Abstract
Background For children with diabetes, metabolic control typically declines across the adolescent years. Objective The longitudinal interplay between supportive relationships with parents and metabolic control were investigated in families that differ in parents’ restrictiveness. Method The time-dependent links between perceived parental social support and metabolic control were investigated in a sample of 109 German adolescents with diabetes. 3 waves of data were collected at annual intervals; metabolic control (indexed by HbA1c) was assayed by physicians annually. Results Family restrictiveness moderated longitudinal associations between metabolic control and perceived social support. For adolescents reporting high family restrictiveness, poorer initial metabolic control predicted greater subsequent declines in perceived parent social support, and lower initial perceived parental social support predicted greater subsequent deterioration in metabolic control. Conclusion The findings add to the growing body of work suggesting that restrictiveness is a risk factor that exacerbates problems associated with low perceived parental support.
Keywords: diabetes, longitudinal links, metabolic control, parental support, restrictive families
Introduction
Diabetes is a chronic disease that affects not only the lives of afflicted adolescents, but also the lives of their parents. Successful treatment of Type 1 diabetes requires family involvement in diet, insulin administration, and blood glucose monitoring (Seiffge-Krenke, 2001). These aspects of the disease can exacerbate the challenges confronting families with adolescent children. Parents of healthy adolescents often struggle to find the right balance of support and limits (Laursen & Collins, 2009). The management of Type 1 diabetes makes the task of providing support more difficult. Children with diabetes and their parents are confronted with relationship alterations prompted by adolescent individuation and autonomy during a time when pubertal changes can prompt dangerous increases in blood glucose (Seiffge-Krenke, 1998a). The present study examines the longitudinal interplay between parent support and metabolic control in families that differ in adolescent perceptions of family restrictiveness. We test the hypothesis that high restrictiveness is a risk factor, prompting a worsening of metabolic control for adolescents who consider parents to be unsupportive.
Metabolic control (i.e., the maintenance of healthy levels of blood glucose) typically declines across the adolescent years, with a commensurate rise in complications such as hyperglycemia and diabetic coma (e.g., Luyckx & Seiffge-Krenke, 2009; Wysocki, 1993). Results from the Diabetes Control and Complications Trial (DCCT, 1995) indicated that improved metabolic control significantly reduces the incidence and progression of microvascular and neuropathic complications for adolescent patients aged >13 years. Successful treatment of Type 1 diabetes rests heavily on how adolescents and their families deal with the many challenges inherent to treatment such as dietary restrictions, exercising regularly, frequent monitoring of blood sugar, and daily insulin administrations. Families of adolescents with Type 1 diabetes, not surprisingly, go to great lengths to maintain optimal blood glucose, as the American Diabetes Association (Silverstein et al., 2005) recommends HbA1c levels of ≤7.5. Adolescents feel constrained by the strict treatment regimen (Pisula & Czaplinska, 2010; Seiffge-Krenke & Stemmler, 2003), and many of them resent the frequent ministrations of physicians and parents, which complicates adherence and self-care (Kyngäs, Hentinen, & Barlow, 1998). Teens in focus groups frequently describe their parents as losing sight of them as people and seeing them solely as “having diabetes” (Weinger, O’Donnell, & Ritholz, 2001). This suggests that perceptions of parents are an important factor in the adolescent’s maintenance of healthy levels of blood glucose.
Even under optimum circumstances, parents struggle with the growing autonomy demands of adolescent children. Parents of adolescents with Type 1 diabetes must balance relationship renegotiation against the prospect of deteriorating metabolic control. A large body of research has investigated links between family variables and outcomes such as treatment adherence and metabolic control. Most studies show that parental emotional support, particularly with regards to the difficulties of living with diabetes, is associated with improved metabolic control, whereas unsupportive parental behavior is correlated with regimen adherence problems and poor metabolic control (Burroughs, Harris, Ponious, & Santiago, 1997; Kyngäs & Rissanen, 2001; Lewin et al., 2006; Levandowski & Drotar, 2007). These studies are representative of research on families of children with diabetes in that they are parent-driven. In parent-driven conceptual models, the child’s well-being is assumed to be a product of parent socialization efforts. Thus, supportive relations between parents and offspring with diabetes may promote medical adherence that can help ameliorate deterioration in metabolic control brought on by puberty.
Child-driven conceptual models hold that children are responsible for changes in parent behavior. Parent support declines over the adolescent years, but the rate of this decline appears to depend on characteristics of the child (Kerr, Stattin, & Pakalniskiene, 2008) and initial characteristics of the parent–child relationship (Laursen, DeLay, and Adams, 2010). Previous studies indicate that parents react negatively to adolescent problem behavior such that externalizing symptoms in early adolescence predict the slope of change in perceived support from mothers and fathers, with the steepest declines in support for those with the highest levels of initial problems (Hafen & Laursen, 2009). In the context of families with an adolescent who has diabetes, it is likely that changes in metabolic control, which are of major concern for parents, may prompt similar changes in parental support during the adolescent years. Rather than struggle to enforce treatment adherence, parents may simply respond to worsening metabolic control by disengaging and withdrawing support. It is important to note that child-driven models are not antithetical to parent-driven models; some have described the conjoint effects as transactional (Sameroff & MacKenzie, 2003).
Although a number of studies have highlighted the protective functions of a positive supporting family climate for adherence and metabolic control (Burroughs et al., 1997; Lewandowski & Drotar, 2007), there is also indication that a certain family climate might be a risk factor during the adolescent years, contributing to a deterioration of metabolic control. Results from a large-scale multicenter study in 19 countries indicate that between a quarter and a third of adolescents consider their parents to be overprotective and complain that their everyday life is governed by too many rules (Cameron et al., 2008). A number of studies found poor levels of metabolic control in adolescents with diabetes who describe their families in terms of high levels of restrictiveness (Davis et al., 2001) and overprotection (Ellis et al., 2008; Mullins et al., 2007). Consistent with previous correlational studies implying that restrictiveness is a risk factor for poor metabolic control (Butler, Skinner, Gelfand, Berg, & Wiebe, 2007; Davis et al., 2001), we focus on family restrictiveness as manifested in excessive and strict rule setting and overcontrol. High levels of restrictiveness may be viewed by adolescents with diabetes as an unwanted intrusion into their autonomy development. Thus, we consider restrictiveness to be a risk factor that amplifies associations arising from low parental support.
Research Questions
Our study examines changes in metabolic control during adolescence as a function of perceived parental support in families that vary in terms of restrictiveness. Using a longitudinal design, we investigate families with an adolescent with diabetes. We focus on the adolescent years because of its high incidence of deteriorating metabolic control. Family relationships are also in transition during adolescence, making this an important developmental turning point.
