Abstract
OBJECTIVE:
The purpose of this study was to assess the utility of preoperative head ultrasound scan (HUS) in a cohort of newborns also undergoing preoperative MRI as part of a prospective research study of brain injury in infants having surgery for congenital heart disease (CHD).
METHODS:
A total of 167 infants diagnosed with CHD were included in this 3-center study. None of the patients had clinical signs or symptoms of preoperative brain injury, and all patients received both HUS and brain MRI before undergoing surgical intervention. HUS and MRI results were reported by experienced neuroradiologists who were blinded to any specific clinical details of the study participants. The findings of the individual imaging modes were compared to evaluate for the presence of brain injury.
RESULTS:
Preoperative brain injury was present on HUS in 5 infants (3%) and on MRI in 44 infants (26%) (P < .001). Four of the HUS showed intraventricular hemorrhage not seen on MRI, suggesting false-positive results, and the fifth showed periventricular leukomalacia. The predominant MRI abnormality was white matter injury (n = 32). Other findings included infarct (n = 16) and hemorrhage (n = 5).
CONCLUSIONS:
Preoperative brain injury on MRI was present in 26% of infants with CHD, but only 3% had any evidence of brain injury on HUS. Among positive HUS, 80% were false-positive results. Our findings suggest that routine HUS is not indicated in asymptomatic term or near-term neonates undergoing surgery for CHD, and MRI may be a preferable tool when the assessment of these infants is warranted.
Keywords: brain imaging, congenital heart disease, head ultrasound, neonate, MRI
What’s Known on This Subject:
Routine head ultrasound scans (HUSs) are frequently performed in the preoperative evaluation of the infants with congenital heart disease, and brain MRI is being increasingly used in the research setting. The utility of HUSs in this population has not yet been established.
What This Study Adds:
This is the first study to prospectively evaluate the utility of routine HUSs compared with MRIs in asymptomatic newborns and young infants undergoing cardiac surgery. Our findings suggest that routine HUS is not indicated in asymptomatic term or near-term neonates undergoing surgery for CHD.
Neurodevelopmental impairment is the most common long-term, noncardiac morbidity affecting neonates undergoing surgery for congenital heart disease (CHD). The impact of this impairment is far-reaching and may not be manifest very early in life but instead may become apparent in later childhood, often extending into adolescence.1–5 Given the potential relationship between brain injury and impaired outcome, and the possibility that aspects of cardiac surgery could further worsen preexisting brain injury, preoperative brain imaging is commonly used in the clinical setting as well as in the context of prospective investigations of brain injury in this high-risk group.
The spectrum of brain injury affecting newborns with heart disease includes stroke or infarct, hemorrhage, and white matter injury (WMI), as well as more subtle maturational, structural, and metabolic abnormalities. Although the impact of these changes on subsequent neurodevelopment is not yet clear, there is an increasing appreciation for the importance of preoperative evaluation in this population. In many centers performing cardiac surgery in young infants, head ultrasound scans (HUSs) have become a routine part of the preoperative evaluation of young infants with CHD because they are relatively inexpensive, easily performed, safe, and readily available. However, in contrast with other neonatal preterm and term populations,6 the utility of HUS to detect brain injury has not been established in the CHD population. Indeed, the overall detection rates of brain injury in infants with CHD range from 5% to 59% in the multiple studies performed.7–11 Over the past decade, the use of MRI in infants with CHD has been increasing, although mostly in the research setting. A number of studies have reported the rate of preoperative brain injury on MRI in young infants undergoing surgery for CHD to be between 25% and 40%.12–16 In noncardiac infant populations, MRI has been shown to be superior to HUS in sensitivity and specificity for detection of brain injury, which included white matter abnormalities or periventricular leukomalacia (PVL),6 ischemia,17 and hemorrhage,6,17 but this comparison has not been made in infants with CHD.
