Abstract
Background. Linezolid is recommended for treatment of pneumonia and other invasive infections caused by methicillin-resistant Staphylococcus aureus (MRSA). The premise underlying this recommendation is that linezolid inhibits in vivo production of potent staphylococcal exotoxins, including Panton-Valentine leukocidin (PVL) and α-hemolysin (Hla), although supporting evidence is lacking.
Methods. A rabbit model of necrotizing pneumonia using MRSA clone USA300 was used to compare therapeutic effects of linezolid (50 mg/kg 3 times/day) and vancomycin (30 mg/kg 2 times/day) administered 1.5, 4, and 9 hours after infection on host survival outcomes and in vivo bacterial toxin production.
Results. Mortality rates were 100% for untreated rabbits and 83%–100% for vancomycin-treated rabbits. In contrast, mortality rates were 25%, 50%, and 100% for rabbits treated with linezolid 1.5, 4, and 9 hours after infection, respectively. Compared with untreated and vancomycin-treated rabbits, improved survival of rabbits treated 1.5 hours after infection with linezolid was associated with a significant decrease in bacterial counts, suppressed bacterial production of PVL and Hla, and reduced production of the neutrophil-chemoattractant interleukin 8 in the lungs.
Conclusions. Across the study interval, only early treatment with linezolid resulted in significant suppression of exotoxin synthesis and improved survival outcomes in a rabbit model of MRSA necrotizing pneumonia.
Keywords: Staphylococcus aureus, MRSA, protein synthesis inhibitor, linezolid, vancomycin, treatment, pneumonia, Panton-Valentine leukocidin, alpha-hemolysin, rabbit model
Staphylococcus aureus has been an infrequent cause of community-acquired pneumonia. However, since 2003 community-associated methicillin-resistant S. aureus (MRSA) strains have gained attention as an important cause of fulminant necrotizing pneumonia affecting young, otherwise healthy persons [1–15]. Community-associated MRSA pneumonia is associated with a high mortality rate despite appropriate antibiotic therapy [10, 11, 16]. In a population-based survey, an estimated 94 360 invasive MRSA infections and 18 650 deaths subsequent to invasive MRSA infections occurred annually in the United States [16]. Of these, 8460 cases were pneumonia due to community-associated or community-onset MRSA [16]. The vast majority of community-associated MRSA disease was due to a single clone, USA300. USA300 has been implicated in unusually severe human diseases, including endocarditis, sepsis, necrotizing fasciitis, and necrotizing pneumonia [6, 17–22]. The hypervirulence phenotype of USA300, especially in the context of pneumonia, has been shown in animal models to be due in part to its hyperproduction of 2 potent staphylococcal toxins, Panton-Valentine leukocidin (PVL) and α-hemolysin (Hla) [23, 24], with the former toxin being epidemiologically linked to lethal necrotizing pneumonia in humans [14]. Inasmuch as PVL and Hla are critical for the pathogenesis of pneumonia, treatment strategies that inhibit bacterial production of these toxins may improve disease outcomes.
In an open-label clinical trial of linezolid, a bacterial protein synthesis inhibitor, versus vancomycin, a cell-wall–active antibiotic, for treatment of nosocomial pneumonia caused by gram-positive bacteria, clinical and microbiological cure rates were equivalent by intent-to-treat analysis [25–27]. However, a retrospective subgroup analysis of 2 randomized clinical trials for hospital-associated pneumonia indicated that linezolid was superior to vancomycin for the treatment of nosocomial pneumonia caused by hospital-associated MRSA [28]. Comparison of linezolid and vancomycin for treatment of severe pneumonia caused by toxigenic strains of community-associated MRSA is not available. As such, treatment guidelines recommend vancomycin, which is still the standard of care, or one of 2 protein synthesis inhibitors, linezolid or clindamycin, for treatment of severe MRSA pneumonia [29, 30]. The debate over the antibiotic of choice for treating severe staphylococcal pneumonia is ongoing, with the Infectious Diseases Society of America guidelines not providing a specific recommendation for a preferred antibiotic for initiating therapy [30].
A case report noted suppression of PVL production in serial sputum samples and disease resolution in a patient with necrotizing pneumonia due to S. aureus after treatment with linezolid, clindamycin, ofloxacin, and intravenous immunoglobulin [31], suggesting that treatment strategies that use antitoxin agents may improve outcomes. Use of clindamycin for treatment of necrotizing pneumonia is not of particular interest because USA300, the predominant MRSA strain in the United States, is increasingly resistant to this antibiotic [22], and strains causing healthcare-associated infections are often resistant, whereas S. aureus remains susceptible to vancomycin and linezolid.
