Abstract
Background. Schistosomiasis elicits cross-regulatory immune responses, but it is unclear how antihelminthic treatment affects this balance. This study integrates data on 13 cytokines elicited by 3 schistosome to examine how praziquantel treatment alters immune polarization and whether post-treatment cytokine profiles influence reinfection status.
Methods. Venous blood from 72 Schistosoma haematobium–exposed participants was cultured with schistosome egg, adult worm, and cercaria antigens pre– and 6 weeks post–praziquantel treatment. Innate inflammatory (tumor necrosis factor α [TNF-α], interleukin(IL-)-6, IL-8), Th1 (interferon γ [IFN-γ], IL-2, IL-12p70), Th2 (IL-4, IL-5, IL-13), Th17 (IL-17A, IL-21, IL-23p19), and regulatory (IL-10) cytokines were quantified via enzyme-linked immunosorbent assay. Cytokine data was integrated using nonmetric multidimensional scaling and factor analysis.
Results. Egg-specific cytokine phenotypes became more proinflammatory post-treatment due to increased TNF-α, IL-6, IL-8, IFN-γ, IL-12p70, and IL-23 levels. Post-treatment cercariae-specific responses were also more proinflammatory reflecting elevated IL-8. In contrast, post-treatment adult worm-specific responses were less inflammatory, reflecting lower post-treatment IL-6. A combination of egg-induced IL-6, IL-12p70, IL-21, and IL-23 and adult worm-induced IL-5 and IL-21 post-treatment was associated with reduced reinfection risk 18 months later.
Conclusions. Praziquantel treatment markedly alters polarization of schistosome-specific cytokine responses, and these changes, particularly in response to egg-stage parasites, may promote resistance to reinfection.
Keywords: Human, helminth, cytokine, schistosomiasis, immune response, praziquantel
Urogenital schistosomiasis is a debilitating disease caused by the parasitic trematode Schistosoma haematobium, which currently infects >100 million people [1]. Humans are exposed to infective larvae (cercariae), which invade via percutaneous penetration; adult male and female worms, which mate in the urogenital tract; and eggs, which are transmitted to the environment in urine. Repeated passage of eggs across the bladder and urogenital tract leads to characteristic immune-mediated morbidities [2, 3]. The World Health Organization advocates schistosomiasis control via treatment with the antihelminthic drug praziquantel (PZQ) [4] due to its efficacy at clearing infection [5, 6] and reducing the prevalence of schistosome-associated morbidities [7].
Praziquantel treatment also alters schistosome-specific immune responses, and removal of infection and/or exposure to antigens released from parasites damaged by treatment may influence responses to subsequent infection [8–10]. Studies showing a decline in the proportion of circulating T regulatory (Treg) cells [11] and increased effector responses to parasite [12, 13] and nonparasite antigens [14, 15] post-treatment indicate that immunoregulatory mechanisms established during infection are alleviated when infection is removed. Furthermore, PZQ-induced changes in antibody levels, CD23+ B cells [16], and cellular proliferation [13] have been associated with reduced Schistosoma mansoni reinfection rates, suggesting that treatment may have longer-term benefits in addition to transient infection clearance.
Taken together, these observations suggest that treatment alters not only the magnitude of the host immune response but also the phenotype of schistosome-specific responses. However, although previous human studies have inferred relative changes in the phenotype of the schistosome-specific cytokine response from changes in individual responses [12, 17], this hypothesis has not been directly assessed. Importantly, experimental models of helminth infection suggest that innate cells and CD4+ T helper (Th) cells (Th1, Th2, Treg, and Th17) cross-regulate one another during infection [18, 19], and the interaction between these responses rather than levels of individual cytokines alone may influence resistance to reinfection [20]. No studies to date have investigated schistosome-specific, Th17-associated cytokine responses in humans despite the role of Th17 in schistosome egg-mediated immunopathologies in mice [21–23] and detectable levels of Th17-type cytokines in plasma samples from S. haematobium–exposed humans [24].
This study addresses 4 key hypotheses relating to the effect of PZQ on schistosome-specific immune responses: (1) treatment influences levels of schistosome-specific, Th17-associated cytokines in addition to the previously assayed cytokines; (2) the cytokine environment (comprising markers associated with innate inflammatory, Th1, Th2, Th17, and regulatory responses) of treated individuals is fundamentally altered relative to pretreatment responses; (3) the effects of treatment on schistosome-specific cytokines differ between responses to cercariae, adult worms and eggs; and (4) cytokine responses elicited 6 weeks post-treatment influence reinfection rates. Our results yield insights into the immunological effects of antihelminthic treatment and how these relate to parasite life history and the development of protective immunity.
MATERIALS AND METHODS
Ethical Permissions
Ethical approval was granted by the Medical Research Council of Zimbabwe and the University of Zimbabwe's Institutional Review Board. Local permission was granted by the Provincial Medical Director. Community members were informed of the study aims and procedures in their local language (Shona), and compliant participants provided written consent or assent from a parent/guardian if aged <18 years.
Study Design
Participants were recruited from Murehwa District an S. haematobium endemic region of Zimbabwe, which is part of ongoing immunoepidemiological studies [14, 25–27]. Recruitment was school-based, and non–school going community members were invited to attend via community meetings. After pretreatment sampling for parasitology and cytokine assays, participants were treated with a single dose of PZQ (40 mg/kg body weight). Blood samples for follow-up cytokine assays were collected 6 weeks post-treatment, and parasitological samples were collected 6 weeks, 6 months, and 18 months post-treatment. To be included in the study, participants had to meet the following 6 criteria: (1) life-long residents of the area; (2) provided at least 2 urine and 2 stool samples on consecutive days for parasitological analysis pretreatment and 6 weeks and 18 months post-treatment; (3) negative for S. mansoni, soil-transmitted helminths, malaria, and human immunodeficiency virus (HIV) at all 3 time points; (4) not previously treated with antihelminthic drugs; (5) received PZQ treatment after baseline sampling and cured of S. haematobium infection (ie, no eggs detectable) 6 weeks post-treatment; and (6) provided sufficient blood for cultures and all cytokine assays before treatment and 6 weeks post-treatment. No cases were excluded for parasite coinfection; 6 HIV-positive cases were excluded, 7 participants refused treatment, and 23 participants provided insufficient sample volume. In accordance with the Medical Research Council of Zimbabwe ethical guidelines on blood volumes that can be collected from young children, children aged <5 years were excluded. Based on these criteria, a total of 72 people were included in this longitudinal study. High community-wide infection levels precluded inclusion of an untreated control group.