The first aim of the study is to replicate the well-established finding that adolescent metabolic control declines across ages 14–16 years (American Diabetes Association, 2010; Helgeson, Siminerio, Escobar, & Becker, 2008; Luyckx & Seiffge-Krenke, 2009). Many adolescents with diabetes do not adhere to their prescribed treatment, which has an adverse impact on metabolic control (Seiffge-Krenke, 2001; Wysocki, 1993). Thus, we expect metabolic control to worsen with age.
A second aim of the study is to examine reciprocal longitudinal associations between deterioration in metabolic control and declining parental support. We know that adolescents from families perceived to be low in diabetes-specific support are less apt to adhere to treatment regimens and more apt to have poor metabolic control (Burroughs et al., 1997; Ryan, 2003). Our study focuses on global (i.e., non-illness specific) support in the everyday life of the adolescent. We assume that global parental support serves as a buffer against stress and should protect against age-related deterioration in metabolic control. We further expect that parents will react to adolescent difficulties associated with maintaining metabolic control the way they react to conduct problems (e.g., Hafen & Laursen, 2009) with diminished support. We therefore use cross-lagged path analyses to determine the temporal sequence between physician reports of metabolic control and adolescent reports of maternal and paternal social support. We hypothesize that low levels of parental support would anticipate deteriorating metabolic control and that poor metabolic control would anticipate declining parental support.
A third aim of the study, approached via a multiple group path analysis, is to examine family restrictiveness as a potential moderator of longitudinal associations between perceived parental support and adolescent metabolic control. Of concern is the level of restrictiveness in families with adolescents with diabetes as a potential moderator. Studies suggest that too much structure and rule setting interferes with the normal developmental process through which adolescents assume increasing responsibility for their own treatment and their everyday life (Butler et al., 2007; Ellis et al., 2008). We hypothesize that restrictiveness would have a paradoxical effect. Associations between declining support and worsening metabolic control are expected to be moderated by family restrictiveness, such that associations would be exacerbated in families high in restrictiveness. We do not necessarily anticipate worse outcomes for youth in families with high restrictiveness compared with those in low- or medium-restrictive families, but we do expect stronger patterns of association between parental support and adolescent metabolic control because excessive and strict rule setting could give rise to a hostile climate that puts adolescents at risk for adverse consequences arising from low support.
Previous studies investigating metabolic control have mainly focused on adolescents and their mothers. We expect similar, although perhaps somewhat weaker patterns of influence for fathers than for mothers, given that fathers tend to be less involved in the treatment of children with diabetes (Phares, Lopez, Fields, Kamboukos, & Duhig, 2005; Seiffge-Krenke, 2002). Internalizing and externalizing symptoms can interfere with adolescents’ treatment adherence and, consequently, glycemic control (Cohen, Lumley, Naar-King, Partridge, & Cakan, 2004). As higher levels of restrictiveness in parents may occur when the child exhibit problem behaviors, we control for the level of externalizing and internalizing symptomatology in the adolescent offspring. Besides behavior problems, physical maturity and disease onset are known to be associated with parental support and metabolic control (Berg et al., 2011; Hoeve, Dubas, Gerris, van der Laan, & Smeek, 2011; Hood et al., 2006). Hence, we also control for each in our analyses.
Method
Participants and Procedure
The adolescent participants were from the German Longitudinal Study on Juvenile Diabetes (Seiffge-Krenke, 2001), which received full Institutional Review Board approval from the first author’s home university. The parents of all participants provided informed consent and adolescent participants provided written assent. A total of 109 patients with Type 1 diabetes (51 girls and 58 boys) were recruited from 17 outpatient pediatric health care facilities in two German cities. The participants had a mean age of 13.77 years (standard deviation [SD] = 1.41) at the outset. Their mothers (N = 109; mean age: 42.21 years, SD = 3.6), fathers (N = 81; mean age: 45.78 years, SD = 4.1), and physicians (N = 100) also participated. Patients had an average of 1.35 (SD = 1.18) siblings. According to parents, children were diagnosed with diabetes 1–4 years (mean [M] = 2.97, SD = 1.15) before the start of the study. Over 90% of the sample was of German descent; the rest had origins in Turkey or Southern Europe. The patients had diverse socioeconomic backgrounds. Self-reports of paternal education indicated that 24% of fathers completed <10 years of schooling, 49% completed 10th–12th grades, and 27% completed 13 grades or attended college. Over 86% of adolescents came from two–biological parent homes; the remainder came from single-mother families.
Three waves of data were collected at annual intervals for two consecutive years. Research project team members visited the homes of the participants and asked the children and parents to complete questionnaires. Questionnaire data were collected from 109 adolescents at age 14 years, 92 adolescents at age 15 years, and 89 adolescents at age 16 years. Participation totals for mothers were 109, 96, and 92; participation totals for fathers were 81, 75, and 69. During these intervals, patients also visited their treating physicians to measure HbA1c levels and assess pubertal status.
Measures
Metabolic Control
Metabolic control was determined from a measure of HbA1c, using the same high-performance liquid chromatographic assay at each site. Questionnaires were sent to the physicians to retrieve metabolic control scores from the patients’ medical records. Metabolic control scores were obtained from 97 patients at age 14 years, 96 patients at age 15 years, and 88 patients at age 16 years. Scores ranged from 4 to 14 (M = 7.59, SD = 2.00) at the outset. Higher scores indicated worse metabolic control.
Family Restrictiveness
At the outset of the study, adolescents completed the Family Environment Scale (Moos & Moos, 1981). The FES includes three subscales. In the present study, we used the 18-item system maintenance subscale as an index of family restrictiveness (e.g., “Our family life is governed by strict rules,” “My family sets strict limits on activities outside the home”). Items were rated on a scale ranging from 1 (little or not true) to 5 (mostly true). The scale had good internal reliability (α = .83)
Perceived Parental Social Support
At each time point, adolescents completed an abbreviated version of the Network of Relationships Inventory (Furman & Buhrmester, 1985), separately describing perceived maternal social support and perceived paternal social support (the same nine items for each relationship). Three of the original eight subscales (affection, instrumental aid, and satisfaction) were included in the present study (e.g., “How much does this person help you when you need to get something done?”). These subscales load on the same social support factor (Adams & Laursen, 2007; Furman, 1996). Items were rated on a scale ranging from 1 (little or none) to 5 (the most). Item scores were averaged to create separate scores for each relationship at each time. Internal reliability was high for perceived maternal social support (α = .80–.82) and perceived paternal social support (α = .77–.81).
Physical Maturity
Pubertal status was determined by reports from adolescents and physicians. We asked adolescents about their age of first menarche (females) and of first pollution (males). The measure at the first two time points was completed by 104 adolescents. In addition, we asked the 100 attending physicians to rate the timing of physical maturity (1 = too early, 2 = on time, 3 = too late) and we also determined a composite of both measures. Approximately 66% of the adolescents matured on time, 20% matured early, and 14% matured late.
