Abstract
Spontaneous pseudoaneurysm regression is a rare event. In particular, the spontaneous regression of a splenic artery pseudoaneurysm has, to our knowledge, been previously documented in only two case reports. Furthermore, the pathophysiological mechanism of this event remains unclear. However, it is fully known that this vascular complication is potentially life-threatening and presents a high mortality rate if untreated. We report the case of a 49-year-old man affected by acute pancreatitis. Computed tomography was performed, and showed a pseudoaneurysm of the splenic artery. This patient underwent endoscopic retrograde cholangiopancreatography to treat the pancreatitis, while the vascular complication was managed with a careful and conservative treatment. On day 6 of hospitalization, a second computed tomography scan was performed and revealed complete regression of the pseudoaneurysm. This case describes the diagnosis and management of splenic artery pseudoaneurysm following acute pancreatitis and its spontaneous regression.
Keywords: vascular complications, acute pancreatitis, pseudoaneurysm, regression
Introduction
Complications occurring during and after an episode of acute pancreatitis are responsible for 2% to 10% of the mortality rate reported in this disease.1 Among these, vascular complications are rarely reported, with incidence ranging from 3% to 10%.2 Hemorrhage secondary to a bleeding pseudoaneurysm is reported with an incidence of 10%.3 Because of its proximity with the inflammatory process, the splenic artery is most frequently affected in about 40% of pseudoaneurysms secondary to pancreatitis.4 The mortality rate in untreated patients can reach 90%.5 To the best of our knowledge, only two cases of spontaneous regression of a splenic artery pseudoaneurysm have been previously documented.6,7
Case report
A 49-year-old man was admitted to hospital, presenting with abdominal pain, emesis, fever, and headache. The patient had been of good health until 1 month before admission, when he began to have abdominal pain. He initially described the pain as sharp and dull, and of moderate intensity (6 on a scale of 0 to 10, with 10 being the most severe), with one episode every 2 days, usually lasting 1 minute; the pain did not radiate, and was localized to the mesogastric region. Gradually, the pain became more frequent (two episodes daily) and progressively more severe (8 on a scale of 0 to 10). The abdominal pain was not associated with positional changes or eating. One week before admission, he developed immediate postprandial bilious vomits, one episode every 3 days, of approximately 50 mL and accompanied by nausea. The patient also had episodes of intermittent holocranial headache that improved with resting. One day before admission, he reported experiencing unquantified fever and chills.
The patient reported that since he was 30 years of age, he has smoked half a pack of cigarettes a week and has consumed more than 100 g of alcohol twice a week. He stopped drinking 10 months prior to admission and denies use of illicit drugs. A review of all the patient’s body systems showed diarrhea, asthenia, choluria, hyporexia, and weight loss (approximately 11 kg since his symptoms began). His medical and family histories were noncontributory. The patient had been taking hyoscine butylbromide, which provided only temporary relief of his abdominal pain.
On physical examination, the patient presented a fever of 40°C. His blood pressure was 100/60 mmHg, his respiratory rate was 35/minute, and his pulse rate was 150 beats/minute. He appeared dehydrated and icteric. The abdomen was slightly distended, nondepressible, painful upon palpation, and with an epigastric mass of approximately 5.0 cm in diameter.
Laboratory studies revealed the following (reference ranges shown parenthetically): serum sodium, 127 mEq/L (137–145 mEq/L); potassium, 4.9 mEq/L (3.5–5.0 mEq/L); total calcium, 7.9 mg/dL (8.5–10.5 mg/dL); glucose, 114 mEq/L (60–110 mEq/L); and creatinine, 0.4 mg/dL (0.8–1.5 mg/dL). A complete blood cell count revealed the following: hemoglobin, 8.9 g/dL (13.5–18.0 g/dL); hematocrit, 26.6% (42.0%–52.0%); mean corpuscular volume, 87 fL (80–100 fL); white blood cells, 11.7 × 109.0/L (5.0–10.0 × 109.0/L); neutrophils, 9.7 × 109.0/L (1.7–7.0 × 109.0/L); and platelets, 242 × 109/L (150–400 × 109/L). Additional studies included the following: lipase, 1200 U/L (22.9–300.0 U/L); and amylase, 380 U/L (30–110 U/L). Serum liver biochemistry results were slightly elevated.