Parasitology and Virology
Schistosoma haematobium, S. mansoni, and soil-transmitted helminth eggs were quantified via microscopic analysis of urine and stool samples using standard urine filtration and Kato–Katz protocols respectively [28, 29]. A minimum of 2 samples per participant collected on consecutive days and 2 slides per stool sample were analyzed to quantify mean infection intensity before treatment and 6 weeks and 18 months post-treatment. Some participants also provided parasitological samples 6 months post-treatment, and these were used to confirm reinfection status. Plasmodium spp. were identified via microscopic examination of blood smears and confirmed with a Paracheck rapid test (Orchid Biomedical Systems). HIV was detected using DoubleCheckGold HIV1&2 Whole Blood test (Orgenics), and positive cases were confirmed using Determine HIV1/2 Ag/Ab Combo (InvernessMedical).
Schistosome Antigens
Schistosoma haematobium cercariae antigen preparation (CAP), whole adult worm homogenate (WWH), and soluble egg antigen (SEA) were obtained from the Theodor Bilharz Institute, Giza, Egypt. Antigens were confirmed endotoxin-free using previously described protocols [30] (Supplementary Figure 1).
Whole Blood Culture
Venous blood samples were collected by trained nurses, diluted 1:3 in culture medium (Roswell Park Memorial Institute medium supplemented with 2 mM L-glutamine and 100 U penicillin/streptomycin; all Lonza), and cultured in duplicate wells coated with 10 µg/mL CAP, WWH, and SEA for 48 hours at 37°C in Anaerogen Compact anaerobic atmosphere generation pouches (OXOID). Unstimulated cultures (antigen-free media) were conducted in parallel. Fewer CAP stimulations were conducted (n = 21) due to sample limitations. Cell-free culture supernatants were frozen and assayed within 12 months.
Cytokine Enzyme-Linked Immunosorbent Assay
Interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin 2 (IL-2,) interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), interleukin 12p70 (IL-12p70), interleukin 13 (IL-13), and interleukin 21 (IL-21) (BD Biosciences) and interleukin 17A (IL-17A) and interleukin 23p19 (IL-23p19) (eBiosciences) were assayed in culture supernatants via enzyme-linked immunosorbent assay. Ninety-six well plates were coated with 1 µg/mL or 2 µg/mL (TNF-α and IL-23 only) capture antibody in serum-free phosphate-buffered saline. Plates were washed 3 times and blocked for 2 hours with phosphate-buffered saline/2% bovine serum albumin (Alpha Diagnostic International). Recombinant cytokine standard dilutions were prepared from starting concentrations of 1 ng/mL (IL-17A), 2 ng/mL (IL-23p19 and IL-4), 5 ng/mL (IFN-γ, TNF-α, IL-5, IL-6, IL-8, and IL-10), 10 ng/mL (IL-13), 20 ng/mL (IL-2 and IL-12p70), and 40 ng/mL (IL-21). Supernatant samples were added to duplicate wells and incubated overnight at 4°C.
After 3 washes, 0.5 µg/mL (IFN-γ, TNF-α, IL-2, IL-5, IL-6, IL-8, IL-12p70, and IL-23) or 1 µg/mL (IL-4, IL-10, IL-13, IL-17A, and IL-21) biotinylated detection antibody was added for 2 hour at 37°C. After 4 washes, 1:6000 streptavidin–horseradish peroxidase (GE Healthcare) was added for 2 hour at 37°C. Plates were washed 4 times and incubated with 3,3′,5,5′-tetramethylbenzidine (TMB)-based streptavidin–horseradish peroxidase substrate solution (Sigma-Aldrich). Assays were developed for 1.5 minutes (IL-8 only) or 5 minutes, stopped with 25% hydrochloric acid, and read at 450 nm using SoftmaxPro spectrophotometer software (Molecular Devices).
Statistical Analysis
Before analysis, mean cytokine concentrations in unstimulated wells were subtracted from those of the corresponding antigen-stimulated cultures for each participant to give antigen-specific cytokine levels.
Cytokine responses to CAP, WWH, and SEA pretreatment and 6 weeks post-treatment were square-root(x + 1) transformed to meet parametric assumptions and compared using repeated measures analysis of variance conducted using IBM SPSS Statistics v.19 software. Pair-wise comparisons were made using Fisher's least significant difference test [31]. Because multiple comparisons may give rise to false positive results, P values from analysis of variance were adjusted using the sequential Bonferroni method [32]. Raw P values are reported, and those significant postcorrection are considered highly significant.
In addition to 1-way analysis of cytokine levels, a data reduction method—multidimensional scaling—was used to visualize the multiple cytokine responses of each participant in a 2-dimensional space. The nonparametric version of this approach, nonmetric multidimensional scaling (NMS) [33], was used because combined data did not meet parametric assumptions. This approach was used to account for multicolinearity and multiplicity between the 13 cytokines and to identify how patterns of cytokine responses (an indicator of cellular immune phenotype) were affected by treatment. A single score was first assigned to each participant corresponding to their combination of CAP-, WWH-, or SEA-specific cytokine responses ranked relative to all other participants before treatment and 6 weeks post-treatment. Scores were then plotted via NMS ordination to give a visual representation of relative similarity and/or dissimilarity in participant cytokine responses [33]. Hypothesis testing comparing pretreatment and post-treatment NMS scores was conducted using the nonparametric multiple response permutation procedure (MRPP) [33]. Pearson's correlation between the original cytokine levels and NMS scores identified responses that varied most between participants. Nonmetric multidimensional scaling and MRPP were implemented using PC-ORD software as described in the Supplementary information.
Factor analysis was used to reduce all square-root(x + 1)–transformed, 6 week post-treatment SEA- and WWH-specific cytokine responses into a smaller number of variables (principal components [PCs]) reflecting cytokine profiles [31]. Only PCs that accounted for a greater than average proportion of variance in the original cytokine data (eigenvalue > 1) and correlated with at least 2 of the original cytokine variables with factor loadings ≥0.5 or ≤ −0.5 were included in subsequent analyses [31]. Binary logistic regression was conducted with sex, age, pretreatment infection intensity, and all SEA- and WWH-specific cytokine profiles (6 week post-treatment PCs) included simultaneously in the model as predictors of reinfection status (reinfected: ≥1 S. haematobium egg detected between 6 and 18 months post-treatment; uninfected: no S. haematobium eggs detected in post-treatment samples). The Log likelihood ratio test statistic (D) and associated P value were calculated for each predictor from the difference between the Log likelihood model fit for the full model to that where the predictor was not included.
RESULTS
Cohort Characteristics Pretreatment
Pretreatment prevalence of S. haematobium infection within the cohort was 58.3% with a mean infection intensity of 30.3 eggs/10 mL urine (standard error of the mean [SEM], 9.82; range, 0–481 eggs/10 mL urine). Participants had a mean age of 9.3 years (SEM, 0.31; range: 5–17 years); 40 were males, and 32 were females. Demographic and infection characteristics of the cohort are representative of the whole community [14].
