. J Physiol. 2009;587:4235–4247. doi: 10.1113/jphysiol.2009.175364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Cull-Candy SG. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci. 2002;22:4428–4436. doi: 10.1523/JNEUROSCI.22-11-04428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J. 1981;102:509–514. doi: 10.1016/0002-8703(81)90739-0. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Penny ML, Murphy D. Cardiovascular regulation of supraoptic neurons in the rat: synaptic inputs and cellular signals. Progress in Biophysics and Molecular Biology Neurohumoral control of cardiovascular function from genes to physiology. 2004;84:183–196. doi: 10.1016/j.pbiomolbio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Dalby NO, Mody I. Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J Neurophysiol. 2003;90:786–797. doi: 10.1152/jn.00118.2003. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the postnatal rat hippocampus. Hippocampus. 1998;8:458–474. doi: 10.1002/(SICI)1098-1063(1998)8:5<458::AID-HIPO6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Davenport AP. International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- Decavel C, Hatton GI. Taurine immunoreactivity in the rat supraoptic nucleus: prominent localization in glial cells. J Comp Neurol. 1995;354:13–26. doi: 10.1002/cne.903540103. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol. 1998;507(Pt 2):463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145:5141–5149. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- Di Scala-Guenot D, Strosser MT. Oxytocin receptors on cultured astroglial cells. Kinetic and pharmacological characterization of oxytocin-binding sites on intact hypothalamic and hippocampic cells from foetal rat brain. Biochem J. 1992;284(Pt 2):491–497. doi: 10.1042/bj2840491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle CA, Slater P. Localization of neuronal and endothelial nitric oxide synthase isoforms in human hippocampus. Neuroscience. 1997;76:387–395. doi: 10.1016/s0306-4522(96)00297-7. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Kehrl JH, Anderson RW, Rieckmann P, Vitkovic L, Coligan JE, Fauci AS. A vasoactive peptide, endothelin-3, is produced by and specifically binds to primary astrocytes. Brain Res. 1991;538:54–58. doi: 10.1016/0006-8993(91)90375-6. [DOI] [PubMed] [Google Scholar]
- Espallergues J, Solovieva O, Techer V, Bauer K, Alonso G, Vincent A, Hussy N. Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus. Neuroscience. 2007;148:712–723. doi: 10.1016/j.neuroscience.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci U S A. 1991;88:5364–5368. doi: 10.1073/pnas.88.12.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing JF, Maines MD. In situ hybridization and immunohistochemical localization of heme oxygenase-2 mRNA and protein in normal rat brain: Differential distribution of isozyme 1 and 2. Mol Cell Neurosci. 1992;3:559–570. doi: 10.1016/1044-7431(92)90068-d. [DOI] [PubMed] [Google Scholar]
- Felder RB, Francis J, Weiss RM, Zhang ZH, Wei SG, Johnson AK. Neurohumoral regulation in ischemia-induced heart failure. Role of the forebrain. Ann N Y Acad Sci. 2001;940:444–453. doi: 10.1111/j.1749-6632.2001.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Naskar K, Perfume G, Iddings JA, Biancardi VC, Vatta MS, Stern JE. Endothelin-mediated calcium responses in supraoptic nucleus astrocytes influence magnocellular neurosecretory firing activity. J Neuroendocrinol. 2012;24:378–392. doi: 10.1111/j.1365-2826.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- Fiske BK, Brunjes PC. Microglial activation in the developing rat olfactory bulb. Neuroscience. 2000;96:807–815. doi: 10.1016/s0306-4522(99)00601-6. [DOI] [PubMed] [Google Scholar]
- Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol. 2011;589:3929–3941. doi: 10.1113/jphysiol.2011.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286:H2264–2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Gomes DA, Giusti-Paiva A, Ventura RR, Elias LL, Cunha FQ, Antunes-Rodrigues J. Carbon monoxide and nitric oxide modulate hyperosmolality-induced oxytocin secretion by the hypothalamus in vitro. Biosci Rep. 2010;30:351–357. doi: 10.1042/BSR2009010. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. J Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci. 2008;28:11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI. Dynamic neuronal-glial interactions: an overview 20 years later. Peptides. 2004;25:403–411. doi: 10.1016/j.peptides.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Haywood JR, Brennan TJ, Hinojosa C. Neurohumoral mechanisms of sodium-dependent hypertension. Fed Proc. 1985;44:2393–2399. [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M. Neuropeptides--an overview. Neuropharmacology. 2000;39:1337–1356. doi: 10.1016/s0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Desarmenien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol. 1997;502(Pt 3):609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Dynamic synapses in the hypothalamic-neurohypophyseal system. Vasopressin and Oxytocin: From Genes to Clinical Applications. 2008;170:119–128. doi: 10.1016/S0079-6123(08)00411-1. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Spillover in the spotlight. Curr Biol. 2000;10:R475–477. doi: 10.1016/s0960-9822(00)00551-0. [DOI] [PubMed] [Google Scholar]
- Iwase K, Miyanaka K, Shimizu A, Nagasaki A, Gotoh T, Mori M, Takiguchi M. Induction of endothelial nitric-oxide synthase in rat brain astrocytes by systemic lipopolysaccharide treatment. J Biol Chem. 2000;275:11929–11933. doi: 10.1074/jbc.275.16.11929. [DOI] [PubMed] [Google Scholar]
- Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson's disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem. 2009;109:656–669. doi: 10.1111/j.1471-4159.2009.05999.x. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Krukoff TL. Central actions of nitric oxide in regulation of autonomic functions. Brain Res Brain Res Rev. 1999;30:52–65. doi: 10.1016/s0165-0173(99)00010-7. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Mactavish D, Jhamandas JH. Activation by hypotension of neurons in the hypothalamic paraventricular nucleus that project to the brainstem. J Comp Neurol. 1997;385:285–296. doi: 10.1002/(sici)1096-9861(19970825)385:2<285::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Langle SL, Poulain DA, Theodosis DT. Induction of rapid, activity-dependent neuronal-glial remodelling in the adult rat hypothalamus in vitro. Eur J Neurosci. 2003;18:206–214. doi: 10.1046/j.1460-9568.2003.02741.x. [DOI] [PubMed] [Google Scholar]
- Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580:373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle Cell KCa channels. Circ Res. 2008;102:234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol. 2002;88:2664–2674. doi: 10.1152/jn.00540.2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience. 2003;118:585–601. doi: 10.1016/s0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Li YW, Dampney RA. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience. 1994;61:613–634. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Lin LH, Taktakishvili O, Talman WT. Identification and localization of cell types that express endothelial and neuronal nitric oxide synthase in the rat nucleus tractus solitarii. Brain Res. 2007;1171:42–51. doi: 10.1016/j.brainres.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, Coote JH. Effects of volume loading on paraventriculo-spinal neurones in the rat. J Auton Nerv Syst. 1988;25:135–140. doi: 10.1016/0165-1838(88)90018-5. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Luo Y, Kaur C, Ling EA. Neuronal and glial response in the rat hypothalamus-neurohypophysis complex with streptozotocin-induced diabetes. Brain Res. 2002;925:42–54. doi: 10.1016/s0006-8993(01)03258-9. [DOI] [PubMed] [Google Scholar]
- Luther JA, Tasker JG. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2000;523 Pt 1:193–209. doi: 10.1111/j.1469-7793.2000.t01-1-00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines MD. Carbon Monoxide: An Emerging Regulator of cGMP in the Brain. Mol Cell Neurosci. 1993;4:389–397. doi: 10.1006/mcne.1993.1049. [DOI] [PubMed] [Google Scholar]
- Mancuso C. Heme oxygenase and its products in the nervous system. Antioxid Redox Signal. 2004;6:878–887. doi: 10.1089/ars.2004.6.878. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Ragazzoni E, Tringali G, Liberale I, Preziosi P, Grossman A, Navarra P. Inhibition of heme oxygenase in the central nervous system potentiates endotoxin-induced vasopressin release in the rat. J Neuroimmunol. 1999;99:189–194. doi: 10.1016/s0165-5728(99)00112-5. [DOI] [PubMed] [Google Scholar]