The objective of our study was to compare linezolid and vancomycin for treatment of necrotizing pneumonia caused by USA300 in a rabbit model, focusing in particular on elucidating the mechanisms of action of linezolid in inhibiting bacterial toxin production in vivo and improving host survival outcomes. Rabbits were used in the present study because this animal species is highly susceptible to toxigenic effects of PVL and Hla [32, 33] and because mechanisms by which these toxins induce injury and inflammation in the rabbit lungs have been well-characterized [23]. Herein we show that the enhanced protective effect of linezolid in the rabbit model of necrotizing pneumonia was strongly correlated with its in vivo suppression of bacterial production of PVL and Hla and a concomitant dampening of interleukin 8 (IL-8)–mediated acute lung inflammation.
METHODS
Bacterial Inoculum
SF8300, a minimally passaged USA300 clinical strain representative of the epidemic clone USA300-0114, was cultured in tryptic soy broth at 37°C with shaking for 6 hours to an exponential phase of growth (OD600, 1.5), harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), resuspended in 0.9% saline containing 10% glycerol to a concentration of 2 × 1010, aliquoted into individual cryovials, and immediately stored at −80°C. Frozen stocks were titered in triplicate on 3 separate occasions to determine the actual concentration of bacteria. For inoculation of rabbits (described below), the concentrated frozen stocks were combined and diluted with 0.9% saline to 5–6 × 109 colony forming units (CFU) per 1.5 mL, and the inoculum was again titered in triplicate to document the actual number of bacteria used for endobronchial instillation.
Rabbit Model of Necrotizing Pneumonia
The 1.5-mL inoculum containing SF8300 strain was delivered directly into the lungs of anesthetized New Zealand white outbred rabbits through a 2.5-mm pediatric endotracheal tube, which was positioned 1 cm above the mainstem bronchi and then removed after instillation of bacterial inoculum. Antibiotic treatment was initiated at 1.5, 4, or 9 hours after infection. Infected rabbits were randomized to one of 3 groups: an untreated control group, a group treated with 0.75 mL vancomycin solution (100 mg/mL) for a 2.5-kg rabbit intravenously 2 times/day (ie, 30 mg/kg intravenously 2 times/day), or a group treated with 50 mg/kg subcutaneously 3 times/day). Because of the poor solubility of linezolid, 125 mg of this substance was dissolved in 50 mL of 0.9% NaCl solution for subcutaneous injection. Rabbits were monitored every 3 hours after infection, and survivors were euthanized at 36 hours. Lungs were removed aseptically from euthanized rabbits and those that were found dead. Lungs were cut into <0.5-cm pieces. Three lung pieces were homogenized in 0.9% NaCl and titered by plating serial dilutions on blood agar to determine the number of bacteria present.
The experimental pneumonia protocol was reviewed and approved by the University of California San Francisco Institutional Animal Care and Use Committee.
Measurements of IL-8, the Chemokine MCP-1, LukS-PV, and Hla Concentrations in Lung Homogenate
To prepare lung homogenate for quantification of host chemokines and bacterial toxins, 4 g of lung pieces was added to 4 mL of PBS containing a protease inhibitor (PBS/PI; Roche) and homogenized using a Tissue-Tearor homogenizer (Biospec Products). The lung homogenate was centrifuged at 5000 × g for 10 minutes at 4°C, and the supernatant was collected. The pellet was resuspended in another 4 mL of PBS/PI. The cell suspension was freeze thawed (20 minutes at −80°C) and homogenized with Tissue-Tearor, and the supernatant was collected by centrifugation as described above. This extraction procedure was repeated to yield a total of 4 supernatants, which were pooled, aliquoted, and stored at −80°C until quantification by enzyme-linked immunosorbent assay (ELISA) of rabbit IL-8 and MCP-1 and LukS-PV as previously described [23].