Cercariae-, Adult Worm–, and Egg-Specific Cytokine Responses Change Post-Treatment
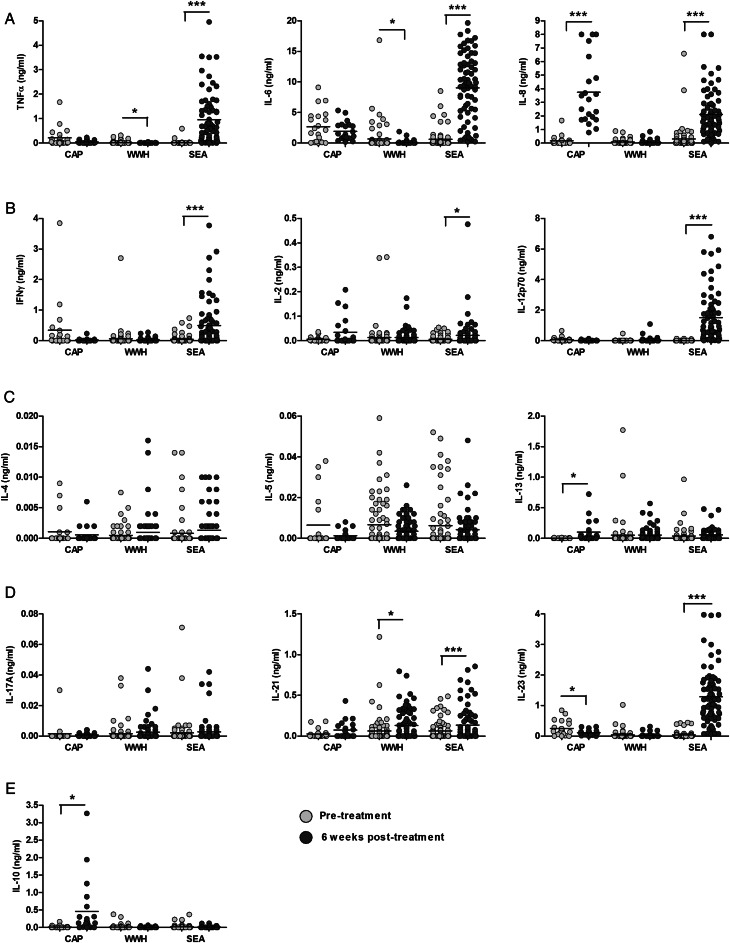
Mean cytokine concentrations showed distinct post-treatment dynamics according to antigen stimulation (Figure 1 and Table 1). Concentrations of all SEA-specific responses, with the exception of IL-5, were higher than pretreatment levels, and this increase was statistically significant for innate inflammatory (TNF-α, IL-6, and IL-8), Th1 (IFN-γ, IL-2, and IL-12p70), and Th17 (IL-21 and IL-23p19) cytokines. Cercariae antigen preparation –specific IL-8, IL-10, and IL-13 responses were significantly higher 6 weeks post-treatment, but CAP-specific IL-23p19 levels were significantly lower 6 weeks post-treatment. Whole adult worm homogenate –specific TNF-α and IL-6 were significantly lower post-treatment than pretreatment. Interleukin 5 responses to all antigens were lower post-treatment than pretreatment, although this trend was not statistically significant. IL-21 responses to all schistosome antigens were higher post-treatment than pretreatment, but only significantly so for SEA and WWH. Changes in SEA-specific TNF-α, IL-6, IL-8, IFN-γ, IL-12p70, IL-21, and IL-23p19 and CAP-specific IL-8 were highly significant (ie, remained significant after Bonferroni correction).
Figure 1.
Levels of innate inflammatory (A), Th1 (B), Th2 (C), Th17 (D), and regulatory (E) cytokines elicited by Schistosoma haematobium antigens changed 6 weeks after curative praziquantel treatment. Cytokine responses were assayed in supernatants from 48 hours whole blood cultures stimulated with S. haematobium cercariae (CAP), adult worm (WWH), and egg (SEA) antigens by enzyme-linked immunosorbent assay (CAP, n = 21; WWH, SEA, and unstimulated, n = 72). Mean pretreatment concentrations (ng/mL) are represented by gray circles and post-treatment cytokine responses are represented by black circles. Levels of cytokine present in parallel unstimulated cultures were subtracted from those present in antigen-stimulated cultures for each participant to account for levels of spontaneously produced cytokine. Pretreatment and 6 week post-treatment responses were compared for each antigen by repeated measures analysis of variance. Horizontal bars indicate median values. IFN-γ, interferon γ; IL-2, interleukin 2; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IL-12p70; interleukin 12p70; IL-13, interleukin 13; IL-17A, interleukin 17A; IL-21, interleukin 21; IL-23, interleukin 23; TNF-α, tumor necrosis factor α. *P < .05, **P < .01, ***P < .001.
Table 1.
Schistosome-Specific Cytokine Responses Differ 6 Weeks After Treatment Relative to Pretreatment Levels
| Phenotype | Cytokine | Antigen |
|||||
|---|---|---|---|---|---|---|---|
| CAP |
WWH |
SEA |
|||||
| F Valuea | P Value | F Valuea | P Value | F Valuea | P Value | ||
| Innate inflammatory | TNF-α | 3.70 | 0.07 | 6.18 | 0.02 | 75.24b | <0.001b |
| IL-6 | 0.82 | 0.38 | 7.78 | 0.01 | 208.47b | <0.001b | |
| IL-8 | 64.92b | <0.001b | 2.38 | 0.13 | 90.49b | <0.001b | |
| Th1 | IFN-γ | 3.53 | 0.07 | 0.95 | 0.33 | 28.70b | <0.001b |
| IL-2 | 4.32 | 0.05 | 0.01 | 0.93 | 4.79 | 0.03 | |
| IL-12p70 | 2.29 | 0.15 | 2.77 | 0.10 | 94.52b | <0.001b | |
| Th2 | IL-4 | 0.76 | 0.39 | 2.70 | 0.10 | 1.34 | 0.25 |
| IL-5 | 3.28 | 0.08 | 3.66 | 0.06 | 1.19 | 0.28 | |
| IL-13 | 7.41 | 0.01 | 0.17 | 0.68 | 1.91 | 0.17 | |
| Regulatory | IL-10 | 9.09 | 0.01 | 3.45 | 0.07 | 1.38 | 0.24 |
| Th17 | IL-17A | 0.07 | 0.79 | 1.04 | 0.31 | 0.26 | 0.61 |
| IL-21 | 4.31 | 0.05 | 7.32 | 0.01 | 8.88b | <0.001b | |
| IL-23 | 5.01 | 0.04 | 1.34 | 0.25 | 190.15b | <0.001b | |
Results of repeated measures analysis of variance comparing mean concentrations (square root(x + 1) transformed) of cytokines present in whole blood cultures stimulated with cercariae (CAP), adult worm (WWH), and egg (SEA) antigens before treatment with those elicited 6 weeks after a single 40 mg/kg body weight dose of praziquantel (CAP, n = 21; WWH, SEA, and unstimulated, n = 72). Significant differences (P < .05) are highlighted in bold.