Internalizing and Externalizing Symptoms
At each time interval, adolescents completed the Youth Self-Report and mothers completed the Child Behavior Checklist (Achenbach, 1991). Items were rated on a scale ranging from 0 (not true) to 2 (very true or often true). Two broadband indexes of adjustment were constructed from narrowband scales. Externalizing symptoms include 30 items that measure aggressive behavior and delinquent behavior (e.g., “gets in many fights”). Internalizing symptoms include 32 items that measure anxiety/depression, somatic complaints, and withdrawn behaviors (e.g., “unhappy, sad, or depressed”). Raw scores were summed for each broadband index. Internal reliability was high for child reports of internalizing (α = .78–.81) and externalizing (α = .79–.82) symptoms, and for mother reports of internalizing (α = .71–.73) and externalizing (α = .79–.85) symptoms.
Plan of Analysis
Missing data accounted for an average of 8.6% of reports for the variables included in this study (range 0%–19.3%). No statistically significant differences on any demographic, control, or study variables were found between those who participated at all three time points and those who did not. Missing data were handled with full information maximum-likelihood estimation, which allowed participants with incomplete data to be included in the models (Schafer & Graham, 2002). Little’s test indicated that data were missing completely at random, χ2(91) = 102.18, p = .20.
Preliminary analyses were conducted to determine patterns of associations between the main study variables. In particular, we were interested in the degree to which metabolic control correlated with maternal support, paternal support, and family restrictiveness. In addition, analysis of variances (ANOVAs) were conducted to determine if adolescents in high (0.5 SD above the mean) restrictiveness families (18 boys and 14 girls) differed from adolescents in moderate (0.5 SD above the mean to 0.5 SD below the mean) restrictiveness families (24 boys and 18 girls) and from adolescents in low (0.5 SD below the mean) restrictiveness families (16 boys and 19 girls) on any study variables (i.e., metabolic control, maternal support, and paternal support), demographic variables (i.e., ethnicity, household structure, and paternal education), and control variables (i.e., onset of illness, physical maturity, internalizing symptoms, and externalizing symptoms).
The main analyses consisted of a series of autoregressive and cross-lagged panel analyses, conducted within a structural equation modeling framework using Amos 19.0 (Arbuckle, 2010). These analyses, also known as residual change models, are used to examine change across consecutive time points (Selig & Little, 2012). The prediction of a Time 2 score by the Time 1 score controls for (or partials out) the influence of the Time 1 score, such that the data from the Time 2 dependent variable is essentially converted into a residual change variable that represents the change in the score between Time 1 and Time 2.
Figure 1 depicts the measurement model. Longitudinal associations between parental social support and adolescent metabolic control were modeled from age 14 to 15 years and from age 15 to 16 years. These analyses were designed to test the hypothesis that parental social support predicted subsequent changes in adolescent metabolic control (see paths ss1mc2 and ss2mc3) and that adolescent metabolic control predicted subsequent changes in parental support (see paths mc1ss2 and mc2ss3). In a residual change model it is customary to define the autoregressive effects as the stability of a variable from one occasion to the next (Selig & Little, 2012). The model estimated the stability of parental social support (see paths ss1-2 and ss2-3) and the stability adolescent metabolic control (see paths mc1-2 and mc2-3) over time. As a consequence, antecedent scores for one variable (e.g., the variance in age 14 years metabolic control scores) predicted changes in cross-lagged scores for the other variable (e.g., the variance in age 15 years maternal social support scores that remains after controlling for the variance attributed to age 14 years maternal social support scores). Separate analyses were conducted for maternal social support and paternal social support. We used standard model fit indices. The chi-square index should be as small as possible; the root mean square error of approximation (RMSEA) should be <.08, and the comparative fit index (CFI) should be >.90 and preferably >.95.
Figure 1.
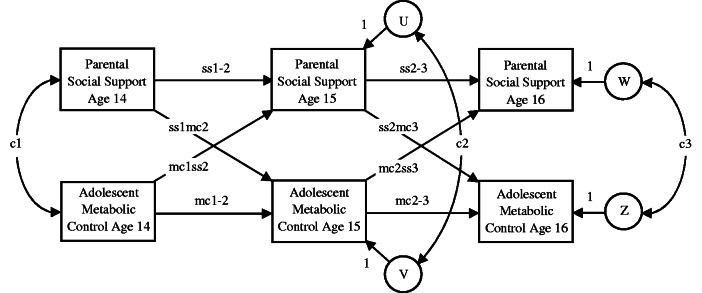
Measurement model of autoregressive and cross-lagged panel analysis describing associations over time between parent social support and adolescent metabolic control. ss1-2 = stability of parental social support from age 14 to age 15 years; mc1-2 = stability of adolescent metabolic control from age 14 to age 15 years; ss2-3 = stability of parental social support from age 15 to age 16 years; mc2-3 = stability of adolescent metabolic control from age 15 to age 16 years; ss1mc2 = influence of age 14 years parental social support on age 15 years adolescent metabolic control; mc1ss2 = influence of age 14 years adolescent metabolic control on age 15 years parental social support; ss2mc3 = influence of age 15 years parental social support on age 16 years adolescent metabolic control; mc2ss3 = influence of age 15 years adolescent metabolic control on age 16 years parental social support. c1 = age 14 years correlation; c2 = age 15 years residual correlation; c3 = age 16 years residual correlation. U = residual variance in age 15 years parental social support; V = residual variance in age 15 years adolescent metabolic control; W = residual variance in age 16 years parental social support; Z = residual variance in age 16 years adolescent metabolic control.
Multiple group analyses contrasted patterns of association across adolescents from high, moderate, and low restrictiveness families. A Satorra-Bentler scaled chi-square difference test compared the three family groups on each of the cross-lagged paths to test the hypothesis that high family restrictiveness is a risk factor, exacerbating the tendency of low parental support to anticipate deteriorating metabolic control and the tendency of poor metabolic control to anticipate declining parental support.
To improve power, a progressive model fitting procedure was used in which temporal constraints were added to the model in a step-wise fashion (Widaman & Thompson, 2003). The initial model included no constraints; all paths in the model were freely estimated within groups. Three additional models were tested: (1) a model in which the paths from age 14 years adolescent metabolic control to age 15 years perceived maternal social support (mc1ss2) and the paths from age 15 years adolescent metabolic control to age 16 years perceived maternal social support (mc2ss3) were set to be equal within family restrictiveness groups (mc1ss2 = mc2ss3); (2) a model in which the paths from age 14 years perceived maternal social support to age 15 years adolescent metabolic control (ss1mc2) and the paths from age 15 years perceived maternal social support to age 16 years adolescent metabolic control (ss2mc3) were set to be equal within family restrictiveness groups (ss1mc2 = ss2mc3); and (3) a model that included equality constraints for both sets of paths (mc1ss2 = mc2ss3 and ss1mc2 = ss2mc3). Constraints were retained if they did not significantly worsen model fit.