For quantification of Hla by sandwich ELISA, using a rabbit anti-Hla immunoglobulin G (IgG) and human anti-Hla monoclonal IgG 2A3 [34]. Briefly, ELISA plates (Nunc) were coated with 2 μg/mL purified rabbit anti-Hla IgG in 100 μL PBS (Invitrogen) and stored overnight at 4°C. Plates were then washed 3 times with PBS and 0.1% Tween (VWR International) and were incubated for 1 hour at room temperature with 200 μL of blocking solution containing PBS and 1% bovine serum albumin. A total of 100 μL of each lung homogenate was added to PBS, starting at dilution of 1:2 and followed by 2-fold serial dilutions. Native Hla, purified from S. aureus Wood strain overnight culture supernatant, was used to generate a standard curve starting at 0.5 μg/mL and followed by 2-fold serial dilutions. After 1 hour of incubation at room temperature and 3 washings, plates were incubated for 1 hour with 2 μg/mL of human anti-Hla monoclonal antibody 2A3 in 100 μL of PBS. Following washing, 100 μL of goat anti-human Fcγ-HRP conjugate (Jackson Immuno-Research) was added at a dilution of 1:10 000. After 1 hour of incubation at room temperature and final washing, 100 μL of 3,3′,5,5′-tetramethylbenzidine substrate (KPL) was added, and the reaction was stopped after 10 minutes with 100 μL of 0.2 M H2S04. Plates were read on a spectrophotometer at OD450, and data were analyzed with SoftMax Pro (Molecular Devices). The nominal range of this assay was 0.1–10 µg/mL. The specificity of ELISA for Hla quantification was demonstrated using lung homogenates from rabbits infected with SF8300Δhla, in which the α-toxin gene was deleted; Hla was not detected in the SF8300Δhla-infected lungs.
Serum Concentrations of Linezolid and Vancomycin
The peak concentrations of linezolid and vancomycin in rabbit serum collected 1 hour after dosing from 3 rabbits in each of the respective treatment group were measured. Assays were performed using high-performance liquid chromatography (Quest Diagnostics).
Statistics
Survival curves were generated using the Kaplan–Meier method, and statistical significance was assessed by means of the log-rank test (GraphPad 5.0). One-way analysis of variance followed by the Dunnett multiple comparisons test (GraphPad 5.0) was used to evaluate for pairwise differences between the linezolid, vancomycin, and no-treatment groups.
RESULTS
Early Treatment With Linezolid Resulted in Improved Survival Outcomes
Two independent experiments were conducted to determine the efficacy of linezolid and vancomycin for treatment of different stages of necrotizing pneumonia (Figure 1). Treatment was initiated at the following 3 distinct time points reflecting different stages of necrotizing pneumonia, as previously described for this rabbit model [23]: 1.5 hours after infection, an early stage prior to the onset of acute lung injury and lung inflammation; 4 hours after infection, an intermediary stage in which significant onset of pulmonary edema and inflammation had occurred; and 9 hours after infection, an end stage in which massive cytokine release, neutrophil influx, pulmonary edema, alveolar hemorrhage, and severe lung necrosis had already occurred.
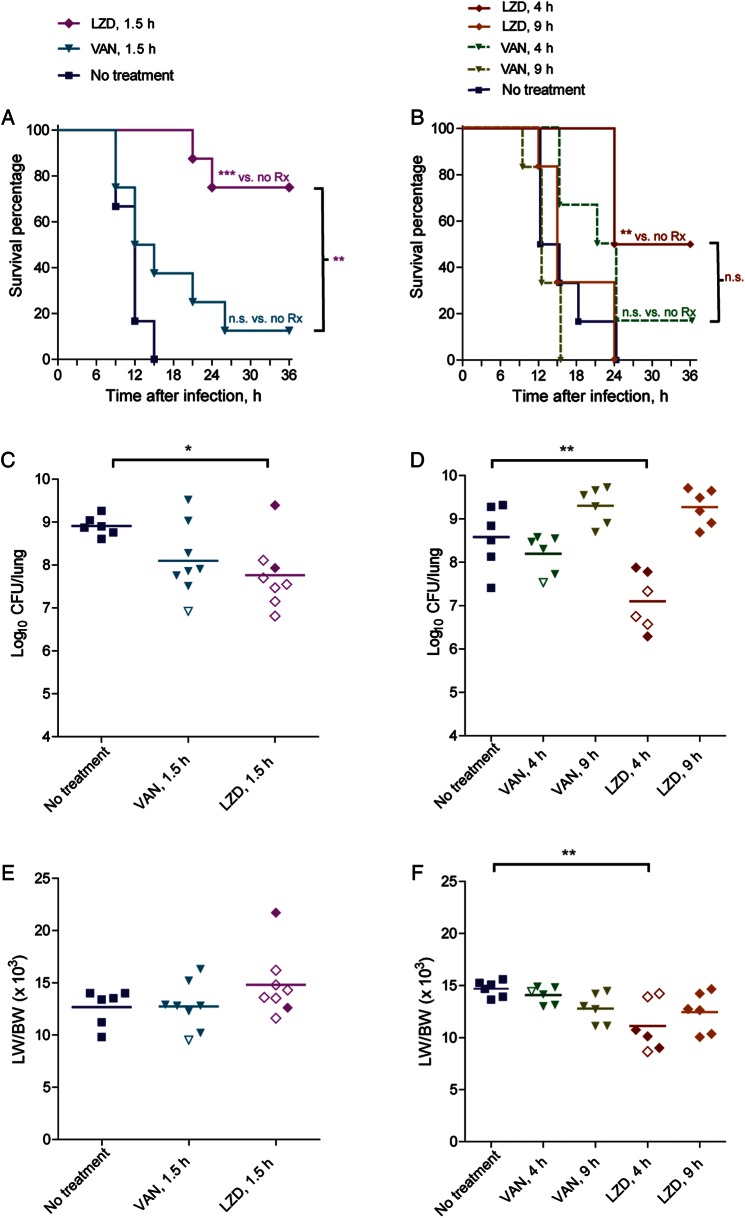
Figure 1.