Abbreviations: IFN-γ, interferon γ; IL-2, interleukin 2; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IL-12p70; interleukin 12p70; IL-13, interleukin 13; IL-17A, interleukin 17A; IL-21, interleukin 21; IL23, interleukin 23; TNF-α, tumor necrosis factor α.
a Error degrees of freedom: 1, 20 (CAP); 1, 71 (WWH and SEA).
b Differences that are significant after Bonferroni adjustment for multiple comparisons.
Consistent with systemic effects of treatment, levels of spontaneously produced IL-12p70 and IL-21 increased and IL-2, IL-5, and IL-8 responses decreased in unstimulated cultures post-treatment (Supplementary Figure 2).
Treatment Alters Polarization of Schistosome-Specific Cytokine Profiles
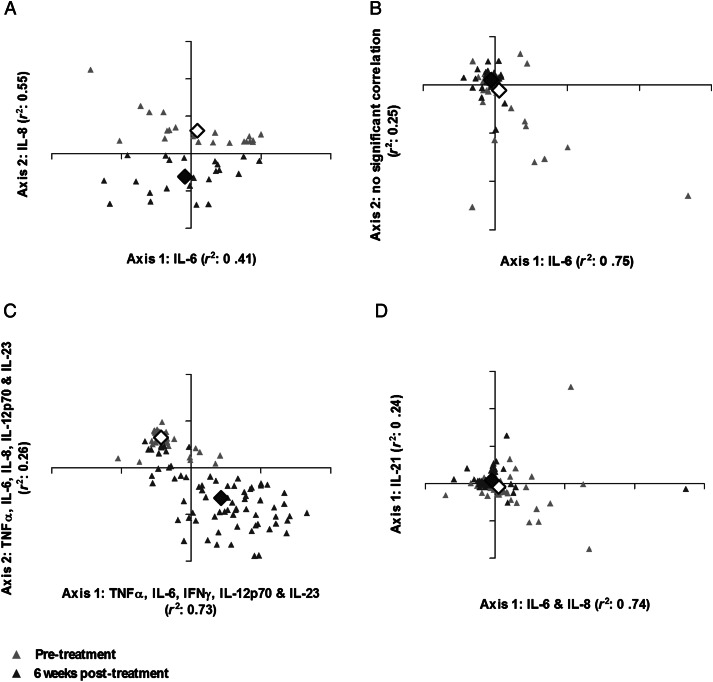
Having identified differences between the levels of individual cytokines following treatment, the implication of these changes for polarization of whole blood cytokine responses was investigated via NMS. Participant scores are plotted according to similarity and/or dissimilarity between cytokine profiles pre- and post-treatment (Figure 2). The individual cytokines that varied most between participants are indicated on spatial axes (correlation coefficients in Supplementary Table 1).
Figure 2.
Cytokine profiles elicited by Schistosoma haematobium cercariae (CAP) (A), adult worm (WWH) (B), and egg (SEA) (C) antigens and spontaneous cytokine production (D) differed 6 weeks post-treatment relative to pretreatment responses. Nonmetric multidimensional scaling ordination plots of participants' combined cytokine responses (interferon γ [IFN-γ], tumor necrosis factor α [TNF-α], interleukin 2 [IL-2], interleukin 4 [IL-4], interleukin 5 [IL-5], interleukin 6 [IL-6], interleukin 8 [IL-8], interleukin 10 [IL-10], interleukin 12p70 [IL-12p70], interleukin 13 [IL-13], interleukin 17A [IL-17A], interleukin 21 [IL-21], and interleukin 23 [IL-23]) before (gray triangles) and 6 weeks after treatment (black triangles) plotted according to 2-dimensional spatial axes (CAP, n = 21; WWH, SEA, and unstimulated, n = 72). The proportion of variance in participant cytokine responses attributable to each axis (Pearson's r2) is shown. Axes are labelled according to the cytokines with which they are most strongly correlated (correlation coefficients for these associations provided in Supplementary Table 1). Axis 2 of the WWH-specific cytokine ordination plot was not strongly associated with any of the pre- or post-treatment cytokine responses assayed. Mean within-group differences reflect the total variation between participant cytokine responses before (white diamond) and after (black diamond) treatment.
Pre- and post-treatment responses to CAP varied predominantly according to IL-6 (axis 1) and IL-8 (axis 2). Distinct groupings along axis 2 (Figure 2A) reflect the significant difference between pre- and post-treatment cytokine polarization (T, −17.25; P < .001; A, 0.199) and particularly the highly significant increase in cercariae-specific IL-8 post-treatment (Table 1).
Variation between pre- and post-treatment WWH-specific cytokine responses (Figure 2B) was mainly due to IL-6 (axis 1), reflecting the post-treatment decline in WWH-specific innate inflammatory cytokines (Figure 1). No cytokines were strongly associated with axis 2, and there was considerable overlap between pre- and post-treatment responses, suggesting that pre- and post-treatment cytokine profiles were similar, consistent with the weakly significant differences in individual WWH-specific cytokines (Table 1). Although MRPP indicated that WWH-specific post-treatment cytokine profiles changed relative to baseline (T, −9.37; P < .001), the effect size of the difference was small (A, 0.029).
Soluble egg antigen–specific cytokine responses exhibited a clear shift post-treatment (Figure 2C) according to a combination of TNF-α, IL-6, IFN-γ, IL-12p70, and IL-23p19 (axis 1) and TNF-α, IL-6, IL-8, IL-12p70, and IL-23p19 (axis 2) and significantly differed relative to pretreatment responses (T, −72.67; P < .001; A, 0.391). The distinct clustering and low overlap of pre- and post-treatment SEA-specific cytokine responses indicate that this post-treatment shift was evident in nearly all participants. The loading of IL-8 onto axis 2 but not axis 1 and IFN-γ onto axis 1 but not axis 2 indicates that participants differed in their IL-8 and IFN-γ responses in addition to the more uniform upregulation of other proinflammatory cytokines.