The analyses were conducted with and without control variables. The following control variables were included in separate models: (a) illness duration; (b) three waves of externalizing symptoms (mother report and child report separately); (c) three waves of internalizing symptoms (mother report and child report separately); (d) three waves of physical maturity (child report and physician report separately); and (e) initial parent marital status were included as control variables.
Results
Preliminary Analyses
Table I presents intercorrelations between metabolic control, perceived maternal social support, perceived paternal social support, and family restrictiveness. Metabolic control was correlated with itself from one year to the next, as was perceived maternal social support and perceived paternal social support. Age 14 years perceived maternal social support and perceived paternal social support were negatively correlated with age 15 years metabolic control. Perceived maternal social support and perceived paternal social support were positively correlated concurrently and over time. No statistically significant correlations were found for age 14 years family restrictiveness.
Table I.
Means, Standard Deviations, and Correlations Between Variables
| Variable | M | SD | N | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age 14 years metabolic control | 7.59 | 2 | 96 | – | ||||||||
| 2. Age 15 years metabolic control | 7.71 | 1.72 | 96 | 0.34** | – | |||||||
| 3. Age 16 years metabolic control | 8.24 | 1.66 | 88 | 0.06 | 0.38** | – | ||||||
| 4. Age 14 years family restrictiveness | 2.99 | 0.46 | 109 | −0.01 | 0.13 | −0.03 | – | |||||
| 5. Age 14 years maternal support | 3.76 | 0.8 | 99 | −0.14 | −0.29** | 0.05 | −0.03 | – | ||||
| 6. Age 15 years maternal support | 3.66 | 0.81 | 92 | −0.19 | −0.09 | 0.16 | −0.02 | 0.62** | – | |||
| 7. Age 16 years maternal support | 3.62 | 0.79 | 89 | −0.04 | −0.14 | 0.11 | 0.08 | 0.62** | 0.77** | – | ||
| 8. Age 14 years paternal support | 3.41 | 0.89 | 98 | −0.09 | −0.31** | 0.03 | 0.09 | 0.64** | 0.35** | 0.40** | – | |
| 9. Age 15 years paternal support | 3.3 | 0.88 | 90 | −0.1 | −0.14 | 0.09 | −0.01 | 0.32** | 0.59** | 0.39** | 0.67** | – |
| 10. Age 16 years paternal support | 3.31 | 0.93 | 87 | −0.04 | −0.1 | 0.07 | 0.01 | 0.37** | 0.49** | 0.58** | 0.64** | 0.80** |
Note. Physician-reported metabolic control ranged from 3.40 to 13.90. Child-reported perceived maternal social support and perceived paternal social support were rated on a scale ranging from 1 (little or none) to 5 (the most). Child-reported family restrictiveness was rated on a scale ranging from 1 (little or not true) to 5 (mostly true).
SD = standard deviation; M = mean.
*p < .05, **p < .01.
A series of repeated measures ANOVAs were conducted with sex and family restrictiveness (high, moderate, and low) as the independent variables and time as the repeated measure. Means and standard deviations are presented in Table I. A main effect of time emerged for metabolic control F(1, 74) = 8.50, p = .005, ηp2 = .10, and maternal support F(1, 78) = 4.66, p = .03, ηp2 = .06. Metabolic control worsened (i.e., HbA1c levels increased) and maternal support declined over time. A similar ANOVA revealed a borderline statistically significant effect for declines in paternal support, F(1,75) = 2.90, p = .09, ηp2 = .04. There were no statistically significant main effects or interactions involving sex or family restrictiveness.
Additional ANOVAs failed to reveal statistically significant family restrictiveness group differences in child and parent reports of internalizing symptoms and externalizing symptoms, illness duration, physical maturity, paternal education, or child age. Chi-square analyses revealed no statistically significant sex or household structure differences between the three groups. The same pattern of results emerged using median splits to identify family restrictiveness groups. In sum, the high, moderate, and low family rule setting groups did not differ on any variables except family restrictiveness.
Longitudinal Associations Between Perceived Maternal Social Support and Adolescent Metabolic Control
In the first step of the analyses, an initial (unconstrained) model was fit to the maternal support data, χ2(12, N = 109) = 17.18, p = .14; CFI = .96; RMSEA = .06. The next step was to add temporal constraints, as described in the plan of analysis. Temporal constraints from metabolic control to subsequent perceived maternal social support (mc1ss2 = mc2ss3) did not worsen model fit and were retained, χ2(15, N = 109) = 18.54, p = .24; CFI = .97; RMSEA = .05. The other two sets of constraints significantly worsened model fit and were omitted.
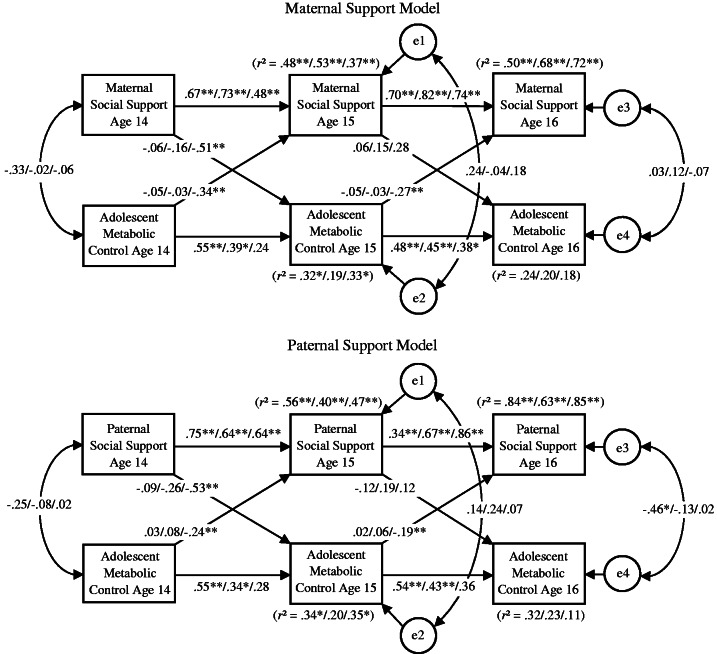
The top of Figure 2 presents results from the multiple group model that included temporal constraints from metabolic control to perceived maternal social support (mc1ss2 = mc2ss3). The cross-lagged paths in the analyses tested the hypothesis that maternal social support predicted subsequent changes in adolescent metabolic control (ss1mc2 and ss2mc3) and that adolescent metabolic control predicted subsequent changes in maternal social support (mc2ss2 and mc2ss3). With one exception (metabolic control from age 14 to age 15 years in high restrictiveness families), stable over time associations emerged for perceived maternal social support (ss1-2 and ss2-3) and adolescent metabolic control (mc1-2 and mc2-3).