Treatment window for linezolid (LZD; 50 mg/kg subcutaneously 3 times/day) and vancomycin (VAN; 30 mg/kg 2 times/day) in a rabbit model of necrotizing pneumonia. Two experiments were conducted. The first experiment evaluated survival outcomes in rabbits treated with LZD or VAN 1.5 hours after infection with 6.4 × 109 colony-forming units (CFU) SF8300 wild-type strain (A, C, and E), and the second experiment evaluated LZD or VAN 4.0 hours and 9.0 hours after infection with 5.2 × 109 CFU SF8300 wild-type strain (B, D, and F). A and B, Kaplan-Meir survival curves for comparison of mortality between the different treatment groups. **P < .01; ***P < .001. NS, not significant. C–F, Comparison of bacterial densities (C and D) and the ratio of lung weight to body weight (LW/BW) × 103 (E and F). Filled symbols represent data from dead rabbits, and open symbols represent data from surviving rabbits that were euthanized 36 hours after infection. *P < .05; **P < .01; comparisons not shown are not significant.
In the untreated control group, all rabbits had fatal, hemorrhagic, necrotizing pneumonia by 15 hours after infection (Figure 1), reflecting the severity of this acute infection. Mortality rates were 88% (7/8), 83% (5/6), and 100% (6/6) for rabbits treated with vancomycin (30 mg/kg 2 times/day) 1.5, 4, and 9 hours after infection, respectively. In contrast, mortality rates were 25% (2/8), 50% (3/6), and 100% (6/6) for rabbits treated with linezolid (50 mg/kg 3 times/day) 1.5, 4, and 9 hours after infection, respectively (Figure 1A and 1B).
One hour after dosing in infected rabbits, mean serum concentrations (±SD; as determined for 3 rabbits per treatment group) were 10.5 ± 2.3 µg/mL for linezolid and 36.1 ± 4.2 µg/mL for vancomycin, which are similar to peak levels observed in humans.
For rabbits treated 1.5 hours after infection, Kaplan-Meier analysis indicates that those treated with linezolid had significantly improved survival as compared to those treated with vancomycin (P < .01) and those that were untreated (P < .001); survival of rabbits treated with vancomycin was not significantly different from those that were untreated (P > .05; Figure 1A). Although linezolid-treated rabbits had significantly decreased bacterial counts in the lungs as compared to untreated rabbits (mean [±SD], 7.76 ± 0.78 log10 CFU vs 8.91 + 0.22 log10 CFU; P < .05; Figure 1C), the difference in bacterial counts between the linezolid- and vancomycin-treatment groups was not significant (mean [±SD], 8.10 ± 0.83 vs 7.76 ± 0.78 log10 CFU, P > .05), suggesting that the decreased mortality rate in the linezolid-treated group was not due to an enhanced antimicrobial killing effect of this antibiotic (Figure 1C).
The ratio of lung weight to body weight (LW/BW) is a quantitative measurement of extravascular lung water and indicates the severity of acute lung injury [23]. Improved survival outcomes in the linezolid-treated group may have been correlated with a decreased LW/BW ratio, although this was the case only when treatment was initiated 4.0 hours, not 1.5 hours, after infection (Figure 1E and 1F). For rabbits treated 1.5 hours after infection, the LW/BW ratio was actually increased in the linezolid-treated group as compared to vancomycin-treated or untreated groups (Figure 1E). A potential explanation for this finding is that because rabbits received 3 doses (for the 2 rabbits that died by 21 and 24 hours after infection) or 4 doses (for the 6 rabbits that survived to 36 hours after infection) of 50-mL linezolid solution subcutaneously (vs 0.75 mL vancomycin solution intravenously), the fluid overload resulted in extravasation of fluid into the lung and formation of greater edema. Rabbits treated with linezolid 4 hours and 9 hours after infection received fewer large-volume doses and did not exhibit an increase in LW/BW ratio (Figure 1F).