The purpose of the current study was to assess the utility of preoperative HUS in a cohort of newborns and infants who also underwent preoperative MRI as part of prospective research studies of brain injury in the setting of surgery for CHD.
Methods
Participants
Participants were enrolled in prospective studies of brain injury related to CHD at 1 of 3 pediatric cardiac centers: Starship Children’s Hospital (SCH), Auckland, New Zealand; The Royal Children’s Hospital (RCH), Melbourne, Australia; and Texas Children’s Hospital (TCH), Houston, Texas. The studies were all approved by the local institutional review boards, and written consent was obtained from parents of all participants. The subgroup of participants included in the current study population were infants with CHD requiring surgical intervention before 8 weeks of age who received both HUS and MRI preoperatively. Infants were excluded if they were <35 weeks’ gestational age at birth, had a recognized genetic or malformation syndrome independently associated with neurodevelopmental impairment, or had an abnormal result on neurologic examination before surgery.
Neuroimaging
HUS was performed at the bedside by using a Zonare ultrasound (Zonare Medical Systems, Inc, Mountain View, CA) or LOGIQ E9 ultrasound (GE Healthcare, Waukesha, WI). An experienced neuroradiologist interpreted each HUS, and the results remained a part of the clinical record. At TCH, MRI scans were performed under general anesthesia and immediately before planned surgery, whereas participants at SCH and RCH were not specifically anesthetized or sedated for the purpose of performing the MRI scans. MRI was performed by using a 1.5- or 3.0-T Magnetom Avanto scanner (Siemens AG, Erlangen, Germany) or a 1.5-T Intera scanner (Philips Medical Systems, Best, the Netherlands). Standardized sequences were used for all studies, including coronal 3D-FLAIR T1-weighted images (1-mm slice thickness), coronal and axial T2-weighted dual-echo, fast spin-echo images (2-mm slice thickness), and axial diffusion-weighted imaging (DWI) (12–20 directions, 4-mm slice thickness). MRI scans were interpreted independently by a neuroradiologist in each institution who was not aware of the clinical details, HUS results, other imaging results, or clinical treatment of any of the infants. The reports for all scans were made available in the medical record so that the treating medical team and parents of enrolled patients could be informed of the results.
For the purposes of this study, the abnormalities that were focused on were focal infarction (stroke), WMI, or hemorrhage (intraventricular or parenchymal). Stroke referred to discrete areas involving the cerebral cortical or deep nuclear gray matter of hyperintensity on DWI, with hypointensity on the corresponding apparent diffusion coefficient scan and/or hyperintensity on T2-weighted images (Fig 1). These were classified as less than one-third, one-third to two-thirds, or more than two-thirds of the vascular territory of the anterior cerebral artery, middle cerebral artery, or posterior cerebral artery in 1 hemisphere.18 WMI referred to discrete, usually punctate, foci of T1 hyperintensity and/or T2 hypointensity. This outcome was classified as normal (no WMI), mild (≤3 foci and all ≤2 mm), moderate (>3 and ≤10 foci or any >2 mm), or severe (>10 foci or 10% white matter).18 Subdural hemorrhage was noted and/or recorded but not considered as brain injury given its frequent occurrence in the healthy neonatal population.19 Hemorrhage was classified according to grade for intraventricular hemorrhage (IVH) and size (1–5 mm, 6–15 mm, and >15 mm) for intraparenchymal hemorrhage.
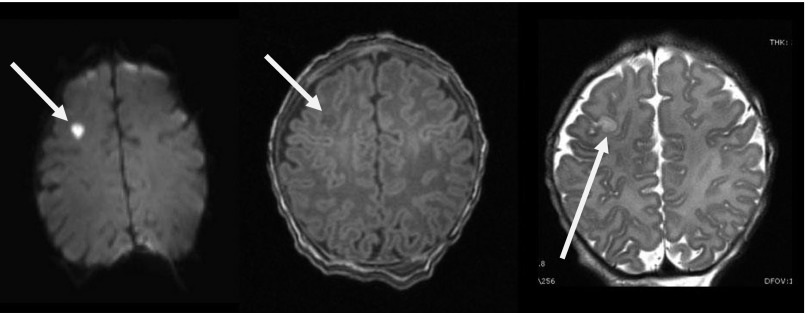
FIGURE 1.