Figure 2.
Associations over time between perceived parental social support and adolescent metabolic control for youth reporting low (n = 35), moderate (n = 42), and high (n = 32) family restrictiveness. Standardized beta weights are reported. Low restrictiveness families are on the left, moderate restrictiveness families are in the middle, and high restrictiveness families are on the right. *p < .05, **p < .01.
In high restrictiveness families, there were several statistically significant cross-lagged associations between metabolic control and maternal social support. Age 14 years perceived maternal social support was negatively associated with age 15 years metabolic control (ss1mc2 β = −.52). Lower levels of initial perceived maternal social support anticipated greater subsequent increases in (i.e., a worsening of) metabolic control from age 14 to age 15 years. Age 14 years metabolic control was negatively associated with age 15 years perceived maternal social support (mc1ss2 β = −.34), and age 15 years metabolic control was negatively associated with age 16 years perceived maternal social support (mc2ss3 β = −.27). In each analysis, higher (i.e., poorer) initial metabolic control anticipated greater declines in perceived maternal social support.
In low and moderate restrictiveness families, there were no statistically significant cross-lagged associations from metabolic control to subsequent maternal social support or from maternal social support to subsequent metabolic control.
Satorra–Bentler scaled chi-square difference tests compared the magnitude of the cross-lagged paths across the high, moderate, and low restrictiveness groups. There were statistically significant differences between the high and the low restrictiveness groups on the path from age 14 years perceived maternal social support to age 15 years metabolic control (ss1mc2); the association was stronger in high restrictiveness families than in low restrictiveness families, Δχ2(1) = 4.03, p = .05. Differences between high and moderate restrictiveness families did not rise to the level of conventional statistical significance, Δχ2(1) = 2.00, p = .16. There were statistically significant differences between high and low restrictiveness families and between high and moderate restrictiveness families on the temporally constrained paths from metabolic control to perceived maternal social support (mc1ss2 and mc2ss3); the association was strongest in high restrictiveness families, Δχ2(1) = 3.84–4.45, p = .04–.05. There were no statistically significant differences between the low and moderate restrictiveness families on any cross-lagged paths.
Longitudinal Associations Between Perceived Paternal Social Support and Adolescent Metabolic Control
In the first step of the analyses, an initial (unconstrained) model was fit to the paternal support data, χ2(9, N = 109) = 14.93, p = .09; CFI = .96; RMSEA = .08. The best-fitting initial model included an additional stability path from age 14 years perceived paternal social support to age 16 years perceived paternal social support. The next step was to add temporal constraints, as described in the plan of analysis. The temporal constraints from metabolic control to subsequent perceived paternal social support (mc1ss2 = mc2ss3) did not worsen model fit and were retained, χ2(12, N = 109) = 16.06, p = .19; CFI = .98; RMSEA = .06. The other two sets of constraints significantly worsened model fit and were omitted.
The bottom of Figure 2 presents results from the multiple group model that included temporal constraints from metabolic control to perceived paternal social support (mc1ss2 = mc2ss3). The cross-lagged paths in the analyses tested the hypothesis that paternal social support predicted subsequent changes in adolescent metabolic control (ss1mc2 and ss2mc3) and that adolescent metabolic control predicted subsequent changes in paternal social support (mc2ss2 and mc2ss3). The additional stability path from age 14 years perceived paternal support to age 16 years perceived paternal support (low β = .64, moderate β = .19, high β = −.03) was omitted from Figure 2 to make the maternal and paternal models comparable. With the exception of metabolic control in high restrictiveness families, stable over time associations emerged for perceived paternal social support (ss1-2 and ss2-3) and adolescent metabolic control (mc1-2 and mc2-3).
In high restrictiveness families, there were several statistically significant cross-lagged associations between metabolic control and paternal social support. Age 14 years perceived paternal social support was negatively associated with age 15 years metabolic control (ss1mc2 β = −.53). Lower levels of initial perceived paternal social support anticipated greater subsequent increases in (i.e., a worsening of) metabolic control from age 14 to age 15 years. Age 14 years metabolic control was negatively associated with age 15 years perceived paternal social support (mc1ss2 β = −.24), and age 15 years metabolic control was negatively associated with age 16 years perceived paternal social support (mc2ss3 β = −.19). In each analysis, higher (i.e., poorer) initial metabolic control anticipated greater declines in perceived paternal social support.
In families with low and moderate restrictiveness, there were no statistically significant associations from metabolic control to subsequent maternal social support or from maternal social support to subsequent metabolic control.
Satorra–Bentler scaled chi-square difference tests compared the magnitude of cross-lagged paths across the high, moderate, and low restrictiveness family groups. There were statistically significant differences between the high and the low restrictiveness groups on the path from age 14 years perceived paternal social support to age 15 years metabolic control (ss1mc2); the association was stronger in high restrictiveness families than in low restrictiveness families, Δχ2(1) = 5.12, p = .02. Differences between high and moderate restrictiveness families were not significant, Δχ2(1) = 1.95, p = .16. There were statistically significant differences between high and low restrictiveness families and between high and moderate restrictiveness families on the temporally constrained paths from metabolic control to perceived maternal social support (mc1ss2 and mc2ss3); the association was strongest in high restrictiveness families, Δχ2(1) = 5.76–9.18, p = .02–.002. There were no statistically significant differences between the low and moderate restrictiveness families on any cross-lagged paths.
Supplemental Analyses
Analyses were repeated using a median split procedure, dividing families into high and low restrictiveness groups. The same pattern of statistically significant associations emerged, but the magnitude of paths weakened somewhat so that scaled chi-square difference tests contrasting high and low restrictiveness families were no longer statistically significant.
Additional multiple group analyses were conducted with sex as a moderator. Statistically significant scaled chi-square differences were not found at levels greater than chance, suggesting that longitudinal associations did not differ for boys and girls.
Additional contrasts examined whether findings differed for mothers and fathers. Specifically, analyses contrasted the magnitude of paths in the maternal social support model with the magnitude of the paths in the paternal social support model. Separate chi-square tests compared each of the cross-lagged paths involving maternal social support and paternal social support. There were no statistically significant differences.
We examined the contribution of several potential confounds. First, illness duration was entered into the model as a control variable. Second, three waves of externalizing symptoms and three waves of internalizing symptoms were separately entered into the model. Third, three waves of physical maturity were included as control variables. Fourth, initial parent marital status was included as a control variable. In each case, model fit significantly weakened and there was no change in the pattern of statistically significant paths.