The patient received fluids and electrolytes, hyoscine butylbromide, dexamethasone, tramadol hydrochloride, metamizole, omeprazole, and imipenem. A nasoenteral feeding tube was inserted. On the second day of hospitalization, a computed tomography (CT) scan was performed (SOMATOM Sensation 64; Siemens AG, Erlangen, Germany). In the arterial phase, the scan detected a pseudoaneurysm of the splenic artery. The total pseudoaneurysm dimension measured 2.1 × 2.5 × 2.6 cm (Figures 1 and 2). CT volumetry of the perfused part of the pseudoaneurysm identified a volume of 7.4 cc. The pancreatic parenchyma exhibited signs of necrosis and intrapancreatic fluid collections with multiple peripancreatic collections that extended into the splenic hilum and the left paracolic gutter. An endoscopic ultrasound showed diffuse areas of necrosis in the pancreatic head and body; a hypoechogenic, irregular ampulla of Vater; and an adjacent hypoechogenic locoregional small ganglion. The elastosonography showed a blue color, suggestive of malignancy in the ampulla and the ganglion.
Figure 1.
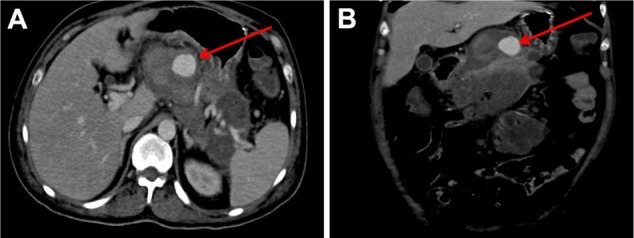
Abdominal CT scan performed on the second day of hospitalization. The arrows point to the splenic artery pseudoaneurysm in the axial (A) and coronal views (B).
Abbreviation: CT, computed tomography.
Figure 2.
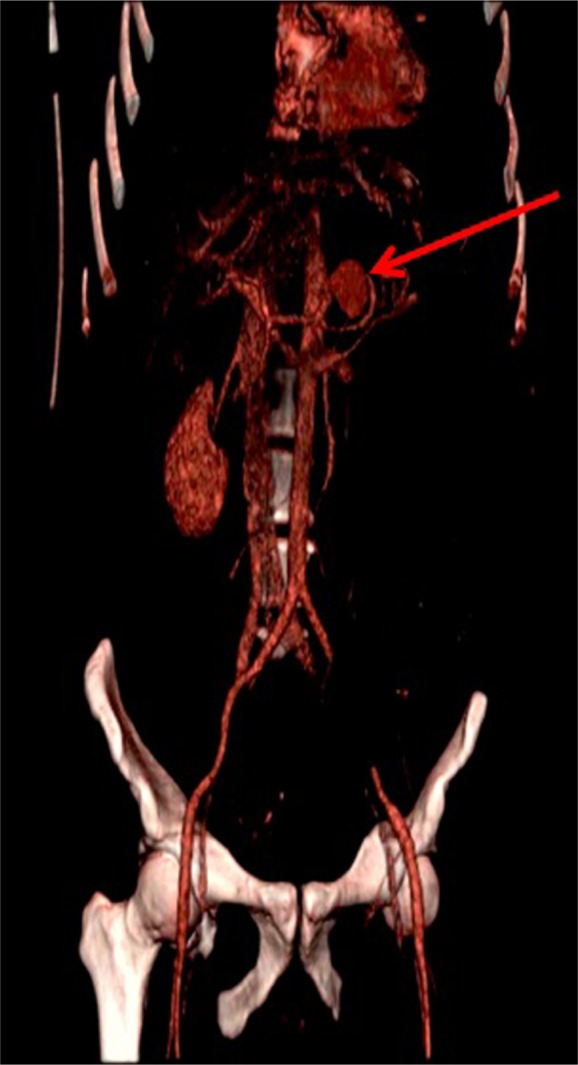
Three-dimensional computed tomography reconstruction performed on the second day of hospitalization.
Note: The arrow points to the splenic artery pseudoaneurysm.
