Abstract
Background. Studies aimed at defining the association between host immune responses and human immunodeficiency virus (HIV) persistence during therapy are necessary to develop new strategies for cure.
Methods. We performed a comprehensive assessment of ultrasensitive plasma HIV RNA levels, cell-associated HIV RNA levels, proviral HIV DNA levels, and T cell immunophenotyping in a cohort of 190 subjects in whom HIV levels were suppressed by highly active antiretroviral therapy.
Results. The median CD4+ T cell count was 523 cells/mm3, and the median duration of viral suppression was 31 months. Cell-associated RNA and proviral DNA levels (but not ultrasensitive plasma HIV RNA levels) were positively correlated with frequencies of CD4+ and CD8+ T cells expressing markers of T-cell activation/dysfunction (CD38, HLA-DR, CCR5, and/or programmed cell death protein 1 [PD-1]) (P < .05). Having a low CD4+ T-cell count despite receipt of virologically suppressive therapy was associated with high cell-associated RNA and proviral DNA levels (P < .01) and higher frequencies of CD4+ T cells expressing CD38, HLA-DR, CCR5, and/or PD-1 (P < .0001).
Conclusions. Cell-based measurements of viral persistence were consistently associated with markers of immune activation and the frequency of PD-1–expressing CD4+ T cells. Treated patients with a low CD4+ T-cell count had higher frequencies of PD-1–expressing CD4+ T cells and cell-based measures of viral persistence, suggesting that HIV infection in these individuals may be more difficult to cure and may require unique interventions.
Keywords: HIV, raltegravir intensification, 2-LTR circles, ongoing viral replication, D-dimer
Despite the effectiveness of long-term therapy, multiple studies have shown that human immunodeficiency virus (HIV) persists indefinitely in plasma [1, 2], peripheral blood mononuclear cells (PBMCs) [3, 4], and tissues [5–7]. Multiple mechanisms contribute to this phenomenon, including the presence of long-lived latently infected CD4+ T cells [8], ongoing cryptic viral replication [9, 10], ineffective HIV-specific responses [11], and persistent immune activation [12–15]. The host immune environment is likely to be a strong determinant of each of these mechanisms. For example, a high density of activated CD4+ T cells in lymphoid tissues could support isolated rounds of de novo infection [10, 16]. In addition, a chronic inflammatory environment might lead to dysfunctional HIV-specific T cells [13, 14] and, hence, to a relative inability to clear infected cells [15]. Finally, the upregulation of certain “negative regulators” of T-cell activation (eg, programmed cell death protein 1 [PD-1]) has been postulated as a mechanism that enables recently infected CD4+ T cells to shift toward a state of persistence rather than one of activation-induced cell death [15]. Expression of PD-1 causes impaired HIV-specific immunity [14, 17], and PD-1high CD4+ T cells are highly enriched in integrated HIV DNA [15]. Given the potential role of the host immune environment in maintaining HIV persistence, we performed a comprehensive assessment of virologic and T-cell immunophenotyping in a large cohort of effectively treated individuals.
METHODS
Study Participants
A total of 190 HIV-infected individuals receiving highly active antiretroviral therapy (HAART) were identified from the University of California San Francisco (UCSF) SCOPE and OPTIONS cohorts. Subjects were required to have had at least 2 consecutive plasma HIV RNA levels below the level of detection by conventional tests (ie, <40–75 copies/mL) while taking antiretroviral therapy. Isolated blips were allowed, as long as they were <1000 copies/mL and preceded and followed by plasma HIV RNA levels <40–75 copies/mL. All subjects provided written informed consent. This study was approved by the UCSF Committee on Human Research.