Discussion
This study adds to the growing literature indicating that family factors are related to metabolic control outcomes in adolescents with diabetes. A special feature of our study was that the perceived support from fathers and mothers was examined in the context of non-illness–specific behaviors. Our findings expand on prior results that tie illness-specific family support to the health outcomes of children with diabetes (Ellis et al., 2008; Mullins et al., 2007; Seiffge-Krenke, 1998b). We found that adolescents from high restrictiveness families are at risk for poor metabolic outcomes, particularly when they perceive a lack of support from parents.
Our findings replicate and extend previous work on the topic. Replication comes in the form of age-related declines in metabolic control and maternal support, which were in line with expectations. Metabolic control worsened in a manner comparable with that reported in other longitudinal studies (e.g., Duke et al., 2008; Grabill et al., 2010; Helgeson et al., 2008). Similarly, several studies of adolescents with diabetes (Lewandowski & Drotar, 2007; Seiffge-Krenke, 2002; Weinger et al., 2001) and on healthy adolescents (for a review see Laursen & Collins, 2009) reported similar decreases in adolescent perceptions of support from mothers and fathers. Replication of normative developmental patterns of change should bolster confidence in the new findings that emerged from our study.
Mean level changes should not be confused with moderated patterns of association. Similar declines in support and metabolic control were found for adolescents in high, medium, and low restrictiveness families. But only in high restrictiveness families did each variable anticipate the other. We found evidence of a downward spiral of declining metabolic control and decreasing parental support among adolescents with diabetes from families with high rule setting. This pattern persisted regardless of duration of the illness, parent marital status, adolescent sex and pubertal status, and adolescent adjustment symptomatology. Thus, restrictiveness serves as a risk factor, exacerbating declines in metabolic control associated with low perceived parental support.
Patterson’s (1982) coercion model can help to understand the mechanisms at the heart of our findings. This model proposes a process of behavioral contingencies in which parent demands for obedience are associated with child refusal to comply, ending with parental submission. In subsequent iterations, coerciveness escalates, creating a hostile family environment. Duke et al. (2008) provided evidence that the model also applies to families with children who have diabetes. Adolescents who perceive their parents as setting too many limits and rules resist parental attempts to control. This may spill over into refusing to adhere to the treatment regimen, causing increases in HbA1c. This negative family climate is more apt to arise in families that are neither warm nor close. When family members are supportive of one another, children may not perceive rule setting as manipulative and a threat to autonomy needs (Laursen & Collins, 2004). Although we did not assess adherence or coercion and thus can only speculate about their role, our data are consistent with a parent-driven model of the effects of social support.
We also found evidence of child-driven effects. We are not the first to suggest that parents react negatively to adolescents’ difficulties in maintaining good blood glucose levels (Duke et al., 2008), but we are the first to find that positive features of parent–child relationships suffer. Our result showing the diminishing support from mothers and fathers in response to deteriorating metabolic control at both time points is important to note. It is worth noting that adverse consequences of behavioral control were reserved for families with high levels of restrictiveness. There were no over-time associations between support and metabolic control at low and medium levels of restrictiveness, suggesting that moderate rule setting is fine in families with an adolescent with diabetes, perhaps because it does not foster a negative family climate.
Clinical Implications
Given that the deterioration in metabolic control seen in many adolescents is an area of great concern for both clinicians and parents (American Diabetes Association, 2010; Duke et al., 2008; Holmes et al., 2006; Luyckx & Seiffge-Krenke, 2009), factors that might be of clinical relevance should be carefully scrutinized. Our study indicates that a focus on non-illness–specific parenting might be helpful. Clinicians should be alert to the dangers of “miscarried helping” (Anderson & Coyne, 1991), whereby parents act in a manner that may ensure good blood glucose levels in children but do so in a manner that is excessive, untimely, or inappropriate. More attention to the ways parenting behavior is affected by an adolescent’s capacity to maintain metabolic control is needed. Of particular concern is the finding that when families are high in restrictiveness, parent support declined when adolescent metabolic control worsened. This problematic reaction can threaten families that already appear to have difficulties coping with the autonomy demands arising from the renegotiation of adolescent roles and responsibilities, resulting in a further deterioration of blood glucose levels. Attending physicians should be prepared to talk to parents about the importance of balancing developmental and illness-specific needs (Seiffge-Krenke, 2001). Physicians should also be prepared to recommend appropriate family discussions whenever blood glucose levels in adolescent children increase (Anderson et al., 1999). As parent-effects and child-effects were likewise strong, this calls on a systemic approach working with families with an adolescent with diabetes.
Perceptions of parental control are particularly problematic for diabetes management in the middle adolescent age range (Horton, Berg, Butner, & Wiebe, 2009; Wiebe et al., 2005). We do not wish to imply that parents should withdraw from the supervision of treatment compliance during these years. Far from it, instead, the findings suggest that overcontrol and overinvolvement may prompt a backlash from adolescent children that may interfere with healthy behavior. The findings suggest that rules and limit setting, as well as activity monitoring, should be supplemented at the onset of adolescence with strategies that emphasize personal responsibility and autonomy. Maintaining warm close relationships is necessary if parental rule setting and monitoring of rules is to succeed. In the absence of positive relationships, high restrictiveness may prove counterproductive.
Limitations
Our study is not without limitations. We used measures of social support and restrictiveness that did not identify specific health-related forms of support and control. Results from studies using diabetes-specific measures of support are inconclusive (e.g., Helgeson et al., 2008), but we would expect that future replications with illness-specific measures would produce even stronger effects. A further limitation is that we relied exclusively on adolescent reports of support and restrictiveness, leaving open the possibility of bias due to shared reporter variance, although this would have no bearing on the main findings of the interplay between perceived parental support and physician reported metabolic control. In contrast to studies of healthy adolescents, we failed to find evidence of sex differences, either in the mean level differences of perceived support or patterns of association involving support (Berg et al., 2011; Laursen et al., 2010). This inconsistency might be because of the small sample we obtained, the fact that children had diabetes, or because of sample characteristics, the vast majority of whom came from white middle class German households. Some may object to the practice of trichotomizing the sample on the basis of parent restrictiveness, for fear that the practice carries risks similar to dichotomization, namely the loss of effect size and power, and the occurrence of spurious associations (MacCallum, Zhang, Preacher, & Rucker, 2002). Unfortunately, the alternatives to this procedure (e.g., Klein & Moosbrugger, 2000) are both complex and inappropriate for samples of this size.
Conclusion
The current study supports a growing body of research documenting that specific parenting behavior may influence adjustment outcomes in chronically ill adolescents. Adolescents with diabetes are a vulnerable population whose special needs have the potential to disrupt optimal parenting practices. This study indicates that in restrictive families, parents may respond to deteriorating medical conditions with diminished support. The potential for a downward spiral is great: These same families also indicated that low levels of support anticipated a worsening of metabolic control.