Stroke on MRI scan. A right cortical stroke (white arrow) identified by a high-signal intensity on DWI (left), a low-signal intensity on T1-weighted imaging (middle), and increased signal intensity on T2-weighted imaging (right) on the MRI scan of an asymptomatic newborn. This finding on MRI resulted in delay of surgery for this patient who had a normal HUS.
Analysis
Data were analyzed by using SAS/STAT software for Microsoft Windows version 9.3 (SAS Institute, Inc, Cary, NC). Continuous variables were summarized according to mean ± SD and medians (interquartile range) as appropriate. Discrete variables were summarized according to frequency and percentage. The primary outcome was presence of preoperative brain injury on HUS versus MRI, and the frequency of positive diagnosis was compared across procedures by using the exact McNemar test. Patient characteristics in neonates with preoperative brain injury on MRI were compared with those without preoperative brain injury by using Fisher’s exact test and the nonparametric Wilcoxon rank-sum test as appropriate. Statistical significance was assessed at the .05 level.
Results
A total of 167 study participants fulfilled the inclusion criteria, had no neurologic abnormalities on examination, and underwent both HUS and MRI scans before surgery. Patient characteristics are given in Table 1. The mean gestational age at birth and birth weight were 38.6 ± 1.4 weeks and 3.2 ± 0.5 kg, respectively. Infants were categorized according to preoperative physiology into 1 of 4 groups as described previously.20 Briefly, the 4 groups were single ventricle (SV; n = 16), single ventricle with arch obstruction (SVA; n = 73), biventricular (2V; n = 63), and biventricular with arch obstruction (2VA; n = 15). Within each group, the most common diagnosis was as follows: SV, pulmonary atresia in 9 (56%) patients; SVA, hypoplastic left heart syndrome in 61 (84%) patients; 2V, transposition of the great arteries in 47 (75%) patients; and 2VA, coarctation of the aorta in 11 (73%) patients.
TABLE 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Birth weight, kg | 3.2 ± 0.5 |
| Gestational age, wk | 38.6 ± 1.4 |
| Head circumference, cm | 33.8 ± 1.6 |
| Age at surgery, d | 7 (4–9) |
| Days between imaging examinations | 3 (1–6) |
| Gender | |
| Male | 99 (59) |
| Female | 68 (41) |
| Mode of deliverya | |
| Cesarean | 61 (37) |
| Vaginal | 105 (63) |
| Diagnostic category | |
| SV physiology | 16 (10) |
| SVA physiology | 73 (44) |
| 2V physiology | 63 (38) |
| 2VA physiology | 15 (9) |
| Enrollment site | |
| SCH | 23 (14) |
| RCH | 51 (31) |
| TCH | 93 (56) |
Data are presented as mean ± SD, median (interquartile range), or absolute numbers (%).
Mode of delivery was unknown in 1 case.
HUS and MRI Findings
HUS was performed before MRI in 137 (82%) of the patients; in the remainder, HUS were performed on the same day or afterward. The mean age at HUS and MRI were 3.8 ± 6.0 and 8.1 ± 7.7 days, respectively, and the interval between scans was 4.3 ± 5.8 days. The interval between the 2 imaging studies was not related to the presence or absence of brain injury (P = .70). Brain injury was diagnosed in 5 infants (3%) by using HUS (Tables 2 and 3). Four patients were diagnosed with grade 1 IVH and 1 with PVL. Overall, brain injury was present on MRI in 44 (26%) infants. The most common abnormality was WMI, which was present in 32 infants; 23 had mild WMI, 8 had moderate WMI, and 1 had severe WMI. Sixteen had evidence of infarct on MRI; 11 had infarcts less than one-third, 4 had infarcts one-third to two-thirds, and 1 had an infarct more than two-thirds of vascular territory. Hemorrhage was present in 5 infants;1 had grade 2 IVH, 3 had intraparenchymal hemorrhages 1 to 5 mm in size, and 1 had intraparenchymal hemorrhage >15 mm in size.