Linezolid Suppressed In Vivo Production of Potent Staphylococcal Toxins
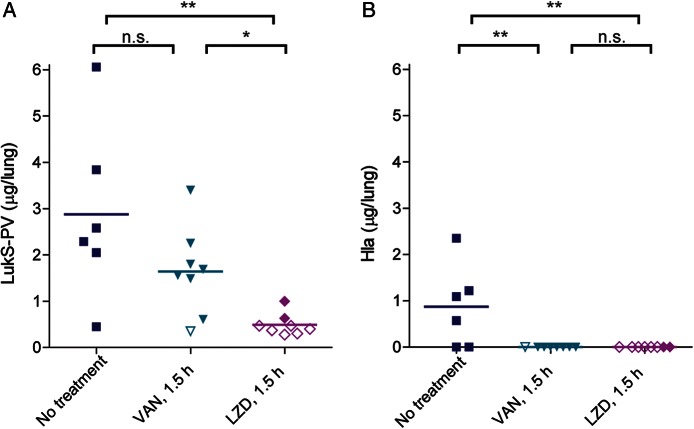
PVL and Hla have been shown to play major roles in the pathogenesis of pneumonia [23, 24], and the protective effects of linezolid could be due to its suppression of bacterial synthesis of these toxins. Measurement of toxin amounts in infected lungs by specific ELISA revealed significantly a lower mean amount (±SD) of the LukS-PV subunit of PVL in linezolid-treated rabbits (0.49 ± 0.23 µg/lung) as compared to vancomycin-treated rabbits (1.64 ± 0.95 µg/lung) and untreated rabbits (2.87 ± 1.90 µg/lung) (Figure 2A). The mean amount (±SD) of Hla in untreated rabbits was 0.87 ± 0.89 µg/lung, whereas this toxin was not detectable by ELISA in lungs of linezolid-treated or vancomycin-treated rabbits (Figure 2B; the ELISA detection limit for Hla is 0.6 ng/mL). The LukS-PV subunit of PVL was produced in greater amounts than Hla in lungs of untreated rabbits (mean [±SD], 2.87 ± 1.90 µg/lung vs 0.87 ± 0.89 µg/lung; P = .041).
Figure 2.
In vivo suppression of Panton-Valentine leukocidin (PVL) and α-hemolysin (Hla) production in lungs of rabbits treated with linezolid (LZD) and vancomycin (VAN) 1.5 hours after infection. LukS-PV component of PVL (A) and Hla (B) quantified by ELISA from rabbit lung homogenates. Filled symbols represent data from dead rabbits, and open symbols represent data from surviving rabbits that were euthanized 36 hours after infection. *P < .05; **P < .01. Abbreviation: NS, not significant.
Correlation Between Linezolid-Mediated Suppression of Toxin Synthesis and Reduced Levels of IL-8
PVL and Hla have been shown to trigger increased production of IL-8 in the lungs [23]. Linezolid-mediated suppression of toxin synthesis may also reduce the level of this potent neutrophil chemoattractant in the lung. Indeed, the mean IL-8 level (±SD) in lungs of linezolid-treated rabbits (1650 ± 1324 ng/lung) was significantly reduced as compared to levels in lungs of vancomycin-treated rabbits (4213 + 2504 ng/lung; P = .023) and untreated rabbits (6013 + 2411 ng/lung; P = .001). There was a strong correlation between levels of LukS-PV subunit of PVL and IL-8 (Pearson correlation coefficient, 0.8876; P = .003) in lungs from linezolid-treated rabbits. MCP-1 levels were not significantly different between groups (Figure 3B).
Figure 3.
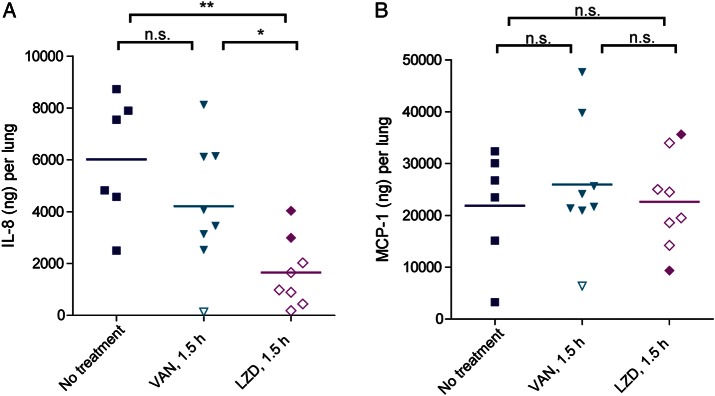
In vivo suppression of proinflammatory cytokines in lungs of rabbits treated with linezolid (LZD) and vancomycin (VAN) 1.5 hours after infection. (A) Interleukin 8 (IL-8; A) and the chemokine MCP-1 (B) were quantified by ELISA from rabbit lung homogenates. Filled symbols represent data from dead rabbits, and open symbols represent data from surviving rabbits that were euthanized 36 hours after infection. *P < .05; **P < .01. Abbreviation: NS, not significant.