Virologic Measurements
Ultrasensitive plasma HIV RNA levels were determined from 1 mL of cryopreserved plasma samples, using the COBAS AmpliPrep/COBAS TaqMan HIV-1 test, version 2.0 (Roche Molecular Diagnostics). The US Food and Drug Administration (FDA)–approved lower reporting level for this assay is 20 copies/mL, with a lower limit of detection (95% hit rate) of 17 copies/mL by probit analysis. For this study, plasma HIV RNA values below the lower reporting range of 20 copies/mL were calculated on the basis of an instrument-derived change in cycle threshold values. The lower limit of detection of this method is 9 copies/mL, using an analogous hit rate used to derive the single-copy assay detection limit reported by others [18].
Cell-associated RNA and proviral DNA levels were measured from cryopreserved PBMCs. The transcription-mediated amplification (TMA) assay (Aptima, Gen-Probe) was used to measure cell-associated RNA levels. The TMA assay is a nucleic acid–amplification test that has been FDA-approved for the early detection of HIV infection in plasma for screening blood donors and has also been validated for clinical diagnostic use [19]. A modified approach of previously published methods for PBMC extraction and TMA amplification of cell-associated hepatitis C virus was used [20, 21]. The output is a signal-to-cutoff ratio (S/Co; range, 0–30), with an S/Co <1.0 considered HIV RNA “negative” and an S/Co ≥1.0 considered “positive.” All S/Co ratios were normalized to 1 million CD4+ T cells (derived from the quantitation of human genomic DNA from a parallel real-time polymerase chain reaction amplification targeting a highly conserved region of the DQ-alpha locus, multiplied by the proportion of PBMCs that were CD4+ T cells at each time point).
Total proviral DNA was extracted from PBMCs, using modifications of previously described methods [21, 22]. This assay has an overall lower limit of detection of 1 copy per 3 μg of input DNA, which is equivalent to approximately 450 000 PBMCs [23, 24]. All proviral DNA levels were normalized to 1 million CD4+ T cells, as described above.
T-Cell Immunophenotyping
PBMCs were isolated from whole blood, cryopreserved, and stored at the UCSF AIDS Specimen Bank. Markers of T-cell activation were measured using flow cytometry at the UCSF Core Immunology Laboratory, using previously described methods that have been optimized and validated for cryopreserved PBMCs [25]. Briefly, cryopreserved PBMCs were rapidly thawed in warm media, counted on an Accuri C6 (BD Biosciences) with the Viacount assay (Millipore), washed, and stained with Aqua Amine Reactive Dye (AARD, Invitrogen) to discriminate dead cells. The average viability of thawed cells was 93% (range, 61%–98%; 80% of samples had a viability >90%). Cells were then stained with the following fluorescently conjugated monoclonal antibodies: CD3-Pacific Blue, CCR5-PECY5 (BD Pharmingen), CD38-PE, HLA-DR-FITC, PD-1-Alexa647 (BD Biosciences), CD4-PE Texas Red, and CD8-QDot 605 (Invitrogen). In each experiment a fluorescence minus one control was included for CD38, HLA-DR, CCR5, and PD-1 to help determine the cutoff for positive staining. Stained cells were washed, fixed in 0.5% formaldehyde (Polyscience), and held at 4°C until analysis. Stained cells were run on a customized BD LSR II (BD Bioscience). A total of 100 000 lymphocytes were collected for each sample. Data were compensated and analyzed using FlowJo (Tree Star) to determine the proportion of CD4+ and CD8+ T cells expressing each of the T-cell markers (CD38, HLA-DR, CCR5, and/or PD-1). Combinations of markers were calculated in FlowJo, using the Boolean gate function.
Statistical Methods
All statistical analyses were conducted with Stata, version 11.1 (StataCorp). Spearman rank correlation coefficients were calculated between virologic and immunologic measurements. Virologic and immunologic parameters were compared between subjects with a low (<350) and high (≥350) CD4+ T-cell count, using the Wilcoxon rank sum test or Fisher exact test.