TABLE 2.
Overall Imaging Findings
| HUS Result | MRI Result | |
|---|---|---|
| Normal | Abnormal | |
| Normal | 121 | 41 |
| Abnormal | 2 | 3 |
TABLE 3.
HUS and MRI Findings
| Result | HUS Positive | MRI Positive | Both Positive |
|---|---|---|---|
| IVH | 4 | 5 | 0 |
| WMI or PVL | 1 | 32 | 1 |
| Infarct | 0 | 16 | 0 |
HUS and MRI Correlation
The overall relationship between HUS and MRI is given in Table 2. The incidence of brain injury on HUS was 3% (95% confidence interval: 1.0–6.9) and on MRI it was 26% (95% confidence interval: 19.8–33.7) (P < .001). When examining the correlation between HUS and MRI findings in further detail (Table 3), of the 4 infants with a grade 1 IVH on HUS, none had a corresponding IVH on MRI, whereas the single infant with PVL on HUS did have corresponding MRI injury. Therefore, the positive predictive value of HUS for the presence of any brain injury was only 20%.
Potential Risk Factors for Brain Injury
When we examined the subset of patients with brain injury according to their physiologic diagnosis, there was MRI-confirmed brain injury in 5 of the 16 infants with SV physiology, in 19 of 73 with SVA, 18 of 63 with 2V, and 2 of 15 with 2VA. The rate of brain injury was not related to diagnostic category (P = .68). For the group as a whole, baseline characteristics (Table 4) were similar in those with brain injury and those without except with respect to gestational age at birth; median gestational age for infants with MRI injury was 1 week less than those without (P = .006).
TABLE 4.
Patient Characteristics by Group
| Characteristic | Normal MRI | Abnormal MRI | Pa |
|---|---|---|---|
| Birth weight, kg | 3.2 ± 0.5 | 3.1 ± 0.5 | .23 |
| Gestational age, wk | 39 (38–39.7) | 38 (37.5–39) | .006 |
| Head circumference, cm | 33.8 ± 1.7 | 33.8 ± 1.5 | .89 |
| Age at surgery, d | 7 (5–11.5) | 7 (4–9.3) | .41 |
| Days between HUS and MRI | 4.7 ± 6.3 | 3.8 ± 3.1 | .70 |
| Female gender | 55 (45) | 13 (30) | .11 |
| Vaginal delivery | 77 (63) | 28 (64) | .99 |
| Diagnostic category | .68 | ||
| SV | 11 (9) | 5 (11) | |
| SVA | 54 (44) | 19 (43) | |
| 2V | 45 (37) | 18 (41) | |
| 2VA | 13 (10) | 2 (5) | |
| Enrollment site | .73 | ||
| SCH | 18 | 5 (22) | |
| RCH | 39 | 12 (24) | |
| TCH | 66 | 27 (29) |
Data are presented as mean ± SD, median (IQR), or absolute numbers (%). For enrollment site data, percentages in parentheses represent row percentages (ie, the percentage of patients with brain injury at each site).
Discussion
To the best of our knowledge, this is the first prospective study of the relationship between HUS and MRI in asymptomatic newborns before surgery for CHD. This study has several important findings relating to brain injury before surgical intervention. First, we showed that brain injury was present in more than one-quarter of our patients. Second, the pattern of brain injury was mostly WMI, with stroke and hemorrhage being much less common. Lastly, but possibly most clinically relevant, we found that HUS was not a reliable screening tool for preoperative brain injury in this patient population.