DISCUSSION
To elucidate mechanisms of action of linezolid for the treatment of staphylococcal necrotizing pneumonia requires experimentation in an animal species that is sensitive to the toxic effects of PVL and Hla. The rabbit is historically known to be exquisitely sensitive to both PVL and Hla [33, 35]. In a rabbit model of necrotizing pneumonia, treatment with linezolid was associated with a 63% decrease in mortality, compared with treatment with vancomycin, but only when treatment was initiated 1.5 hours after infection, prior to onset of significant pulmonary edema formation and inflammation [23]. Because of the rapidly lethal course of necrotizing pneumonia in this model, in which one-third of the rabbits died of hemorrhagic pneumonia by 6–9 hours after infection (Figure 1A and 1B), delaying linezolid treatment to 4 hours and 9 hours after infection decreased or abolished its effectiveness in preventing death (Figure 1B). In contrast, treatment with vancomycin did not improve survival outcomes regardless of when treatment was initiated, despite the fact that peak vancomycin serum levels in infected rabbits were within range of 30–40 µg/mL recommended for humans.
United Kingdom treatment guidelines recommend use of linezolid for treatment of patients with severe staphylococcal necrotizing pneumonia [29]. As a protein synthesis inhibitor, linezolid may improve disease outcomes by suppressing in vivo toxin production. Protein synthesis inhibitors have been shown to improve disease outcomes in other toxin-mediated diseases, including those caused by Streptococcus pyogenes [36, 37] and Clostridium perfringens [38, 39]. Indirect experimental evidence from in vitro studies showed that subinhibitory concentrations of linezolid, but not vancomycin, inhibit production of PVL by S. aureus [40, 41]. Direct experimental evidence to support in vivo suppression of toxin production by linezolid is lacking because it is difficult to measure amounts of bacterial toxins at the infection site before and after antimicrobial treatment. Using specific and sensitive ELISA, we showed that USA300 MRSA produced toxic amounts of PVL (2.87 µg/lung) and Hla (0.87 µg/lung) in the infected lungs of untreated rabbits. Treatment with linezolid 1.5 hours after infection resulted in 83% reduction in PVL amounts (0.49 µg/lung vs 2.87 µg/lung; P < .01), whereas treatment with vancomycin 1.5 hours after infection resulted in 43% reduction in PVL amounts (1.64 µg/lung vs 2.87 µg/lung; P > .05, not significant). Among the 8 rabbits treated with linezolid 1.5 hours after infection, the 6 rabbits that survived had significantly decreased PVL amounts in the lungs as compared to the 2 rabbits that did not survive (0.82 µg/lung vs 0.38 µg/lung; P = .007). Thus, improved survival outcomes after linezolid treatment 1.5 hours after infection is correlated with in vivo suppression of PVL production. USA300 produced Hla in lesser amounts than PVL in the rabbit lungs (Figure 2A and 2B). Following antimicrobial therapy, Hla levels were reduced below the lower limit of quantitation. Because of the low initial levels of Hla, it is unclear whether the reduced Hla levels resulted from a reduction in bacterial numbers or a suppression of Hla expression. Regardless of the mechanism, Hla levels were reduced by antimicrobial therapy.
Linezolid has been shown to be superior to vancomycin for treatment of experimental pneumonia in mouse and pig models, although mechanisms of action of linezolid are poorly characterized in these models [42–45]. A recent study by Yanagihara et al [43], using a mouse model of hematogenous pulmonary infection with a PVL-positive MRSA strain, found a correlation between enhanced therapeutic effects of linezolid on survival outcomes and suppression of proinflammatory cytokines in the mouse lungs, which the authors speculated could be due to linezolid's suppression of PVL production. PVL is known to have proinflammatory activities because of its cytotoxic effects on phagocytic cells. However, it should be noted that the mouse is relatively insensitive to PVL, making this species less useful for dissecting mechanism of action of linezolid on PVL-positive MRSA infection [33, 35]. In a rabbit model of necrotizing pneumonia, PVL has been shown previously to induce acute lung inflammation via IL-8–mediated recruitment of neutrophils into the lungs [23]. In the present rabbit study, linezolid-mediated in vivo suppression of PVL was strongly correlated with decreased levels of IL-8 in the rabbit lungs (Figure 3A). In fact, of the 8 rabbits treated with linezolid 1.5 hours after infection, the 2 rabbits that did not survive had higher levels of PVL and IL-8, compared with the 6 rabbits that survived (Figure 2 and Figure 3A). As such, linezolid can have indirect effects on modulating host inflammatory response in the lungs through inhibiting the production of potent bacterial toxins like PVL.