Funding
The first author received a grant from Bundesminster for Forschung and Technologie (BMFT, Grant No 0706567) and Deutsche Forschungsgemeinschaft (DFG, Grant No se 408/10-1) for conducting the study. Brett Laursen received support from the U.S. National Institute of Child Health and Human Development (HD068421) and the U.S. National Science Foundation (0923745, 0909733).
Conflicts of interest: None declared.
References
- Achenbach T M. Youth Self Report (YSR) and Child Behavior Checklist (CBCL) Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Adams R E, Laursen B. The correlates of conflict: Disagreement is not always detrimental. Journal of Family Psychology. 2007;21:445–458. doi: 10.1037/0893-3200.21.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Executive summary: Standards of medical care in diabetes. Diabetes Care. 2010;33(Suppl. 1):S4–S10. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Brackett B J, Ho J, Laffel L M B. An office-based intervention to maintain parent-adolescent teamwork in diabetic management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22:713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- Anderson B J, Coyne J C. “Miscarried helping” in families of children and adolescents with chronic diseases. In: Johnson J H, Johnson S B, editors. Advances in child health psychology. Gainesville, FL: University of Florida Press; 1991. pp. 166–177. [Google Scholar]
- Arbuckle J L. Amos. 2010. (Version 19.0) [Computer Program]. Chicago: SPSS. [Google Scholar]
- Berg C A, King P S, Butler J, Pham P, Palmer D, Wiebe D J. Parental involvement and adolescents’ diabetes management: The mediating role of self-efficacy and externalizing and internalizing behaviors. Journal of Pediatric Psychology. 2011;36:329–339. doi: 10.1093/jpepsy/jsq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs T E, Harris M A, Ponious S L, Santiago J V. Research on social support in adolescents with IDDM: A critical review. The Diabetes Educator. 1997;23:438–448. doi: 10.1177/014572179702300409. [DOI] [PubMed] [Google Scholar]
- Butler J M, Skinner M, Gelfand D, Berg C A, Wiebe D J. Maternal parenting style and adjustment in adolescents with type I diabetes. Journal of Pediatric Psychology. 2007;32:1227–1237. doi: 10.1093/jpepsy/jsm065. [DOI] [PubMed] [Google Scholar]
- Cameron F J, Skinner C E, de Beaufort C E, Hoey H, Swift P G F, Aanstoot H, Aman J, Martul P, Chiarelli F, Daneman D, Danne T, Dorchy H, Kaprio EA, Kaufman F, Kocova M, Mortensen HB, Njølstad PR, Phillip M, Robertson KJ, Schoenle EJ, Urakami T, Vanelli M, Ackermann RW, Skovlund S E. Are family factors universally related to metabolic outcomes in adolescents with type 1 diabetes? Diabetic Medicine. 2008;25:463–468. doi: 10.1111/j.1464-5491.2008.02399.x. [DOI] [PubMed] [Google Scholar]
- Cohen D M, Lumley M A, Naar-King S, Partridge T, Cakan N. Child behavior problems and family functioning as predictors of adherence and glycemic control in economically disadvantaged children with type 1 diabetes: A prospective study. Journal of Pediatric Psychology. 2004;29:174–184. doi: 10.1093/jpepsy/jsh019. [DOI] [PubMed] [Google Scholar]
- Davis C L, Delamater A M, Shaw K H, LaGreca A, Eidson M S, Perez-Rodriguez J E, Nemery R. Brief report: Parenting styles, regimen adherence, and glycemic control in 4-to 10 year old children with diabetes. Journal of Pediatric Psychology. 2001;26:123–129. doi: 10.1093/jpepsy/26.2.123. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Study (DCCT) Research Group. Adverse events and their associations with treatment regimens in the Diabetes Control and Complications Trial. Diabetes Care. 1995;18:1415–1427. doi: 10.2337/diacare.18.11.1415. [DOI] [PubMed] [Google Scholar]
- Duke D C, Geffken G R, Lewin A B, Williams L B, Storch E A, Silverstein J H. Glycemic control in youth with type 1 diabetes: Family predictors and mediators. Journal of Pediatric Psychology. 2008;33:719–727. doi: 10.1093/jpepsy/jsn012. [DOI] [PubMed] [Google Scholar]
- Ellis D C, Geffken G R, Lewin A B, Williams L B, Storch E A, Silverstein J H. Towards conceptual clarity in a critical parenting construct: Parental monitoring in youth with chronic illness. Journal of Pediatric Psychology. 2008;33:799–808. doi: 10.1093/jpepsy/jsn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman W. The measurement of friendship perceptions: Conceptual and methodological issues. In: Bukowski W M, Newcomb A F, Hartup W W, editors. The company they keep: Friendship in childhood and adolescence. New York: Cambridge University Press; 1996. pp. 41–65. [Google Scholar]
- Furman W, Buhrmester D. Children's perception of the personal relationships in their social networks. Developmental Psychology. 1985;21:1016–1024. [Google Scholar]
- Grabill K M, Geffken G R, Duke A, Lewin A, Willimas L, Storch E, Silverstein J. Family functioning and adherence in youth with type 1 diabetes: A latent growth model of glycemic control. Children’s Health Care. 2010;39:279–295. [Google Scholar]
- Hafen C A, Laursen B. More problems and less support: Early adolescent adjustment forecasts changes in perceived support from parents. Journal of Family Psychology. 2009;23:193–202. doi: 10.1037/a0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson V S, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A 4-year longitudinal study. Journal of Pediatric Psychology. 2008;34: 254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeve M, Dubas J S, Gerris J R M, van der Laan P H, Smeek W. Maternal and paternal parenting styles: Unique and combined links to adolescent and early adult delinquency. Journal of Adolescence. 2011;34:813–827. doi: 10.1016/j.adolescence.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Holmes C S, Chen R, Streisand R, Marschall D E, Souter S, Swift E E. Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. Journal of Pediatric Psychology. 2006;31:770–784. doi: 10.1093/jpepsy/jsj083. [DOI] [PubMed] [Google Scholar]
- Hood K K, Huestis S, Maher A, Butler D, Volkening L, Staffel L M B. Depressive symptoms in children and adolescents with type I diabetes. Diabetes Care. 2006;29:1389–1391. doi: 10.2337/dc06-0087. [DOI] [PubMed] [Google Scholar]
- Horton D, Berg C A, Butner J, Wiebe D. The role of parental monitoring in metabolic control: Effect on adherence and externalizing behavior during adolescence. Journal of Pediatric Psychology. 2009;34:1008–1018. doi: 10.1093/jpepsy/jsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M, Stattin H, Pakalniskiene V. Parents react to adolescent problem behaviors by worrying more and monitoring less. In: Kerr M, Stattin H, Engels R C M E, editors. What can parents do? New insights into the role of parents in adolescent problem behavior. New York: Wiley; 2008. pp. 91–112. [Google Scholar]
