Abstract
NADH-nitrate reductase activity in excised embryos of Agrostemma githago develops in response to nitrate as well as benzyladenine. Induction of nitrate reductase by benzyladenine was much more susceptible to inhibition by a mixture of amino acid analogues and by cordycepin than induction by nitrate. In contrast, only induction of nitrate-nitrate reductase was decreased by chloramphenicol.
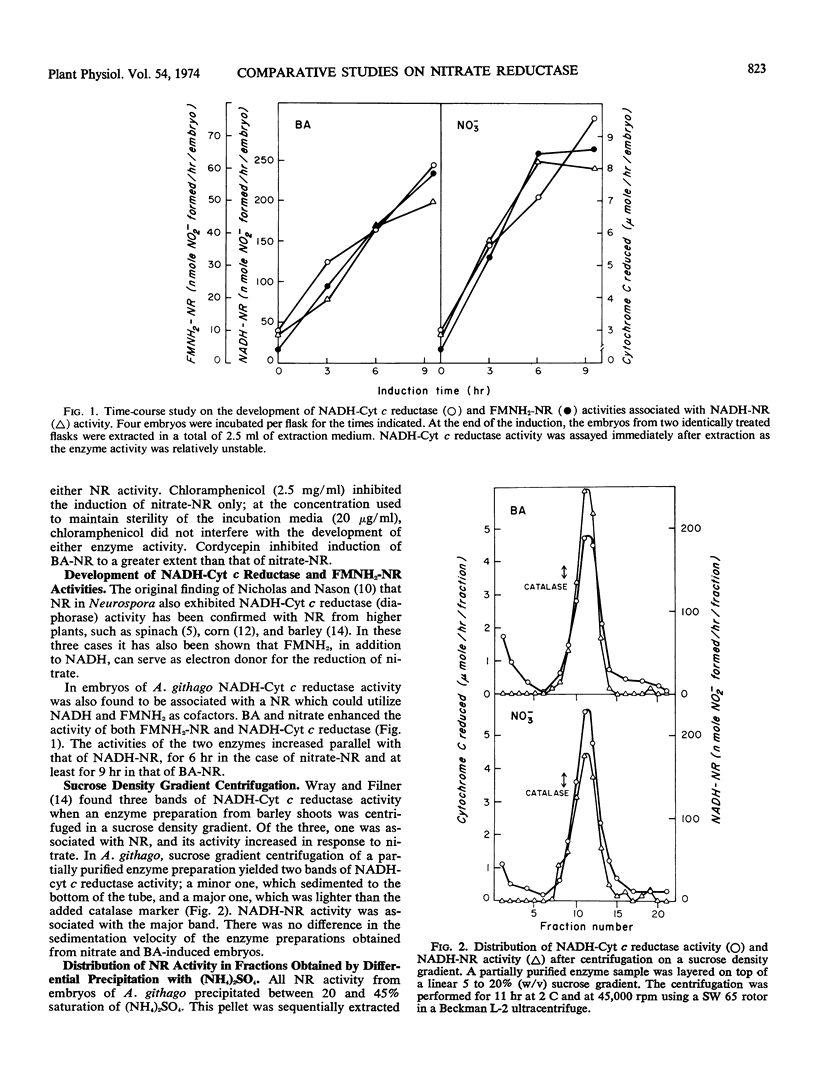
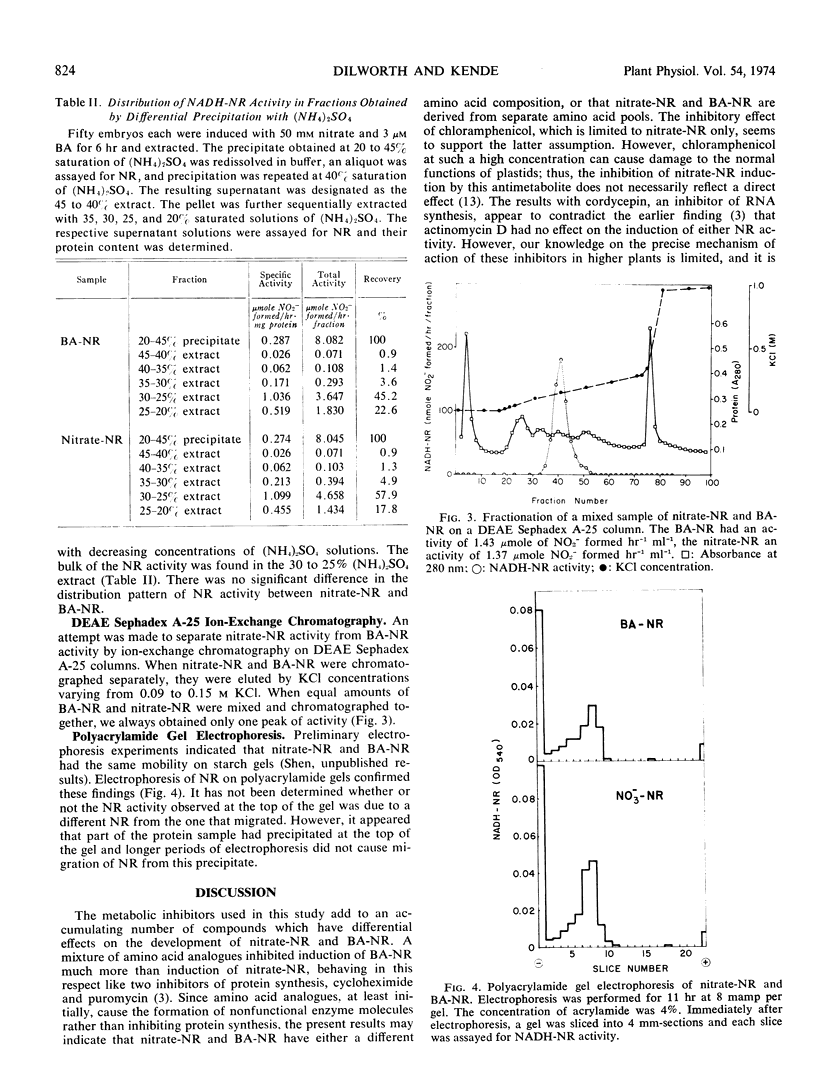
NADH-cytochrome c reductase and reduced flavin mono-nucleotide-nitrate reductase activities were found to be associated with NADH-nitrate reductase and were induced by both nitrate and benzyladenine. When a partially purified enzyme sample was centrifuged in a linear 5 to 20% sucrose density gradient, a minor and a major band of NADH-cytochrome c reductase activity were observed. NADH-nitrate reductase cosedimented with the major band.
The characteristics of nitrate-nitrate reductase and benzyl-adenine-nitrate reductase were compared by four methods but no differences could be detected: (a) Both enzymes sedimented with the same velocity during sucrose density gradient centrifugation. (b) Their distribution among fractions obtained by differential precipitation with (NH4)2SO4 was identical. (c) The elution profile of nitrate-nitrate reductase and benzyl-adenine-nitrate reductase after chromatography on diethyl-aminoethyl Sephadex A-25 columns showed no significant difference. (d) On polyacrylamide gel, the electrophoretic migration of the two enzymes was also identical.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kende H., Hahn H., Kays S. E. Enhancement of Nitrate Reductase Activity by Benzyladenine in Agrostemma githago. Plant Physiol. 1971 Dec;48(6):702–706. doi: 10.1104/pp.48.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Shen T. C. Nitrate reductase in Agrostemma githago. Comparison of the inductive effects of nitrate and cytokinin. Biochim Biophys Acta. 1972 Nov 24;286(1):118–125. doi: 10.1016/0304-4165(72)90097-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Mechanism of action of nitrate reductase from Neurospora. J Biol Chem. 1954 Nov;211(1):183–197. [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. I. Reduction of Nitrate to Nitrite. Plant Physiol. 1964 May;39(3):416–422. doi: 10.1104/pp.39.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Ritenour G. L., Eilrich G. L., Hageman R. H. Some characteristics of nitrate reductase from higher plants. Plant Physiol. 1968 Jun;43(6):930–940. doi: 10.1104/pp.43.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]