In summary, data from a rabbit model of necrotizing pneumonia established a treatment window in which early treatment with linezolid was associated with a significant decrease in bacterial counts, suppression of PVL and Hla production in the lungs, reduction in toxin-induced IL-8 production, and improved survival outcomes in a rabbit model of MRSA necrotizing pneumonia. Because of the rapid progression of necrotizing pneumonia, delayed treatment with linezolid decreased or abolished its protective effects. These experimental data support the use of a protein synthesis inhibitor for treatment of severe necrotizing pneumonia caused by community-associated MRSA, but the apparently narrow treatment window may be a practical limitation.
Notes
Disclaimer. The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Financial support. This work was supported by Pfizer (ASPIRE Young Investigator award) and the US Public Health Service (grant NIH NIAID R01 AI087674) to B. A. D.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Palavecino E. Community-acquired methicillin-resistant Staphylococcus aureus infections. Clin Lab Med. 2004;24:403–18. doi: 10.1016/j.cll.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Jones TF, Creech CB, Erwin P, et al. Family outbreaks of invasive community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;42:e76–8. doi: 10.1086/503265. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert M, MacDonald J, Gregson D, et al. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ. 2006;175:149–54. doi: 10.1503/cmaj.051565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micek ST, Dunne M, Kollef MH. Pleuropulmonary complications of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus: importance of treatment with antimicrobials inhibiting exotoxin production. Chest. 2005;128:2732–8. doi: 10.1378/chest.128.4.2732. [DOI] [PubMed] [Google Scholar]
- 5.Frazee BW, Salz TO, Lambert L, Perdreau-Remington F. Fatal community-associated methicillin-resistant Staphylococcus aureus pneumonia in an immunocompetent young adult. Ann Emerg Med. 2005;46:401–4. doi: 10.1016/j.annemergmed.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–7. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez BE, Hulten KG, Dishop MK, et al. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis. 2005;41:583–90. doi: 10.1086/432475. [DOI] [PubMed] [Google Scholar]
- 8.Dickson RP, Martinez SM, Ortiz JR. A Case of rapidly progressive necrotizing pneumonia caused by community-acquired methicillin-resistant Staphylococcus aureus. Respir Care. 2008;53:1223–6. [PubMed] [Google Scholar]
- 9.McAdams RM, Ellis MW, Trevino S, Rajnik M. Spread of methicillin-resistant Staphylococcus aureus USA300 in a neonatal intensive care unit. Pediatr Int. 2008;50:810–5. doi: 10.1111/j.1442-200X.2008.02646.x. [DOI] [PubMed] [Google Scholar]
- 10.Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza–Louisiana and Georgia, December 2006–January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:325–9. [PubMed] [Google Scholar]
- 11.Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis. 2006;12:894–9. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adam H, McGeer A, Simor A. Fatal case of post-influenza, community-associated MRSA pneumonia in an Ontario teenager with subsequent familial transmission. Can Commun Dis Rep. 2007;33:45–8. [PubMed] [Google Scholar]
- 13.Enayet I, Nazeri A, Johnson LB, et al. Community-associated methicillin-resistant Staphylococcus aureus causing chronic pneumonia. Clin Infect Dis. 2006;42:e57–60. doi: 10.1086/501125. [DOI] [PubMed] [Google Scholar]
- 14.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 15.Boussaud V, Parrot A, Mayaud C, et al. Life-threatening hemoptysis in adults with community-acquired pneumonia due to Panton-Valentine leukocidin-secreting Staphylococcus aureus. Intensive Care Med. 2003;29:1840–3. doi: 10.1007/s00134-003-1918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 17.Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:752–60. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 18.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 19.Diep BA, Carleton HA, Chang RF, et al. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2006;193:1495–503. doi: 10.1086/503777. [DOI] [PubMed] [Google Scholar]
- 20.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin Infect Dis. 2008;46:1637–46. doi: 10.1086/587893. [DOI] [PubMed] [Google Scholar]