Spontaneous cytokine production was also affected by treatment predominantly due to variation in IL-6 and IL-8 (axis 1) and IL-21 (axis 2) after treatment relative to baseline (Figure 2D). Pre- and post-treatment cytokine responses in unstimulated cultures differed significantly (T, −2.804; P = .02), although the effect size of this difference was low (A, 0.008).
Cytokine Profiles 6 Weeks Post-Treatment Predict Reinfection Status
Of the study participants, 18 were reinfected within 18 months of treatment. Table 2 summarizes the characteristics of the reinfected participants relative to those that remained uninfected. Having identified reinfection within the cohort, we sought to investigate whether the risk of reinfection was related to the cytokine profiles mounted in response to S. haematobium adult worm and egg antigens 6 weeks post-treatment.
Table 2.
Demographic and Schistosoma haematobium Infection Characteristics of the Study Cohort 18 Months After Praziquantel Treatment
| Reinfection status |
||
|---|---|---|
| Characteristics | Uninfected | Reinfecteda |
| No. | 54 | 18 |
| Mean age (SEM) | 9.2 (0.37) | 9.3 (0.53) |
| Males, females | 27, 27 | 13, 5 |
| Mean pretreatment infection intensitya | 28.6 (11.0) | 35.6 (21.9) |
| Pretreatment infection rangeb | 0–481 | 0–395 |
| Pretreatment infection prevalence | 57.4% | 61.1% |
| Mean post-treatment infection intensityb (SEM) | 0 | 10.2 (4.5) |
| Post-treatment infection rangeb (SEM) | 0 | 0–65 |
Abbreviation: SEM, standard error of the mean.
a Reinfection classified as presence of ≥1 S. haematobium egg in ≥1 urine sample between 6 months and 18 months post-treatment.
b Infection intensity quantified as mean S. haematobium egg counts/10 mL urine.
Factor analysis identified 4 profiles of 6 weeks post-treatment cytokine responses to WWH and SEA (Table 3). Principle component 1, which accounted for the most variation between cytokine profiles (15.6%), reflects regulatory and Th2-polarized cytokine responses due to its positive loading with SEA- and WWH-specific IL-10 and IL-13 and negative loading with SEA-specific TNF-α, IL-6 and IL-23. Principle component 2 reflects a combination of SEA-specific proinflammatory and WWH-specific Th2- and Th17-polarized responses. Principle component 3 reflects proinflammatory cytokine responses to WWH- (IL-6, IL-8, and IL-23p19) and SEA-specific IL-17A and was negatively loaded with Th2 cytokines. Principle component 4 comprised SEA-specific TNF-α and IL-13, both of which are associated with schistosome-associated immunopathology [34, 35].
Table 3.
Adult Worm and Egg-Specific Cytokine Profiles 6 Weeks After Treatment
| Phenotype | Cytokine | Antigen | Principal Component |
|||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Th2/Regulatory | SEA Proinflammatory/WWH Th2/Th17 | WWH Proinflammatory | SEA Proinflammatory/Th2 | |||
| Innate inflammatory | TNF-α | SEA | −0.5 | 0.3 | 0.1 | 0.5 |
| WWH | 0.3 | 0.4 | 0.1 | −0.1 | ||
| IL-6 | SEA | −0.7 | 0.5 | 0.1 | 0.2 | |
| WWH | −0.3 | 0.1 | 0.7 | −0.2 | ||
| IL-8 | SEA | −0.1 | 0.1 | 0.0 | 0.3 | |
| WWH | −0.2 | 0.1 | 0.7 | −0.4 | ||
| Th1 | IFN-γ | SEA | −0.4 | 0.4 | 0.1 | 0.4 |
| WWH | 0.1 | 0.1 | 0.1 | 0.1 | ||
| IL-2 | SEA | 0.4 | 0.1 | 0.1 | 0.2 | |
| WWH | 0.6 | 0.1 | 0.2 | 0.2 | ||
| IL-12p70 | SEA | −0.4 | 0.5 | 0.1 | 0.4 | |
| WWH | 0.2 | 0.1 | 0.0 | 0.0 | ||
| Th2 | IL-4 | SEA | 0.2 | 0.4 | −0.5 | −0.2 |
| WWH | 0.1 | 0.4 | −0.5 | −0.3 | ||
| IL-5 | SEA | 0.1 | 0.4 | −0.5 | −0.3 | |
| WWH | −0.1 | 0.6 | −0.4 | 0.0 | ||
| IL-13 | SEA | 0.6 | 0.0 | 0.1 | 0.5 | |
| WWH | 0.7 | 0.1 | 0.1 | 0.3 | ||
| Regulatory | IL-10 | SEA | 0.7 | 0.1 | 0.2 | 0.4 |
| WWH | 0.5 | 0.1 | 0.3 | 0.4 | ||
| Th17 | IL-17A | SEA | 0.2 | 0.4 | 0.5 | −0.5 |
| WWH | 0.3 | 0.4 | 0.3 | −0.5 | ||
| IL-21 | SEA | 0.2 | 0.6 | −0.1 | 0.0 | |
| WWH | 0.4 | 0.7 | −0.1 | −0.1 | ||
| IL-23 | SEA | −0.5 | 0.5 | 0.1 | 0.4 | |
| WWH | 0.0 | 0.0 | 0.5 | −0.2 | ||
| % Total variance | 15.6 | 12.3 | 15.4 | 12.5 | ||
Antigen-specific cytokine factor loadings for principal components (PCs) 1–4 extracted by factor analysis of all Schistosoma haematobium whole worm homogenate (WWH)– and soluble egg antigen (SEA)–specific cytokine responses 6 weeks after praziquantel treatment (n = 72). Cytokines with factor loadings ≥0.5 or≤−0.5 for an extracted PC are highlighted in bold. The cellular immune phenotype with which the cytokines are associated is given for each PC. The percentage of total variance in cytokine responses accounted for by each PC is given at the bottom of the relevant column.
Abbreviations: IFN-γ, interferon γ; IL-2, interleukin 2; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IL-12p70; interleukin 12p70; IL-13, interleukin 13; IL-17A, interleukin 17A; IL-21, interleukin 21; IL23, interleukin 23; TNF- α, tumor necrosis factor α.
Binary logistic regression analysis showed that participant age, sex, and pretreatment infection intensity were not significant predictors of reinfection status, but high PC2 factor scores (reflecting a combination of SEA-specific proinflammatory [IL-6, IL-12p70, IL-21, and IL-23p19] and WWH-specific Th2- (IL-5) and Th17-associated [IL-21] responses) were significantly associated with a lower risk of reinfection (Table 4). None of the other post-treatment cytokine profiles were significant predictors of reinfection status (Table 4).
Table 4.