RESULTS
The median CD4+ T-cell count was 523 cells/mm3 (interquartile range [IQR], 249–728 cells/mm3) (Table 1). The median duration of viral suppression during HAART was 31 months (IQR, 14–66 months). Approximately half (53%) of the cohort had undetectable plasma HIV RNA levels, using the ultrasensitive Roche assay. The median plasma HIV RNA level for the entire cohort was 0 copies/mL; of those with detectable HIV RNA, the median value was 14 copies/mL.
Table 1.
Baseline Characteristics Among 190 Study Subjects
| Characteristic | Value |
|---|---|
| Age, years | 51 (44–57) |
| Male sex, % of patients | 92 |
| CD4+ T-cell count, cells/mm3 | 523 (249–728) |
| T cells expressing CD4, % | 23 (16–32) |
| CD8+ T-cell count, cells/mm3 | 844 (606–1185) |
| T cells expressing CD8, % | 48 (37–57) |
| Nadir CD4+ T-cell count, cells/mm3 | 113 (29–227) |
| Duration of HAART suppression, months | 31 (14–66) |
| Agent included in HAART, no. of patients | |
| Protease inhibitor | 124 |
| NNRTI | 73 |
| Raltegravir | 14 |
| Maraviroc | 1 |
| Enfuvirtide | 3 |
Data are median (interquartile range), unless otherwise indicated.
Abbreviations: HAART, highly active antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor.
T-Cell Activation and Viral Persistence
We found no associations between ultrasensitive plasma HIV RNA levels and any of our measures of T-cell activation (defined by coexpression of CD38 and HLA-DR, HLA-DR alone, or CCR5 alone, in either CD4+ or CD8+ T cells) (all P > .40) (Figure 1). In addition, when we compared individuals with an undetectable plasma HIV RNA level to those with low but detectable levels, we found no significant differences in any of these immunologic measurements between the 2 groups (data not shown).
Figure 1.
No associations between ultrasensitive plasma human immunodeficiency virus (HIV) RNA levels and T-cell activation. Ultrasensitive plasma HIV RNA levels were measured using the COBAS AmpliPrep/COBAS TaqMan HIV-1 test, version 2.0. All P values are >.40.
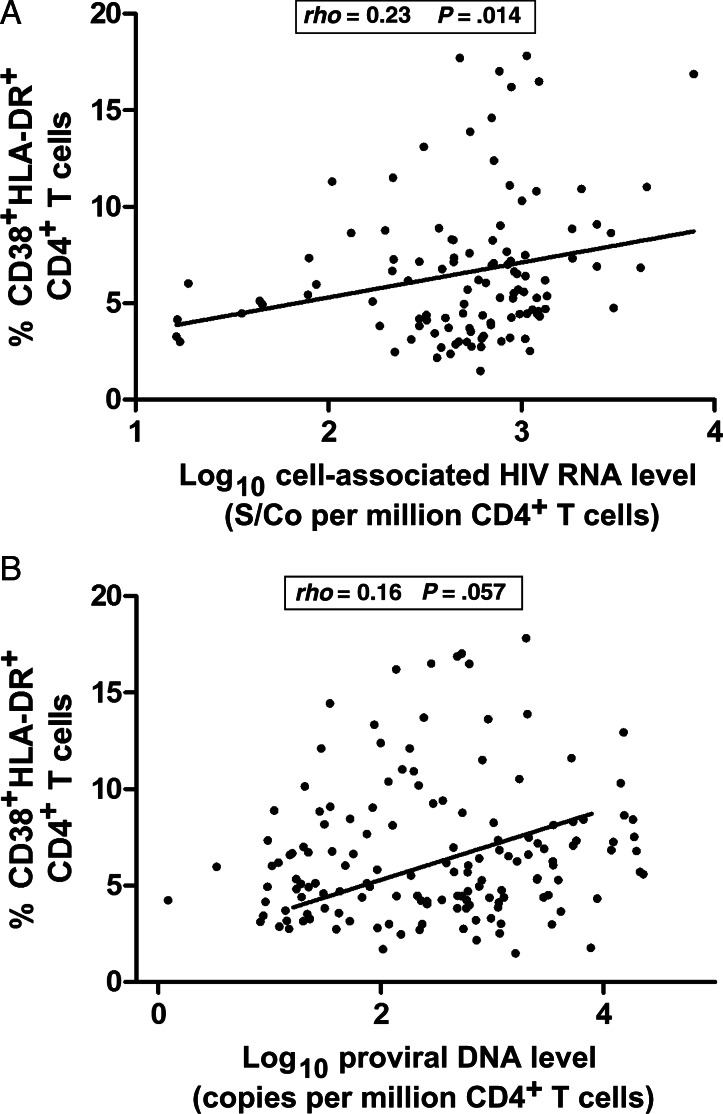
In contrast, cell-associated RNA and proviral DNA levels were positively correlated with frequencies of T cells expressing these activation markers (Figure 2). However, these relationships were modest, suggesting that there are other virologic and immunologic factors contributing to this relationship.