- Martin DL, Madelian V, Shain W. Osmotic sensitivity of isoproterenol- and high-[K+]o-stimulated taurine release by cultured astroglia. Prog Clin Biol Res. 1990;351:349–356. [PubMed] [Google Scholar]
- McKinley MJ, Hards DK, Oldfield BJ. Identification of neural pathways activated in dehydrated rats by means of Fos-immunohistochemistry and neural tracing. Brain Res. 1994;653:305–314. doi: 10.1016/0006-8993(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Mark AL. The treatment of heart failure: the role of neurohumoral activation. Intern Med. 1998;37:112–122. doi: 10.2169/internalmedicine.37.112. [DOI] [PubMed] [Google Scholar]
- Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci. 1996;16:6255–6264. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Naruse M, Naruse K, Shioda S, Nakai Y, Uemura H. Colocalization of immunoreactive endothelin-1 and neurohypophysial hormones in the axons of the neural lobe of the rat pituitary. Endocrinology. 1993;132:530–533. doi: 10.1210/endo.132.2.8425473. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Bicknell RJ, McAllen RM, Weisinger RS, McKinley MJ. Intravenous hypertonic saline induces Fos immunoreactivity in neurons throughout the lamina terminalis. Brain Res. 1991;561:151–156. doi: 10.1016/0006-8993(91)90760-s. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Panatier A, Piet R, Mothet JP, Poulain DA, Theodosis DT. Neuron-glia interactions in the rat supraoptic nucleus. Prog Brain Res. 2008a;170:109–117. doi: 10.1016/S0079-6123(08)00410-X. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA, Theodosis DT. Glial modulation of synaptic transmission: Insights from the supraoptic nucleus of the hypothalamus. Glia. 2004;47:258–267. doi: 10.1002/glia.20032. [DOI] [PubMed] [Google Scholar]
- Oliet SHR, Panatier A, Piet R, Mothet JP, Poulain DA, Theodosis DT. Neuron-glia interactions in the rat supraoptic nucleus. In: Landgraf IDNaR., editor. Progress in Brain Research Advances in Vasopressin and Oxytocin -- From Genes to Behaviour to Disease. Vol. 170. Elsevier; 2008b. pp. 109–117. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Elekes I, Lang T, Patthy A. Taurine levels in discrete brain nuclei of rats. J Neurochem. 1986;47:1333–1335. doi: 10.1111/j.1471-4159.1986.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Panatier A, Gentles SJ, Bourque CW, Oliet SH. Activity-dependent synaptic plasticity in the supraoptic nucleus of the rat hypothalamus. J Physiol. 2006a;573:711–721. doi: 10.1113/jphysiol.2006.109447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006b;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol. 2009;587:4645–4660. doi: 10.1113/jphysiol.2009.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Son S, Stern JE. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons 10.1113/jphysiol.2007.133223. J Physiol. 2007;582:539–551. doi: 10.1113/jphysiol.2007.133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Stern JE. Characterization of a novel tonic gamma-aminobutyric acid(A) receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147:3746–3760. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- Parpura V, Baker BJ, Jeras M, Zorec R. Regulated exocytosis in astrocytic signal integration. Neurochem Int. 2010;57:451–459. doi: 10.1016/j.neuint.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Moran J, Schousboe A. Taurine release associated to cell swelling in the nervous system. Prog Clin Biol Res. 1990;351:369–376. [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. GLIA modulates synaptic transmission. Brain Res Rev. 2010;63:93–102. doi: 10.1016/j.brainresrev.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Perez-Tilve D, Stern JE, Tschop M. The brain and the metabolic syndrome: Not a wireless connection. Endocrinology. 2006;147:1136–1139. doi: 10.1210/en.2005-1586. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered Astrocyte Glutamate Trasporter Regulation of Hypothalamic Neurosecretory Neurons in Heart Failure Rats. Am J Physiol Regul Integr Comp Physiol. 2012;303(3):R291–300. doi: 10.1152/ajpregu.00056.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Raju VS, McCoubrey WK, Jr, Maines MD. Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochim Biophys Acta. 1997;1351:89–104. doi: 10.1016/s0167-4781(96)00183-2. [DOI] [PubMed] [Google Scholar]
- Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- Rana I, Stebbing M, Kompa A, Kelly DJ, Krum H, Badoer E. Microglia activation in the hypothalamic PVN following myocardial infarction. Brain Res. 2010;1326:96–104. doi: 10.1016/j.brainres.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Randolph RR, Li Q, Curtis KS, Sullivan MJ, Cunningham JT. Fos expression following isotonic volume expansion of the unanesthetized male rat. Am J Physiol. 1998;274:R1345–1352. doi: 10.1152/ajpregu.1998.274.5.R1345. [DOI] [PubMed] [Google Scholar]
- Reis WL, Biancardi VC, Son S, Antunes-Rodrigues J, Stern JE. Enhanced expression of heme oxygenase-1 and carbon monoxide excitatory effects in oxytocin and vasopressin neurones during water deprivation. J Neuroendocrinol. 2012;24:653–663. doi: 10.1111/j.1365-2826.2011.02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tong WM, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Richard D, Bourque CW. Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol. 1995;489(Pt 2):567–577. doi: 10.1113/jphysiol.1995.sp021073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Rey P, Parga JA, Munoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31:58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Rossi NF. Regulation of vasopressin secretion by ETA and ETB receptors in compartmentalized rat hypothalamo-neurohypophysial explants. Am J Physiol Endocrinol Metab. 2004;286:E535–541. doi: 10.1152/ajpendo.00344.2003. [DOI] [PubMed] [Google Scholar]
- Rossi NF, Beierwaltes WH. Nitric oxide modulation of ET(B) receptor-induced vasopressin release by rat and mouse hypothalamo-neurohypophyseal explants. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1218–1215. doi: 10.1152/ajpregu.00701.2005. [DOI] [PubMed] [Google Scholar]
- Rossi NF, Chen H. PVN lesions prevent the endothelin 1-induced increase in arterial pressure and vasopressin. Am J Physiol Endocrinol Metab. 2001;280:E349–356. doi: 10.1152/ajpendo.2001.280.2.E349. [DOI] [PubMed] [Google Scholar]
- Rossi NF, O'Leary DS, Chen H. Mechanisms of centrally administered ET-1-induced increases in systemic arterial pressure and AVP secretion. Am J Physiol. 1997a;272:E126–132. doi: 10.1152/ajpendo.1997.272.1.E126. [DOI] [PubMed] [Google Scholar]
- Rossi NF, O'Leary DS, Scislo TJ, Caspers ML, Chen H. Central endothelin 1 regulation of arterial pressure and arginine vasopressin secretion via the AV3V region. Kidney Int. 1997b;(61):S22–26. [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll R. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Santos RM, Lourenco CF, Ledo A, Barbosa RM, Laranjinha J. Nitric oxide inactivation mechanisms in the brain: role in bioenergetics and neurodegeneration. Int J Cell Biol. 2012;2012:391914. doi: 10.1155/2012/391914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Hoffman GE, Stricker EM, Sved AF. Decreases in arterial pressure activate oxytocin neurons in conscious rats. Am J Physiol. 1997;273:R1484–1483. doi: 10.1152/ajpregu.1997.273.4.R1474. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Gupta A, Szarek WA. Suppression of glial HO-1 activity as a potential neurotherapeutic intervention in AD. Curr Alzheimer Res. 2009;6:424–430. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- Schrader LA, Tasker JG. Presynaptic modulation by metabotropic glutamate receptors of excitatory and inhibitory synaptic inputs to hypothalamic magnocellular neurons. J Neurophysiol. 1997;77:527–536. doi: 10.1152/jn.1997.77.2.527. [DOI] [PubMed] [Google Scholar]
- Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek CD, Badre SE, Morsette DJ, Sidorowicz HE. Role of non-NMDA receptors in osmotic and glutamate stimulation of vasopressin release: effect of rapid receptor desensitization. J Neuroendocrinol. 1998;10:897–903. doi: 10.1046/j.1365-2826.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Jaffrey SR, Zakhary R. Nitric oxide and carbon monoxide: parallel roles as neural messengers. Brain Res Brain Res Rev. 1998;26:167–175. doi: 10.1016/s0165-0173(97)00032-5. [DOI] [PubMed] [Google Scholar]
- Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol. 2007;582:1219–1238. doi: 10.1113/jphysiol.2007.134379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE. Nitric oxide and homeostatic control: an intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog Biophys Mol Bio. 2004;84:197–215. doi: 10.1016/j.pbiomolbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Stern JE, Ludwig M. NO inhibits supraoptic oxytocin and vasopressin neurons via activation of GABAergic synaptic inputs. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1815–1822. doi: 10.1152/ajpregu.2001.280.6.R1815. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol. 2005;563:249–263. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R729–725. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker EM, Callahan JB, Huang W, Sved AF. Early osmoregulatory stimulation of neurohypophyseal hormone secretion and thirst after gastric NaCl loads. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1720–1717. doi: 10.1152/ajpregu.00548.2001. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980a;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980b;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol. 2012;24:566–576. doi: 10.1111/j.1365-2826.2011.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Montagnese C, Rodriguez F, Vincent JD, Poulain DA. Oxytocin induces morphological plasticity in the adult hypothalamo-neurohypophysial system. Nature. 1986;322:738–740. doi: 10.1038/322738a0. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Neuronal-glial and synaptic plasticity in the adult rat paraventricular nucleus. Brain Research. 1989;484:361–366. doi: 10.1016/0006-8993(89)90382-x. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol. 1999;468:175–182. doi: 10.1007/978-1-4615-4685-6_14. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Topel I, Stanarius A, Wolf G. Distribution of the endothelial constitutive nitric oxide synthase in the developing rat brain: an immunohistochemical study. Brain Res. 1998;788:43–48. doi: 10.1016/s0006-8993(97)01506-0. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011a;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011b;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Defining the role of astrocytes in neuromodulation. Neuron. 2007;54:497–500. doi: 10.1016/j.neuron.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien RY. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- van den Pol A, Wuarin J, Dudek F. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation 10.1126/science.1978759. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. J Comp Neurol. 1982;206:317–345. doi: 10.1002/cne.902060402. [DOI] [PubMed] [Google Scholar]
- Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B, Dominguez-Meijide A, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Involvement of microglial RhoA/Rho-Kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors. Neurobiol Dis. 2012;47:268–279. doi: 10.1016/j.nbd.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Das S, Maines MD. Brain heme oxygenase isoenzymes and nitric oxide synthase are co-localized in select neurons. Neuroscience. 1994;63:223–231. doi: 10.1016/0306-4522(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Watts SW. Endothelin receptors: what's new and what do we need to know? Am J Physiol Regul Integr Comp Physiol. 2010;298:R254–260. doi: 10.1152/ajpregu.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J, Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yuan H, Gao B, Duan L, Jiang S, Cao R, Xiong YF, Rao ZR. Acute hyperosmotic stimulus-induced Fos expression in neurons depends on activation of astrocytes in the supraoptic nucleus of rats. J Neurosci Res. 2010;88:1364–1373. doi: 10.1002/jnr.22297. [DOI] [PubMed] [Google Scholar]
- Zampronio AR, Kuzmiski JB, Florence CM, Mulligan SJ, Pittman QJ. Opposing actions of endothelin-1 on glutamatergic transmission onto vasopressin and oxytocin neurons in the supraoptic nucleus. J Neurosci. 2010;30:16855–16863. doi: 10.1523/JNEUROSCI.5079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H995–1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol. 1997;273:R874–872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gong N, Wang W, Xu L, Xu TL. Bell-shaped D-serine actions on hippocampal long-term depression and spatial memory retrieval. Cereb Cortex. 2008;18:2391–2401. doi: 10.1093/cercor/bhn008. [DOI] [PubMed] [Google Scholar]
- Zheng H, Mayhan WG, Bidasee KR, Patel KP. Blunted Nitric Oxide-Mediated Inhibition Of Sympathetic Nerve Activity Withinthe Paraventricular Nucleusin Diabetic Rats. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00363.2005. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]