Adult Worm– and Egg-Specific Post-Treatment Cytokine Responses Influence the Risk of Reinfection 18 Months After Curative Treatment
| Predictor | D | P Value | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Sex | 2.54 | .11 | 2.65 | .77–9.10 |
| Age in years | 0.06 | .81 | 0.97 | .76–1.24 |
| Pretreatment infection intensity | 0.31 | .58 | 1.00 | 1.00–1.01 |
| Th2 (PC1) | 2.36 | .63 | 1.56 | .88–2.77 |
| SEA proinflammatory/WWH Th2 (PC2) | 5.67 | .02 | 0.48 | .25–.93 |
| WWH proinflammatory (PC3) | 0.62 | .43 | 0.75 | .35–1.60 |
| SEA proinflammatory/Th2 (PC4) | 0.35 | .56 | 0.81 | .41–1.61 |
Results of adjusted binary logistic regression analysis (n = 72) with reinfection status (uninfected or reinfected) as the dependent variable and sex, age, pretreatment infection intensity (mean egg count/10 mL urine), and factor scores for whole blood cytokine profiles 6 weeks after treatment identified via factor analysis (Table 2) included as linear predictors. Analysis of each predictor was conducted adjusting for all other predictors. Significant predictors (P < .05) of reinfection status are highlighted in bold. Hosmer–Lemeshow statistic (full model): 7.794; P = .45.
Abbreviations: CI, confidence interval; D, Log likelihood ratio statistic (difference between model excluding the predictor and the full model); PC, principle component.
DISCUSSION
Praziquantel treatment is known to affect levels of individual schistosome-specific immune responses and may do so directly by killing parasites and exposing their antigens to immune recognition [36, 37] and/or indirectly by removing immunosuppressive mechanisms mediated by live parasites [11]. The current study shows that the effects of treatment on schistosome-specific cytokine responses are dependent both on parasite life-cycle stage and the cellular phenotype with which they are associated. In particular, cercariae- and egg-specific, but not adult worm–specific, cytokine responses become polarized toward a more proinflammatory phenotype after treatment than that observed prior to treatment. Interestingly, participants who became reinfected within 18 months of curative treatment had lower PC scores for a combination of egg-specific proinflammatory and adult worm–specific IL-5 and IL-21 than their uninfected counterparts, suggesting that the boost in proinflammatory responses to egg antigens after treatment may promote resistance to reinfection. Importantly, quantification of innate-, Th1-, Th2-, Treg- and Th17-associated cytokine families allowed a more comprehensive analysis of how treatment affects schistosome-specific cytokine profiles than those described in previous studies. Use of a multivariate statistical approach also facilitated meaningful interpretation of highly complex responses, which are known to interact and cross-regulate one another during helminth infections [18, 19].
As initially hypothesized, levels of Th17-associated cytokines (IL-21 and IL-23) changed markedly 6 weeks post-treatment; however, the 2 cytokines had distinct antigen-specific dynamics. Soluble egg antigen–specific IL-21 and IL-23 increased in the context of a range of other proinflammatory cytokines, cercariae-specific IL-21 was unchanged, cercariae-specific IL-23 declined, and adult worm-induced IL-21, but not IL-23, increased post-treatment. Interestingly, despite being present at detectable levels in response to all antigens, IL-17A levels did not differ post-treatment, suggesting that IL-21 and IL-23 may have IL-17A– (or indeed Th17-) independent roles despite their shared association with the Th17 lineage. For example, murine IL-21 has also been implicated in Th2 function and alternative activation of macrophages [38]. Furthermore, murine dendritic, cell-derived, schistosome-specific IL-23 promotes T cell IL-17 in vitro, and heightened IL-23 levels may also elicit tissue-derived proinflammatory cytokines in vivo [23]. Further studies are required to ascertain the cellular sources and functions of IL-17, IL-21, and IL-23 in human schistosomiasis.
Distinct post-treatment responses to schistosome life-cycle stages suggest that the exposure of stage-specific antigens differs following treatment and is evident despite the overlap in somatic antigens [39]. For example, the low cytokine responsiveness to WWH post-treatment may be due to rapid removal of adult worms from the circulation [40], whereas ongoing contact with cercariae-infected water [41] and prolonged excretion of eggs from host tissues [5] may have maintained exposure to these life-cycle stages during the 6 weeks following treatment. Thus, our results do not preclude the possibility that cellular immune responses to adult worms are “boosted” at earlier post-treatment timepoints, as observed in S. mansoni studies [17, 42], but indicate that these effects decline within 6 weeks without repeated treatment.
Egg- and cercariae-specific proinflammatory responses may also increase post-treatment due to disruption of immunosuppression mediated by live parasites during infection [43]. This hypothesis is supported by observations that circulating Treg numbers decrease postclearance of S. mansoni [11], suggesting that infection elevates peripheral immunoregulation. However, observed changes in SEA-specific cytokines were not due to changes in egg-specific IL-10, which was unaffected by treatment. Thus, treatment-dependent elevation of SEA-specific proinflammatory cytokines may reflect changes in alternative mechanisms (eg, Tregs [35, 44]) regulating SEA-specific responses during infection. Consistent with the latter hypothesis, levels of spontaneous IL-10 were lower post-treatment than pretreatment, and we have previously shown that serological responses to non-parasite antigens increase post-treatment in the same population [14]. Regulation of egg-specific responses prior to treatment may be particularly important due to their immunopathological potential. For example, IL-13 and TNF-α, which are associated with egg-induced immunopathology [34, 35] and Th1 cytokines (IFN-γ and IL-12p70), which tend to be lower in chronically infected than uninfected humans [45, 46], were significantly higher 6 weeks post-treatment. The elevated IL-21 and IL-23 responses to SEA post-treatment are also consistent with a “rebound” in potentially pathological responses after removal of infection because Th17-associated responses exacerbate egg-induced granulomas in experimental schistosomiasis [21, 22].
This study also tested the hypothesis that post-treatment cytokine profiles influence the acquisition of new schistosome infections. Whereas some human helminth studies have focused on how pretreatment immune responses relate to reinfection [12, 47], we focused on post-treatment responses because reinfection occurs in the context of this altered immune environment. High post-treatment PC2 scores (comprising SEA-specific proinflammatory cytokines and WWH-specific IL-5 and IL-21) were associated with a reduced risk of reinfection within 18 months of treatment, consistent with an immune-mediated component to protective immunity rather than age-related changes in water contacts or immune physiology alone [48]. Only 18 participants were reinfected within 18 months of treatment, and thus reinfection analysis may have lacked statistical power to detect other significant associations. Nonetheless, our data provide the first evidence that Th17-associated IL-23 and IL-21 may contribute to protection from schistosome infection in addition to their putative immunopathological effects [21], and thus our data advocate further study of these cytokines in human schistosomiasis. The association between adult worm-specific Th2 cytokines and resistance to reinfection has been noted previously [9, 13] but never assessed in the context of innate inflammatory, Th1, Th17, and regulatory cytokines which can cross-regulate Th2 responses [19].