Figure 2.
Cell-based measures of viral persistence are modestly associated with immune activation.
PD-1 Expression and Viral Persistence
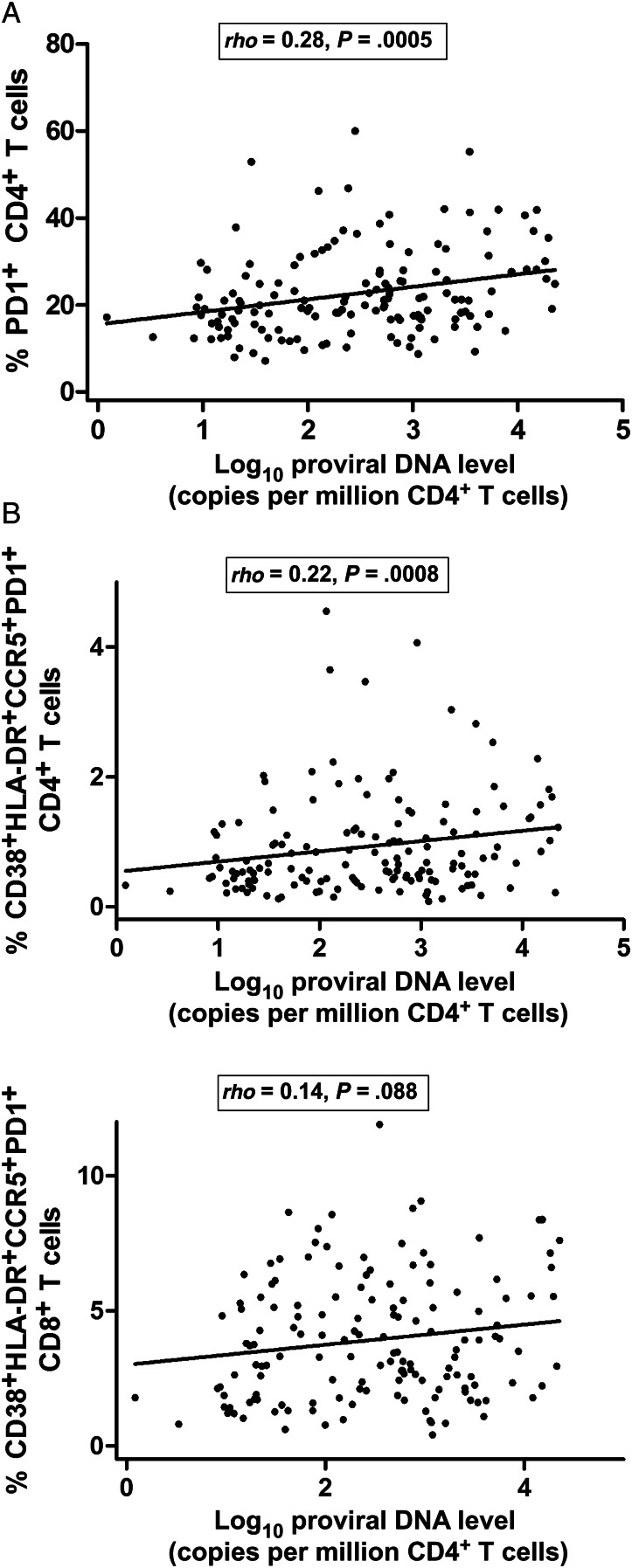
We observed a statistically significant association between proviral DNA levels and the frequency of PD-1–expressing CD4+ T cells (ρ = 0.28, P = .0005) (Figure 3A). We also observed an association between proviral DNA levels and the frequency of CD4+ T cells expressing all markers of activation (CD38, HLA-DR, CCR5, and PD-1) (ρ = 0.22, P = .008) (Figure 3B). On the other hand, there were no consistent associations between ultrasensitive plasma HIV RNA levels and the frequency of PD-1–expressing CD4+ or CD8+ T cells (data not shown).