- Klein A, Moosbrugger H. Maximum likelihood estimation of latent interaction effects with the LMS method. Psychometrika. 2000;65:457–474. [Google Scholar]
- Kyngäs H, Hentinen M, Barlow J H. Adolescents' perceptions of physicians, nurses, parents and friends: Help or hindrance in compliance with diabetes self-care? Journal of Advanced Nursing. 1998;27:760–769. doi: 10.1046/j.1365-2648.1998.00608.x. [DOI] [PubMed] [Google Scholar]
- Kyngäs H, Rissanen M. Support as a crucial predictor of good compliance of adolescents with a chronic disease. Journal of Clinical Nursing. 2001;10:767–774. doi: 10.1046/j.1365-2702.2001.00538.x. [DOI] [PubMed] [Google Scholar]
- Laursen B, Collins W A. Parent-child communication during adolescence. In: Vangelisti A L, editor. Handbook of family communication. Hillsdale, NJ: Erlbaum; 2004. pp. 333–348. [Google Scholar]
- Laursen B, Collins W A. Parent–adolescent relationships during adolescence. In: Lerner R M, Steinberg L, editors. Handbook of adolescent psychology. 3rd edn. Vol. 2. Hoboken, NJ: Wiley; 2009. pp. 3–42. [Google Scholar]
- Laursen B, DeLay D, Adams R E. Trajectories of perceived support in mother-adolescent relationships: The poor (quality) get poorer. Developmental Psychology. 2010;46:1792–1798. doi: 10.1037/a0020679. [DOI] [PubMed] [Google Scholar]
- Lewandowski A, Drotar D. The relationship between parent-reported social support and adherence to medical treatment in families of adolescents with type I diabetes. Journal of Pediatric Psychology. 2007;32:427–436. doi: 10.1093/jpepsy/jsl037. [DOI] [PubMed] [Google Scholar]
- Lewin A B, Heidgerken A D, Geffken G R, Williams L B, Stroch E A, Gelfand K M, Silverstein J H. The relations between family factors and metabolic control: The role of diabetes adherence. Journal of Pediatric Psychology. 2006;31:174–183. doi: 10.1093/jpepsy/jsj004. [DOI] [PubMed] [Google Scholar]
- Luyckx K, Seiffge-Krenke I. Continuity and change in glycemic control trajectories from adolescence to emerging adulthood: Relationships with family climate and self-concept in type 1 diabetes. Diabetes Care. 2009;32:797–801. doi: 10.2337/dc08-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum R C, Zhang S, Preacher K J, Rucker D D. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Moos R H, Moos B S. Family environment scale manual. Palo Alto, CA: Consulting Psychologists Press; 1981. [Google Scholar]
- Mullins L L, Wolfe-Christensen C, Hoff A L, Carpentier M, Gillaspy S, Cheek J, Page M. The relationship of parental overprotection, perceived child vulnerability, and parenting stress to uncertainty in youth with chronic illness. Journal of Pediatric Psychology. 2007;32:973–982. doi: 10.1093/jpepsy/jsm044. [DOI] [PubMed] [Google Scholar]
- Patterson G R. Coercive family processes. Eugene, OR: Castalia; 1982. [Google Scholar]
- Phares V, Lopez E, Fields S, Kamboukos D, Duhig A M. Are fathers involved in pediatric psychology research and treatment? Journal of Pediatric Psychology. 2005;30:631–643. doi: 10.1093/jpepsy/jsi050. [DOI] [PubMed] [Google Scholar]
- Pisula E, Czaplinska C. Coping with stress in adolescents with type 1 diabetes and their mothers. European Journal of Medical Research. 2010;15:115–119. doi: 10.1186/2047-783X-15-S2-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C M. Psychological factors and diabetes mellitus. In: Pichup J C, Williams G, editors. Textbook of diabetes. Vol. 2. Oxford, UK: Blackwell Science; 2003. pp. 1–24. [Google Scholar]
- Sameroff A J, Mackenzie M J. Research strategies for capturing transactional models of development: The limits of the possible. Development and Psychopathology. 2003;15:613–640. doi: 10.1017/s0954579403000312. [DOI] [PubMed] [Google Scholar]
- Schafer J L, Graham J W. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Seiffge-Krenke I. Chronic disease and perceived developmental progression in adolescence. Developmental Psychology. 1998a;34:1073–1084. doi: 10.1037//0012-1649.34.5.1073. [DOI] [PubMed] [Google Scholar]
- Seiffge-Krenke I. The highly structured climate in families of adolescents with diabetes: Functional or dysfunctional for metabolic control? Journal of Pediatric Psychology. 1998b;23:313–322. doi: 10.1093/jpepsy/23.5.313. [DOI] [PubMed] [Google Scholar]
- Seiffge-Krenke I. Adolescents with diabetes and their families: Stress, coping, and adaptation. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Seiffge-Krenke I. “Come on, say something, Dad!”: Communication and coping in fathers of diabetic adolescents. Journal of Pediatric Psychology. 2002;27:439–450. doi: 10.1093/jpepsy/27.5.439. [DOI] [PubMed] [Google Scholar]
- Seiffge-Krenke I, Stemmler M. Coping with everyday stress and links to medical and psychosocial adaptation in diabetic adolescents. Journal of Adolescent Health. 2003;33:180–188. doi: 10.1016/s1054-139x(02)00707-3. [DOI] [PubMed] [Google Scholar]
- Selig J P, Little T D. Autoregressive and cross-lagged panel analysis for longitudinal data. In: Laursen B, Little T D, Card N A, editors. Handbook of developmental research methods. New York: Guilford; 2012. pp. 265–278. [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Deeb L, Grey M, Anderson B, Holzmeister LA, Clark N. Care of children and adolescents with type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Weinger K, O’Donnell K A, Ritholz M D. Adolescent views of diabetes-related parent conflict and support: A focus group analysis. Journal of Adolescent Health. 2001;29:330–336. doi: 10.1016/s1054-139x(01)00270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widaman K F, Thompson J S. On specifying the null model for incremental fit indices in structural equation modeling. Psychological Methods. 2003;8(1):16–37. doi: 10.1037/1082-989x.8.1.16. [DOI] [PubMed] [Google Scholar]
- Wiebe D J, Berg C, Korbel C, Palmer D L, Berveridge R M, Upchurch R, Lindsay R, Swinyard MT, Donaldson D L. Children’s appraisal of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. Journal of Pediatric Psychology. 2005;30:167–178. doi: 10.1093/jpepsy/jsi004. [DOI] [PubMed] [Google Scholar]
- Wysocki T. Associations among teenager-parent-relationships, metabolic control, and adjustment to diabetes in adolescents. Journal of Pediatric Psychology. 1993;18:441–445. doi: 10.1093/jpepsy/18.4.441. [DOI] [PubMed] [Google Scholar]