Because eggs are only produced after an infection has already been established, immune responses targeting egg antigens alone are unlikely to directly reduce reinfection. What seems more likely is that post-treatment, egg-specific, proinflammatory cytokine levels correspond to responses that limit de novo invasion by cercariae or the fitness and/or fecundity of adult worms. For example, SEA contains a range of antigens that cross-react with CAP, WWH, and schistosomules [39], and therefore increased reactivity to egg-stage parasites may also promote anti-larval and anti–adult worm responses [49]. Consistent with our observations, individuals with low peripheral blood mononuclear cell proliferative responses to S. mansoni SEA (and CAP) were more likely to be reinfected independent of age, sex, and pretreatment infection intensity in an Egyptian cohort [50]. Interestingly, we show that CAP-specific IL-8, IL-10, and IL-13 increase post-treatment; however, the limited number of cases for which CAP stimulation was conducted meant their relationship with reinfection rates could not be assessed.
This study provides the most comprehensive analysis of schistosome-specific cytokine responses in human schistosomiasis to date and the first description of human Th17 cytokine responses to schistosome antigens. Our results demonstrate that a single dose of PZQ markedly alters the polarization of schistosome-specific cytokine responses and that these changes, particularly in response to egg-stage parasites, may contribute to the development of resistance to reinfection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. This study would not have been possible without the participation of Magaya community and the students and teachers of Magaya primary and secondary schools. We also acknowledge the support of technical specialists from the National Institute of Health Research, Harare, and the University of Zimbabwe, Harare, for collection and analysis of all parasitological samples; the nursing staff of Murehwa District Hospital, Murehwa, who collected all blood samples and administered treatment; and Noah Paul and students from the University of Zimbabwe, who assisted with whole blood sample processing at the field site. We thank the director and staff of Murehwa District Hospital for hosting our research; Dr Margo Chase Topping, University of Edinburgh, Edinburgh, for her advice on NMS; and Dr Adrian Mountford for comments on the manuscript.
Financial support. This work was supported by the Carnegie Trust for the Universities of Scotland; Tenovus Scotland; the University of Edinburgh Moray Endowment Fund; the Thrasher Foundation, the World Health Organization (grant RPC264); and the Wellcome Trust (grant WT082028MA). C. D. B. was funded by a studentship from the Biotechnology and Biological Sciences Research Council and a travel grant from the British Society of Parasitology Garnham Expeditionary Fund. These funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fenwick A, Savioli L, Engels D, Robert Bergquist N, Todd MH. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends Parasitol. 2003;19:509–15. doi: 10.1016/j.pt.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Kjetland EF, Kurewa EN, Mduluza T, et al. The first community-based report on the effect of genital Schistosoma haematobium infection on female fertility. Fertil Steril. 2010;94:1551–3. doi: 10.1016/j.fertnstert.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 3.Mostafa MH, Sheweita SA, O'Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva, Switzerland: WHO; 2006. pp. 1–56. [Google Scholar]
- 5.Tchuem Tchuente LA, Shaw DJ, Polla L, Cioli D, Vercruysse J. Efficacy of praziquantel against Schistosoma haematobium infection in children. Am J of Trop Med and Hyg. 2004;71:778–82. [PubMed] [Google Scholar]
- 6.Midzi N, Sangweme D, Zinyowera S, et al. Efficacy and side effects of praziquantel treatment against Schistosoma haematobium infection among primary school children in Zimbabwe. Trans R Soc Trop Med Hyg. 2008;102:759–66. doi: 10.1016/j.trstmh.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Koukounari A, Gabrielli AF, Toure S, et al. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis. 2007;196:659–69. doi: 10.1086/520515. [DOI] [PubMed] [Google Scholar]
- 8.Mutapi F. Heterogeneities in anti-schistosome humoral responses following chemotherapy. Trends Parasitol. 2001;17:518–24. doi: 10.1016/s1471-4922(01)02118-3. [DOI] [PubMed] [Google Scholar]
- 9.Medhat A, Shehata M, Bucci K, et al. Increased interleukin-4 and interleukin-5 production in response to Schistosoma haematobium adult worm antigens correlates with lack of reinfection after treatment. J Infect Dis. 1998;178:512–9. doi: 10.1086/515630. [DOI] [PubMed] [Google Scholar]
- 10.van den Biggelaar AHJ, Borrmann S, Kremsner P, Yazdanbakhsh M. Immune responses induced by repeated treatment do not result in protective immunity to Schistosoma haematobium: interleukin (IL)–5 and IL-10 responses. J Infect Dis. 2002;186:1474–82. doi: 10.1086/344352. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe K, Mwinzi PN, Black CL, et al. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J of Trop Med and Hyg. 2007;77:676–82. [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph S, Jones FM, Walter K, et al. Increases in human T helper 2 cytokine responses to Schistosoma mansoni worm and worm-tegument antigens are induced by treatment with praziquantel. J Infect Dis. 2004;190:835–42. doi: 10.1086/422604. [DOI] [PubMed] [Google Scholar]
- 13.Roberts M, Butterworth AE, Kimani G, et al. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993;61:4984–93. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutapi F, Imai N, Nausch N, et al. Schistosome infection intensity is inversely related to auto-reactive antibody levels. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Biggelaar AHJ, Rodrigues LC, van Ree R, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004;189:892–900. doi: 10.1086/381767. [DOI] [PubMed] [Google Scholar]