Figure 3.
A, Highly significant association between proviral DNA levels and frequency of programmed cell death protein 1 (PD-1)–expressing CD4+ T cells. B, Associations between proviral DNA levels and the frequency of CD4+ and CD8+ T cells expressing all markers of activation (CD38, HLA-DR, CCR5, and PD-1).
T-Cell Activation, T-Cell Dysfunction, and Viral Persistence in Low and High CD4+ T-Cell States
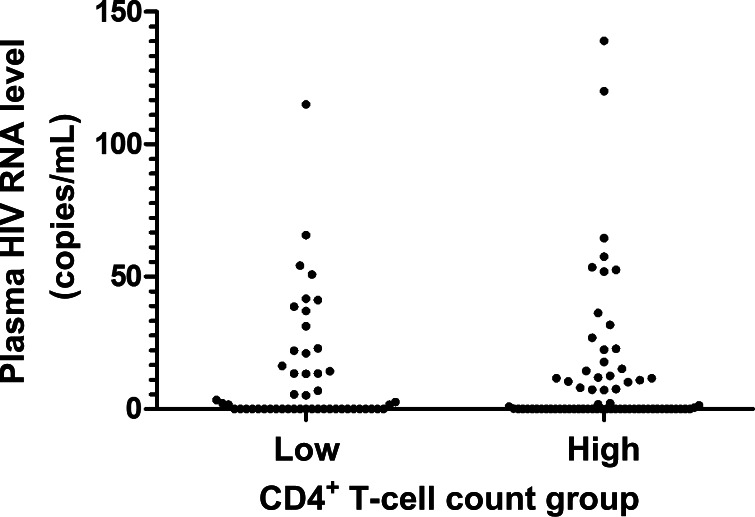
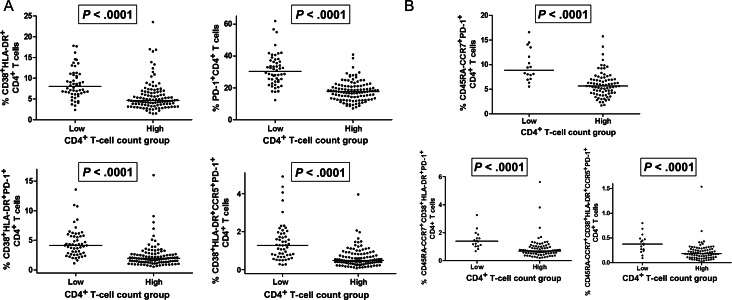
There is growing recognition that the peripheral CD4+ T-cell count during long-term antiretroviral therapy is a major predictor of morbidity and mortality [26, 27]. The detrimental effects of having a low CD4+ T-cell count despite receipt of suppressive HAART have been most readily seen among individuals with a CD4+ T-cell count <350 cells/mm3 [26]; indeed, this threshold has often been used by our group and others to define “immunologic failure,” or “incomplete CD4+ T cell recovery” [11, 28, 29]. To explore host-virus interactions in individuals with low versus high CD4+ T-cell counts [29], we performed an analysis comparing measures of viral persistence and immune activation/dysfunction in subjects with low (<350 cells/mm3) and those with high (≥350 cells/mm3) CD4+ T-cell counts. Although ultrasensitive plasma HIV RNA levels were similar between low and high CD4+ T-cell count groups (P > .50) (Figure 4), cell-associated RNA levels (878 vs 620 S/Co per million CD4+ T cells) and proviral DNA levels (600 vs 204 copies per million CD4+ T cells) were higher in the low CD4+ T-cell count group (P < .01) (Figure 5). As expected, the low CD4+ T-cell count group had lower frequencies of naive CD4+ T cells and higher frequencies of CD4+ T cells expressing CD38, HLA-DR, and/or CCR5 (P < .0001) (Figure 6A). The low CD4+ T-cell count group also had remarkably higher frequencies of PD-1–expressing CD4+ T cells (P < .0001) (Figure 6A); this was most consistently observed in the central memory compartment (P < .0001) (Figure 6B). These relationships held true even after adjustment for duration of viral load suppression and nadir CD4+ T-cell count.
Figure 4.
Ultrasensitive plasma human immunodeficiency virus (HIV) RNA levels were similar between low (<350 cells/mm3) and high (≥350 cells/mm3) CD4+ T-cell count groups. Ultrasensitive plasma HIV RNA levels were measured using the COBAS AmpliPrep/COBAS TaqMan HIV-1 test, version 2.0 All p-values are >.50.
Figure 5.