It is important to note that this study evaluated the use of HUS as a routine screening tool in the preoperative management of asymptomatic neonates with CHD, and it cannot be extrapolated to guide postoperative decision-making or perioperative management of patients deemed to be more fragile.
The rate of preoperative brain injury diagnosed by using MRI in our patients was 26%. Previous studies report incidences of preoperative brain injury ranging from 29% to 43%.12,14,15,21 It is important to note that our cohort did not include infants with any clinical manifestations or suspicion of brain injury, nor did we consider for the purpose of this report more subtle abnormalities such as maturational changes. Block et al15 showed that preoperatively identified brain injury did not progress or extend from preoperative to postoperative MRI analysis. This is an important fact when determining routine imaging need in asymptomatic infants before surgical intervention because a preoperative imaging study (eg, HUS) can be avoided, awaiting instead a more comprehensive postoperative imaging analysis if deemed necessary by clinical status.
The nature of injury in this study population of neonates with CHD was predominantly WMI, with an overall incidence of 19%. Infarct was present in 10% and hemorrhage was present in 3% of patients in our analysis. Our findings are in keeping with previously published studies that report incidences of WMI ranging from 10% to 38% and infarcts or hemorrhage ranging from 0% to 33%.12,14,15,20
In our analysis, HUS proved to not only be unreliable but also potentially misleading in the preoperative evaluation of the CHD population, with a false-positive rate of 80%. False-positive findings on HUS have the potential of increasing parental stress and anxiety, and of potentially influencing management decisions. Fortunately for our patients, this discrepancy was able to be detected after confirmatory MRI testing revealed the error, but in the nonresearch setting, without a confirmatory MRI, this finding could have very different ramifications.
There were a number of findings reported on each imaging modality that have not been included in our study. Incidental HUS findings in our cohort that were not included in our analysis were choroid plexus cyst, lenticulostriate vasculopathy (LSV), periventricular echogenicity, and prominence of choroid plexus. These are all common findings on HUS, are not indicative of brain injury, and would not be expected to affect clinical management or patient outcome.22,23 When considering MRI analysis, we did not include abnormalities of maturation that we have reported elsewhere24 or some specific abnormalities of anatomy or function. Although these findings could be of significance to the patient, a clear HUS corollary does not exist due to the intrinsic nature of the 2 modalities.
Studies range widely when examining the rates of abnormalities found on preoperative HUS in infants with CHD. In 1994, Krull et al7 reported abnormalities on preoperative HUS in 9% of patients. These abnormalities included structural abnormalities and variable increases in ventricular size; importantly, this cohort included infants with coexisting prematurity and asphyxial injury. Another study reported a 42% incidence of abnormalities on HUS, with the majority having ventriculomegaly or enlargement of the subarachnoid space, LSV, or calcifications in the basal nuclei.8 Gonzalez et al9 reported that 21% of infants with CHD had significant or marginally significant abnormalities on HUS. One of the most frequently cited articles about findings on HUS in CHD indicates an incidence of brain injury of 59% diagnosed with HUS, with the majority again demonstrating LSV and/or cerebral atrophy.11 None of these studies used MRI as a gold standard tool for comparison, and the majority of published series include patients with other significant risk factors for brain injury, including prematurity and birth asphyxia. Therefore, it is difficult and overspeculative to draw many conclusions when comparing their findings with ours. Glauser et al25 recommended screening examinations of intracranial anatomy in all patients with hypoplastic left heart syndrome due to a somewhat elevated incidence of cerebral abnormalities found on autopsy. Of note, their cohort included infants with significant genetic or malformation syndromes that could be associated with neurodevelopmental impairment. Although this feature would contrast with our patient cohort (because significant syndromes was an exclusion criterion for the study), this important article was among the first to recommend routine screening in the complex CHD population. In summary, our cohort of 167 patients is among the largest preoperative CHD populations that have been studied to date7,8,10–12,14,15,18,21 and unique in that we were able to compare and contrast HUS with MRI.