An endoscopic retrograde cholangiopancreatography performed 4 days after the patient’s admission showed stenosis of the sphincter of Oddi, common bile duct dilation, and the absence of stones. An endoscopic sphincterotomy was performed for effective bile drainage, with insertion of a biliary stent and multiple forceps biopsies of the ampulla. Pathology examination showed chronic ampullitis, but no evidence of a malignant process. On the sixth day of hospitalization, a second CT scan was performed and revealed complete regression of the pseudoaneurysm (Figures 3 and 4).
Figure 3.
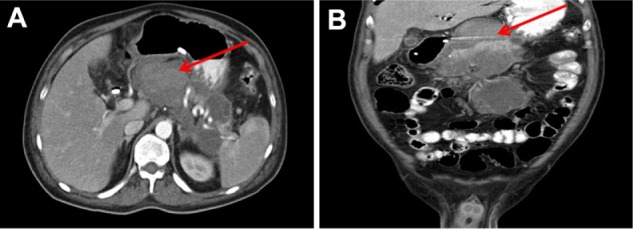
Abdominal CT performed on the sixth day of hospitalization. The arrows in the axial (A) and coronal view (B) point to the area where the pseudoaneurysm was prior to its spontaneous regression.
Abbreviation: CT, computed tomography.
Figure 4.
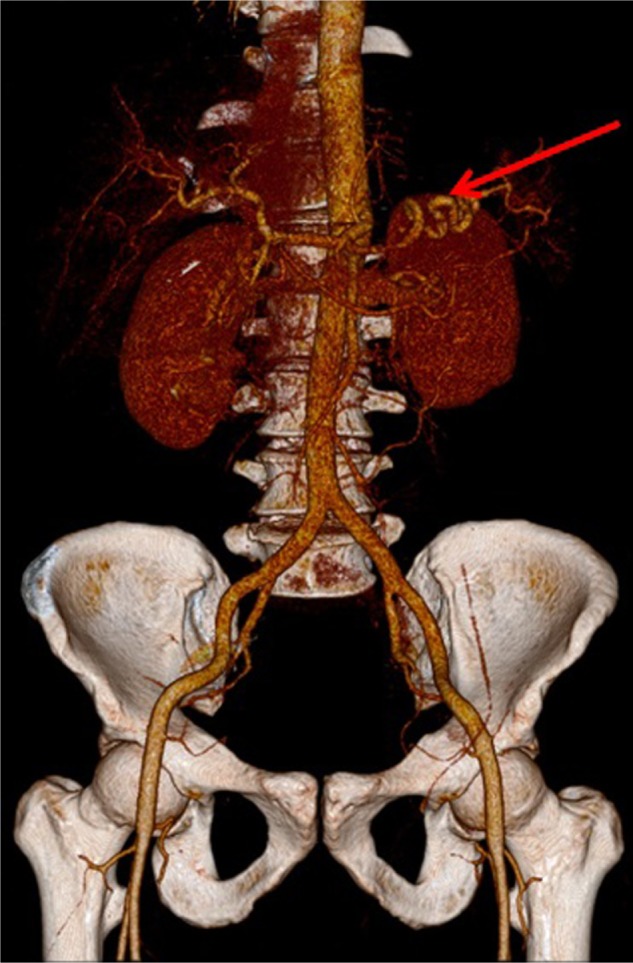
Three-dimensional computed tomography reconstruction performed on the sixth day of hospitalization.
Note: The arrow points to the splenic artery without the pseudoaneurysm.
Discussion
Acute pancreatitis is an acute inflammatory process of the pancreas. It is usually associated with severe acute upper abdominal pain and elevated blood levels of pancreatic enzymes.8 A few patients may develop severe complications affecting the peripancreatic vessels. Pseudoaneurysm is a rare but potentially fatal complication of acute pancreatitis. The risk of rupture can be as high as 37%.9 The arteries involved include, in order of frequency, the splenic (40%), gastroduodenal (20%), pancreaticoduodenal (20%), gastric (5%), and hepatic arteries (2%).10 The pathogenetic mechanism is secondary to degradation of the vessel wall by pancreatic enzymes released from a destroyed pancreatic duct, resulting in a primary formation of a pseudoaneurysm or rupture of the vessel into a preexisting pseudocyst, which then converts into a pseudoaneurysm.2 Pseudoaneurysms usually present symptoms such as gastrointestinal bleeding (60%), abdominal pain (50%), and splenomegaly or pulsatile abdominal tumors (<5%).11 In our case, the patient reported abdominal pain without gastrointestinal bleeding; therefore, the pain was more likely associated with his initial pancreatitis process, rather than with the pseudoaneurysm itself.