Cell-associated RNA and proviral DNA levels were significantly higher in the low (<350 cells/mm3) CD4+ T-cell count group. Abbreviation: S/Co, signal-to-cutoff ratio.
Figure 6.
A, The low (<350 cells/mm3) CD4+ T-cell count group had significantly higher frequencies of CD4+ T cells expressing CD38, HLA-DR, CCR5, and/or programmed cell death protein 1 (PD-1). B, The low (<350 cells/mm3) CD4+ T-cell count group had significantly higher frequencies of central memory CD4+ T cells expressing CD38, HLA-DR, CCR5, and/or PD-1.
DISCUSSION
Given the widely assumed role of the host environment in determining the size and persistence of the HIV reservoir during effective antiretroviral therapy, we conducted a comprehensive cross-sectional study of virologic and immunologic measurements in a large cohort of long-term–treated individuals. We observed no associations between ultrasensitive plasma HIV RNA levels and measures of immune activation/dysfunction. The lack of correlation between ultrasensitive plasma HIV RNA levels and cellular markers of immune activation has been observed by other groups, using more-sensitive assays [30]. This is in contrast to the correlations between these measurements in untreated individuals [13]. We did, however, observe modest but consistent associations between our cell-based measures of viral persistence and T-cell activation, suggesting a common mechanistic pathway linking these measurements. We also found strong associations between proviral HIV DNA levels and the frequency of PD-1–expressing CD4+ T cells, a finding that is consistent with prior studies suggesting that PD-1–expressing CD4+ T cells are a preferential reservoir of HIV during effective HAART [15]. Collectively, these data suggest that the host inflammatory environment might be a cause or consequence of HIV persistence. Work aimed at defining the mechanisms for these associations could lead to novel ways to reduce the size of the viral reservoir and/or the degree of persistent immune activation/dysfunction. If, as we predict, immune activation enables a greater degree of viral persistence (through a number of possible mechanisms, including increased target cell availability, loss of T-cell function, and upregulation of PD-1), then almost any intervention that directly affects the inflammatory process might also have secondary effects on HIV persistence. Our extended group is now pursuing this hypothesis through a number of studies that directly affect immune activation in antiretroviral-treated individuals.