The major limitation of the study relates to the variable interval between HUS and MRI scans. This limitation was in part related to the approach to preoperative MRI, which was immediately before surgery in 1 of the 3 institutions, whereas in the other 2, this was not the case. Overall, the MRI scans were usually performed ∼4.3 days after the HUS, whereas ideally, the 2 imaging studies would be performed as close as possible to each other. However, the studies were performed closer together in those with brain injury than those without, and importantly there was no significant difference in the rates of injury between centers. A second limitation is that given the very different jurisdictions and financial models that exist in our population, we could not apply an accurate financial analysis to this study. However, it is likely that a more targeted investigational approach would be associated with significant cost savings.
Conclusions
Brain injury was a common finding on MRI in these infants before surgery for CHD. The majority of brain injury diagnosed was WMI, and arterial ischemic strokes and hemorrhages were less common in this population. Brain injury was rarely seen on HUS, and the majority of these abnormal findings were subsequently found to be false-positive results. The single instance of true PVL diagnosed on HUS would not affect surgical plans. Therefore, routine HUS is not indicated in asymptomatic term and near-term inants undergoing surgery for CHD.
Acknowledgments
We acknowledge Dr Terrie Inder for the original design of the study and Dr Ayton Hope for his assistance with MRI analysis. We also thank Marcie Meador for her help with study coordination.
Glossary
- 2V
biventricular
- 2VA
biventricular with arch obstruction
- CHD
congenital heart disease
- DWI
diffusion-weighted imaging
- HUS
head ultrasound scan
- IVH
intraventricular hemorrhage
- LSV
lenticulostriate vasculopathy
- PVL
periventricular leukomalacia
- RCH
Royal Children’s Hospital
- SCH
Starship Children’s Hospital
- SV
single ventricle
- SVA
single ventricle with arch obstruction
- TCH
Texas Children’s Hospital
- WMI
white matter injury
Footnotes
Dr Rios was responsible for the collation of data, data analysis and interpretation of results, drafting and revising the initial manuscript, and approval of the final manuscript as submitted; Dr Welty was responsible for research supervision, data preparation and interpretation of the results, and assisted in revising the final manuscript; Dr Gunn was responsible for the acquisition and collation of data, analysis and interpretation of results, manuscript preparation and revision, and approval of the final manuscript; Dr Beca was responsible for the initiation of the study, acquisition and collation of data and interpretation of results, as well as preparation and revision and approval of the final manuscript; Dr Minard was responsible for data analysis, interpretation of results, writing the statistical analysis sections, and contributing to manuscript revisions; Ms Goldsworthy was responsible for data acquisition and entry, and contributed to data analysis, revision, and approval of the final manuscript; Drs Coleman and Hunter were responsible for MRI and head ultrasound scan interpretation, scoring and analysis, as well as contributing to the revision and approval of the final manuscript; Dr Andropoulos was responsible for the study design and data collection and interpretation, as well as preparation, revision, and approval of the final manuscript; and Dr Shekerdemian was responsible for the initiation of the study, the study design, as well as the amalgamation of data, interpretation of results, and preparation, revision, and approval of the manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Dan L. Duncan Institute for Clinical and Translational Research at Baylor College of Medicine, Houston, Texas; the National Heart Foundation of Australia; the Heart Foundation of New Zealand; the Green Lane Research and Education Fund; Australian and New Zealand Intensive Care Foundation; the Murdoch Childrens Research Institute; the Victorian Government’s Operational Infrastructure Support Program; US National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant 1R21-HD55501–01; Charles A. Dana Foundation, Brain and Immuno-Imaging Grant; Baylor College of Medicine General Clinical Research Center Grant #0942, funded by NIH M01 RR00188; and Texas Children’s Hospital Pediatric Anesthesiology Research Fund. Funded by the National Institutes of Health (NIH).