- 22.Diep BA, Chambers HF, Graber CJ, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–57. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 23.Diep BA, Chan L, Tattevin P, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A. 2010;107:5587–92. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bubeck Wardenburg J, Bae T, Otto M, et al. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–6. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 25.Stevens DL, Herr D, Lampiris H, et al. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34:1481–90. doi: 10.1086/340353. [DOI] [PubMed] [Google Scholar]
- 26.Kollef MH, Rello J, Cammarata SK, et al. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 2004;30:388–94. doi: 10.1007/s00134-003-2088-1. [DOI] [PubMed] [Google Scholar]
- 27.Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32:402–12. doi: 10.1086/318486. [DOI] [PubMed] [Google Scholar]
- 28.Wunderink RG, Rello J, Cammarata SK, et al. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–97. [PubMed] [Google Scholar]
- 29.Nathwani D, Morgan M, Masterton RG, et al. Guidelines for UK practice for the diagnosis and management of methicillin-resistant Staphylococcus aureus (MRSA) infections presenting in the community. J Antimicrob Chemother. 2008;61:976–94. doi: 10.1093/jac/dkn096. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 31.Rouzic N, Janvier F, Libert N, et al. Prompt and successful toxin-targeting treatment of three patients with necrotizing pneumonia due to Staphylococcus aureus strains carrying the Panton-Valentine leukocidin genes. J Clin Microbiol. 2010;48:1952–5. doi: 10.1128/JCM.01892-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladstone GP, van Heyningen WE. Staphylococcal leukocidins. Br J Exp Pathol. 1957;38:123–37. [PMC free article] [PubMed] [Google Scholar]
- 33.Szmigielski S, Prevost G, Monteil H, et al. Leukocidal toxins of staphylococci. Zentralbl Bakteriol. 1999;289:185–201. doi: 10.1016/s0934-8840(99)80105-4. [DOI] [PubMed] [Google Scholar]
- 34.Tkaczyk C, Hua L, Varkey R, et al. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol. 2012;19:377–85. doi: 10.1128/CVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loffler B, Hussain B, Grundmeier M, et al. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens DL, Gibbons AE, Bergstrom R, Winn V. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis. 1988;158:23–8. doi: 10.1093/infdis/158.1.23. [DOI] [PubMed] [Google Scholar]
- 37.Stevens DL, Bryant AE, Hackett SP. Antibiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis. 1995;20(Suppl 2):S154–7. doi: 10.1093/clinids/20.supplement_2.s154. [DOI] [PubMed] [Google Scholar]
- 38.Stevens DL, Maier KA, Laine BM, Mitten JE. Comparison of clindamycin, rifampin, tetracycline, metronidazole, and penicillin for efficacy in prevention of experimental gas gangrene due to Clostridium perfringens. J Infect Dis. 1987;155:220–8. doi: 10.1093/infdis/155.2.220. [DOI] [PubMed] [Google Scholar]
- 39.Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987;31:213–8. doi: 10.1128/aac.31.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumitrescu O, Boisset S, Badiou C, et al. Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob Agents Chemother. 2007;51:1515–9. doi: 10.1128/AAC.01201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens DL, Ma Y, Salmi DB, et al. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis. 2007;195:202–11. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 42.Akinnusi ME, Hattemer A, Gao W, El-Solh AA. Does linezolid modulate lung innate immunity in a murine model of methicillin-resistant Staphylococcus aureus pneumonia? Crit Care Med. 2011;39:1944–52. doi: 10.1097/CCM.0b013e31821bd79e. [DOI] [PubMed] [Google Scholar]
- 43.Yanagihara K, Kihara R, Araki N, et al. Efficacy of linezolid against Panton-Valentine leukocidin (PVL)-positive meticillin-resistant Staphylococcus aureus (MRSA) in a mouse model of haematogenous pulmonary infection. Int J Antimicrob Agents. 2009;34:477–81. doi: 10.1016/j.ijantimicag.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Yanagihara K, Kaneko Y, Sawai T, et al. Efficacy of linezolid against methicillin-resistant or vancomycin-insensitive Staphylococcus aureus in a model of hematogenous pulmonary infection. Antimicrob Agents Chemother. 2002;46:3288–91. doi: 10.1128/AAC.46.10.3288-3291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez-Olondris P, Rigol M, Soy D, et al. Efficacy of linezolid compared to vancomycin in an experimental model of pneumonia induced by methicillin-resistant Staphylococcus aureus in ventilated pigs. Crit Care Med. 2012;40:162–8. doi: 10.1097/CCM.0b013e31822d74a2. [DOI] [PubMed] [Google Scholar]