Our secondary objective was to assess the relationship between CD4+ T-cell treatment response and T-cell activation/dysfunction. A significant proportion of individuals are unable to achieve a normal CD4+ T-cell count despite prolonged viral suppression with effective HAART (“immunologic nonresponders”) [31]. Moreover, having a suboptimal CD4+ T-cell response has been associated with significant clinical consequences, including increased AIDS-related and non–AIDS-related morbidity and mortality [26, 27, 32, 33]. We therefore investigated the relationship between CD4+ T-cell treatment response and immune activation/dysfunction. We found that treated subjects with a low CD4+ T-cell count (<350 cells/mm3) during therapy not only had higher measures of viral persistence as compared to those with a high CD4+ T-cell count (≥350 cells/mm3), but they also had an expansion of CD4+ T cells expressing CD38, HLA-DR, CCR5, and/or PD-1; this effect was most consistently observed in the central memory subset (P < .0001). This is consistent with findings from a study by Lederman et al, which showed that immunologic failure despite suppressive HAART was associated with increased immune activation and turnover of memory CD4+ T cells [29].
The association between HIV persistence, chronic immune activation, T-cell dysfunction, and suboptimal CD4+ T-cell gains is expected to be complex. Given the growing recognition that inflammation and immune dysfunction predict and presumably cause excess morbidity and mortality during otherwise effective therapy, detailed mechanistic studies in humans are clearly needed to untangle these complex associations. Perhaps the only way to truly understand how these factors interact is to intervene directly with either antiretroviral drugs (to reduce any residual replication) or immune-based therapies. Such studies are ongoing. On the basis of the general inability of intensification studies to affect systemic inflammation [11, 34], we generally favor a model in which residual immune dysfunction is the most proximal cause of our findings. Theoretically, persistent T-cell activation may be causally related to the inability to reconstitute normal CD4+ T-cell counts due to its deleterious effects on lymphoid tissue architecture [35]. The degree of collagen deposition in lymphoid tissues has been shown to prevent access to T-cell survival factors such as interleukin 7 [36, 37] and has also been shown to predict the degree of treatment-mediated CD4+ T-cell recovery [38, 39]. Collectively, these data suggest that suboptimal CD4+ T-cell recovery despite prolonged and effective HAART may be a consequence of delayed initiation of effective antiretroviral therapy, and they argue for very early initiation of antiretroviral therapy [40–42].
Understanding the causes of viral persistence and immune activation/dysfunction in the setting of otherwise effective HAART is also necessary to develop new strategies for cure. Future studies aimed at eradication of HIV should focus on effects on cell-based measures of viral persistence, rather than on plasma-based measurements of HIV RNA load. The frequency of PD-1–expressing CD4+ T cells and cell-based measures of viral persistence were elevated in treated patients with low CD4+ T-cell counts. This suggests that when cure strategies are being studied, these individuals may be more difficult to cure and may require unique interventions.
Notes
Acknowledgments. We thank Dr Nicolas Chomont and Dr Rafick Sekaly for their helpful discussions about this work.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by grants from the National Institute of Allergy and Infectious Diseases (R01 AI087145, K23 AI075985, K24 AI069994), the DARE: Delaney AIDS Research Enterprise (U19 AI0961090), the American Foundation for AIDS Research (106710–40-RGRL), the UCSF/Gladstone Institute of Virology & Immunology CFAR (P30 AI027763), the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), the Center for AIDS Prevention Studies (P30 MH62246), and the CFAR Network of Integrated Systems (R24 AI067039). J. M. M. is a recipient of the NIH Director's Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant DPI OD00329.