- 16.Mwinzi PNM, Ganley-Leal L, Black CL, Evan Secor W, Karanja DMS, Colley DG. Circulating CD23+ B cell subset correlates with the development of resistance to Schistosoma mansoni reinfection in occupationally exposed adults who have undergone multiple treatments. J Infect Dis. 2009;199:272–9. doi: 10.1086/595792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzsimmons CM, Joseph S, Jones FM, et al. Chemotherapy for schistosomiasis in Ugandan fishermen: treatment can cause a rapid increase in interleukin-5 levels in plasma but decreased levels of eosinophilia and worm-specific immunoglobulin E. Infect Immun. 2004;72:4023–30. doi: 10.1128/IAI.72.7.4023-4030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–88. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 19.Diaz A, Allen JE. Mapping immune response profiles: the emerging scenario from helminth immunology. Eur J Immunol. 2007;37:3319–26. doi: 10.1002/eji.200737765. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MS, Cheever AW, White SD, Thompson RW, Wynn TA. IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni. Plos Pathog. 2011;7:e1002171. doi: 10.1371/journal.ppat.1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, Stadecker MJ. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J Immunol. 2008;180:2486–95. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- 22.Rutitzky LI, Smith PM, Stadecker MJ. T-bet protects against exacerbation of schistosome egg-induced immunopathology by regulating Th17-mediated inflammation. Eur J Immunol. 2009;39:2470–81. doi: 10.1002/eji.200939325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol. 2008;181:8559–67. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milner T, Reilly L, Nausch N, et al. Circulating cytokine levels and antibody responses to human Schistosoma haematobium: IL-5 and IL-10 levels depend upon age and infection status. Parasite Immunol. 2010;32:710–21. doi: 10.1111/j.1365-3024.2010.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai N, Rujeni N, Nausch N, et al. Exposure, infection, systemic cytokine levels and antibody responses in young children concurrently exposed to schistosomiasis and malaria. Parasitology FirstView, 2011:1–15. doi: 10.1017/S0031182011001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mutapi F, Rujeni N, Bourke C, et al. Schistosoma haematobium treatment in 1–5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Negl Trop Dis. 2011;5 doi: 10.1371/journal.pntd.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rujeni N, Nausch N, Midzi N, Mduluza T, Taylor DW, Mutapi F. Schistosoma haematobium infection levels determine the effect of praziquantel treatment on anti-schistosome and anti-mite antibodies. Parasite Immunol. 2012;34:330–40. doi: 10.1111/j.1365-3024.2012.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mott KE. A reusable polyamide filter for diagnosis of S. haematobium infection by urine filtration. Bull Soc Pathol Exot. 1983;76:101–4. [PubMed] [Google Scholar]
- 29.Katz N, Chavez A, Pellegring J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–402. [PubMed] [Google Scholar]
- 30.Jenkins SJ, Hewitson JP, Ferret-Bernard S, Mountford AP. Schistosome larvae stimulate macrophage cytokine production through TLR4-dependent and -independent pathways. Int Immunol. 2005;17:1409–18. doi: 10.1093/intimm/dxh319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd ed. New York, NY: W.H. Freeman & Co Ltd; 1995. [Google Scholar]
- 32.Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–5. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 33.McCune B, Grace JB. Oregon,: 2002. Analysis of ecological communities. MJM software design. [Google Scholar]
- 34.Caldas IR, Campi-Azevedo AC, Oliveira LFA, Silveira AMS, Oliveira RC, Gazzinelli G. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Tropica. 2008;108:109–17. doi: 10.1016/j.actatropica.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Wamachi AN, Mayadev JS, Mungai PL, et al. Increased ratio of tumor necrosis factor-alpha to interleukin-10 production is associated with Schistosoma haematobium–induced urinary-tract morbidity. J Infect Dis. 2004;190:2020–30. doi: 10.1086/425579. [DOI] [PubMed] [Google Scholar]
- 36.Harnett W, Kusel JR. Increased exposure of parasite antigens at the surface of adult male Schistosoma mansoni exposed to praziquantel in vitro. Parasitology. 1986;93:401–5. doi: 10.1017/s0031182000051568. [DOI] [PubMed] [Google Scholar]
- 37.Mutapi F, Burchmore R, Mduluza T, et al. Praziquantel treatment of individuals exposed to Schistosoma haematobium enhances serological recognition of defined parasite antigens. J Infect Dis. 2005;192:1108–18. doi: 10.1086/432553. [DOI] [PubMed] [Google Scholar]
- 38.Pesce J, Kaviratne M, Ramalingam TR, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–55. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curwen RS, Ashton PD, Johnston DA, Wilson RA. The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages. Mol Biochem Parasitol. 2004;138:57–66. doi: 10.1016/j.molbiopara.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Hassan MM, Medhat A, Makhlouf MM, et al. Detection of circulating antigens in patients with active Schistosoma haematobium infection. Am J of Trop Med and Hyg. 1998;59:295–301. doi: 10.4269/ajtmh.1998.59.295. [DOI] [PubMed] [Google Scholar]
- 41.Butterworth AE, Capron M, Cordingley JS, et al. Immunity after treatment of human schistosomiasis mansoni 0.2. Identification of resistant individuals and analysis of their immune responses. Trans R Soc Trop Med Hyg. 1985;79:393–408. doi: 10.1016/0035-9203(85)90391-8. [DOI] [PubMed] [Google Scholar]
- 42.Reimert CA, Fitzsimmons CM, Joseph S, et al. Eosinophil activity in Schistosoma mansoni infections in vivo and in vitro in relation to plasma cytokine profile pre- and posttreatment with praziquantel. Clin Vaccine Immunol. 2006;13:584–93.. doi: 10.1128/CVI.13.5.584-593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 44.Nausch N, Midzi N, Mduluza T, Maizels RM, Mutapi F. Regulatory and activated T cells in human Schistosoma haematobium infections. PLoS One. 2011;6:e16860. doi: 10.1371/journal.pone.0016860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson S, Jones FM, Mwatha JK, et al. Hepatosplenomegaly is associated with low regulatory and Th2 responses to schistosome antigens in childhood schistosomiasis and malaria coinfection. Infect Immun. 2008;76:2212–8. doi: 10.1128/IAI.01433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silveira AMS, Gazzinelli G, Alves-Oliveira LF, et al. Human schistosomiasis mansoni: intensity of infection differentially affects the production of interleukin-10, interferon-gamma and interleukin-13 by soluble egg antigen or adult worm antigen stimulated cultures. Trans R Soc Trop Med Hyg. 2004;98:514–9. doi: 10.1016/j.trstmh.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Mutapi F, Ndhlovu PD, Hagan P, Woolhouse MEJ. A comparison of re-infection rates with Schistosoma haematobium following chemotherapy in areas with high and low levels of infection. Parasite Immunol. 1999;21:253–9. doi: 10.1046/j.1365-3024.1999.00227.x. [DOI] [PubMed] [Google Scholar]
- 48.Hagan P. Reinfection, exposure and immunity in human schistosomiasis. Parasitol Today. 1992;8:12–6. doi: 10.1016/0169-4758(92)90303-j. [DOI] [PubMed] [Google Scholar]
- 49.Woolhouse MEJ. Immunoepidemiology of human schistosomes: taking the theory into the field. Parasitol Today. 1994;10:196–202. doi: 10.1016/0169-4758(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 50.Colley DG, Barsoum IS, Dahawi HSS, Gamil F, Habib M, Elalamy MA. Immune responses and immunoregulation in relation to human schistosomiasis in Egypt 0.3. Immunity and longitudinal-studies of in vitro responsiveness after treatment. Trans R Soc Trop Med Hyg. 1986;80:952–7. doi: 10.1016/0035-9203(86)90268-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.