References
- 1.Dittrich H, Bührer C, Grimmer I, Dittrich S, Abdul-Khaliq H, Lange PE. Neurodevelopment at 1 year of age in infants with congenital heart disease. Heart. 2003;89(4):436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126(5):1385–1396 [DOI] [PubMed] [Google Scholar]
- 3.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121(4). Available at: www.pediatrics.org/cgi/content/full/121/4/e759 [DOI] [PubMed] [Google Scholar]
- 4.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114(5). Available at: www.pediatrics.org/cgi/content/full/114/5/e572 [DOI] [PubMed] [Google Scholar]
- 5.Snookes SH, Gunn JK, Eldridge BJ, et al. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics. 2010;125(4). Available at: www.pediatrics.org/cgi/content/full/125/4/e818 [DOI] [PubMed] [Google Scholar]
- 6.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58(12):1726–1738 [DOI] [PubMed] [Google Scholar]
- 7.Krull F, Latta K, Hoyer PF, Ziemer G, Kallfelz HC. Cerebral ultrasonography before and after cardiac surgery in infants. Pediatr Cardiol. 1994;15(4):159–162 [DOI] [PubMed] [Google Scholar]
- 8.Te Pas AB, van Wezel-Meijler G, Bökenkamp-Gramann R, Walther FJ. Preoperative cranial ultrasound findings in infants with major congenital heart disease. Acta Paediatr. 2005;94(11):1597–1603 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez JH, Shirali GS, Atz AM, et al. Universal screening for extracardiac abnormalities in neonates with congenital heart disease. Pediatr Cardiol. 2009;30(3):269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosti L, Giamberti A, Chessa M, et al. Pattern of cerebral ultrasound in neonatal heart surgery. Pediatr Med Chir. 2011;33(3):124–128 [PubMed] [Google Scholar]
- 11.van Houten JP, Rothman A, Bejar R. High incidence of cranial ultrasound abnormalities in full-term infants with congenital heart disease. Am J Perinatol. 1996;13(1):47–53 [DOI] [PubMed] [Google Scholar]
- 12.Petit CJ, Rome JJ, Wernovsky G, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119(5):709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beca J, Gunn J, Coleman L, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009;53(19):1807–1811 [DOI] [PubMed] [Google Scholar]
- 14.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 suppl 1):I109–I114 [PubMed] [Google Scholar]
- 15.Block AJ, McQuillen PS, Chau V, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140(3):550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andropoulos DB, Easley RB, Brady K, et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. Ann Thorac Surg. 2012;94(4):1250–1255, discussion 1255–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blankenberg FG, Loh NN, Bracci P, et al. Sonography, CT, and MR imaging: a prospective comparison of neonates with suspected intracranial ischemia and hemorrhage. AJNR Am J Neuroradiol. 2000;21(1):213–218 [PMC free article] [PubMed] [Google Scholar]
- 18.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139(3):543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looney CB, Smith JK, Merck LH, et al. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 2007;242(2):535–541 [DOI] [PubMed] [Google Scholar]
- 20.Clancy RR, McGaurn SA, Wernovsky G, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119(2):347–357 [DOI] [PubMed] [Google Scholar]
- 21.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–1938 [DOI] [PubMed] [Google Scholar]
- 22.Bernier FP, Crawford SG, Dewey D. Developmental outcome of children who had choroid plexus cysts detected prenatally. Prenat Diagn. 2005;25(4):322–326 [DOI] [PubMed] [Google Scholar]
- 23.Weber K, Riebel T, Nasir R. Hyperechoic lesions in the basal ganglia: an incidental sonographic finding in neonates and infants. Pediatr Radiol. 1992;22(3):182–186 [DOI] [PubMed] [Google Scholar]
- 24.Beca J, Gunn J, Coleman L, et al. New white matter injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest [published online ahead of print January 31, 2013]. Circulation. [DOI] [PubMed] [Google Scholar]
- 25.Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85(6):984–990 [PubMed] [Google Scholar]