Potential conflicts of interest. H. H. has received research grant support from Roche Molecular Diagnostics. T. D. D. is an employee of Roche Molecular Diagnostics. Measurement of ultrasensitive plasma HIV RNA levels was performed by Roche Molecular Diagnostics at no cost to the study. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatano H, Delwart EL, Norris PJ, et al. Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals. AIDS. 2010;24:2535–9. doi: 10.1097/QAD.0b013e32833dba03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 4.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 5.Chun TW, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–20. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 6.Yukl SA, Gianella S, Sinclair E, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202:1553–61. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anton PA, Mitsuyasu RT, Deeks SG, et al. Multiple measures of HIV burden in blood and tissue are correlated with each other but not with clinical parameters in aviremic subjects. Aids. 2003;17:53–63. doi: 10.1097/00002030-200301030-00008. [DOI] [PubMed] [Google Scholar]
- 8.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 9.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 10.Yukl SA, Shergill AK, McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. Aids. 2010;24:2451–60. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatano H, Hayes TL, Dahl V, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203:960–8. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 13.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 14.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 15.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigal A, Kim JT, Balazs AB, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–8. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 17.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nugent CT, Dockter J, Bernardin F, et al. Detection of HIV-1 in alternative specimen types using the APTIMA HIV-1 RNA Qualitative Assay. J Virol Methods. 2009;159:10–4. doi: 10.1016/j.jviromet.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardin F, Tobler L, Walsh I, et al. Clearance of hepatitis C virus RNA from the peripheral blood mononuclear cells of blood donors who spontaneously or therapeutically control their plasma viremia. Hepatology. 2008;47:1446–52. doi: 10.1002/hep.22184. [DOI] [PubMed] [Google Scholar]
- 21.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–35. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TH, el-Amad Z, Reis M, et al. Absence of HIV-1 DNA in high-risk seronegative individuals using high-input polymerase chain reaction. Aids. 1991;5:1201–7. doi: 10.1097/00002030-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Lee TH, Paglieroni T, Utter GH, et al. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005;45:1280–90. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee TH, Chafets DM, Reed W, et al. Enhanced ascertainment of microchimerism with real-time quantitative polymerase chain reaction amplification of insertion-deletion polymorphisms. Transfusion. 2006;46:1870–8. doi: 10.1111/j.1537-2995.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair E, Tan QX, Sharp M, et al. Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon- gamma and interleukin-2 and have a CD8+ cell early maturational phenotype. J Infect Dis. 2006;194:1537–46. doi: 10.1086/508997. [DOI] [PubMed] [Google Scholar]
- 26.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 27.Moore DM, Hogg RS, Chan K, Tyndall M, Yip B, Montaner JS. Disease progression in patients with virological suppression in response to HAART is associated with the degree of immunological response. Aids. 2006;20:371–7. doi: 10.1097/01.aids.0000196180.11293.9a. [DOI] [PubMed] [Google Scholar]
- 28.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–83. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–26. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun TW, Murray D, Justement JS, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204:135–8. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–94. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–8. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000321. e1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schacker TW, Nguyen PL, Martinez E, et al. Persistent abnormalities in lymphoid tissues of human immunodeficiency virus-infected patients successfully treated with highly active antiretroviral therapy. J Infect Dis. 2002;186:1092–7. doi: 10.1086/343802. [DOI] [PubMed] [Google Scholar]
- 36.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng M, Southern PJ, Reilly CS, et al. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8:e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schacker TW, Reilly C, Beilman GJ, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS. 2005;19:2169–71. doi: 10.1097/01.aids.0000194801.51422.03. [DOI] [PubMed] [Google Scholar]
- 39.Schacker TW, Brenchley JM, Beilman GJ, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–60. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boulassel MR, Chomont N, Pai NP, et al. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol. 2012;53:29–32. doi: 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Jain V, Hartogensis W, Bacchetti P, et al. ART initiation during acute/early HIV infection compared to later ART initiation is associated with improved immunologic and virologic parameters during suppressive ART [abstract 517]. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections (Boston). [Google Scholar]
- 42.Schacker T, Little S, Connick E, et al. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis. 2000;181:354–7. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]