The diagnosis of acute pancreatitis was established by the clinical history of abdominal pain, vomiting, and fever; elevation of pancreatic enzymes; and absence of calcifications in the abdominal CT.
CT scan is the most important imaging test for the diagnosis of acute pancreatitis and its intraabdominal complications.8 Ultrasound offers several benefits, including lower costs and no need for contrast material.12 It can distinguish between a pseudoaneurysm and a pancreatic pseudocyst, but it is operator-dependent and is of limited value in obese patients.13 Angiography, however, remains the gold standard and the procedure of choice for imaging pseudoaneurysms,14 with a sensitivity of 94%.15 In our case, the palpation of an epigastric mass led us to perform an abdominal CT and an endoscopic ultrasound to confirm the presence of a pseudoaneurysm.
Once the diagnosis was established, the therapeutic plan was discussed. All visceral pseudoaneurysms should be treated, regardless of size.16 Endovascular therapy is the elective treatment in asymptomatic patients with a pseudoaneurysm > 1.5 cm and can be used even in patients with bleeding (except in unstable patients where surgery is preferred).17 The procedure consists of supraselective catheterization of the involved artery with distal and proximal embolization to the lesion and the endoluminal sac of the pseudoaneurysm, mainly with the use of coils or N-butyl-2-cyanoacrylate.13
With the persistence of systemic inflammatory response syndrome, a second abdominal CT scan was performed. We found that the pseudoaneurysm had undergone complete spontaneous regression. Some authors suggest that thrombosis is the primary mechanism in its development;18 however, further studies are needed to elucidate the underlying pathophysiology and understand the natural progression of this disorder.
Conclusion
Splenic artery pseudoaneurysm is a rare vascular complication of acute pancreatitis. Angiography is considered the gold standard and the procedure of choice for imaging pseudoaneurysms; however, conservative physicians prefer a CT scan rather than the invasive procedure. Endovascular therapy should be considered as the first-line option for asymptomatic patients with a pseudoaneurysm > 1.5 cm and can be used in hemodynamically stable patients with bleeding. The natural evolution of the splenic artery pseudoaneurysm secondary to acute pancreatitis is not well known. With few cases of spontaneous regression published before,6,7 the pathophysiological mechanism remains unclear. However, it is fully known that the mortality rate in untreated patients can be as high as 90%;5 therefore, it is essential to establish an early and appropriate diagnosis, treatment, and follow-up.
Acknowledgments
The authors want to thank Drs Sonia Buist, Ana Menezes, Angel Alvarado, and MT Maiko Zambrano for their valuable help and guidance. They also wish to thank Dr Eduardo Zambrano and Ms Martha Swain for their valuable contributions in the editing of this paper. Responsibility for any errors remains with the authors.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Balthazar EJ. Complications of acute pancreatitis: clinical and CT evaluation. Radiol Clin North Am. 2002;40(6):1211–1227. doi: 10.1016/s0033-8389(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 2.Li Destri G, Innao V, Petrillo G, Di Cataldo A. A rare pancreatic pseudoaneurysm in patient with acute pancreatitis and multiple myeloma. Ann Ital Chir. 2011;82(4):301–304. [PubMed] [Google Scholar]
- 3.White AF, Baum S, Buranasiri S. Aneurysms secondary to pancreatitis. AJR Am J Roentgenol. 1976;127(3):393–396. doi: 10.2214/ajr.127.3.393. [DOI] [PubMed] [Google Scholar]
- 4.Boudghène F, L’Herminé C, Bigot JM. Arterial complications of pancreatitis: diagnostic and therapeutic aspects in 104 cases. J Vasc Interv Radiol. 1993;4(4):551–558. doi: 10.1016/s1051-0443(93)71920-x. [DOI] [PubMed] [Google Scholar]
- 5.Bergert H, Hinterseher I, Kersting S, Leonhardt J, Bloomenthal A, Saeger HD. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery. 2005;137(3):323–328. doi: 10.1016/j.surg.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Alobeidi F, Aldin Z, Tait P. Spontaneous resolution of post pancreatitis splenic artery pseudoaneurysm. European Journal of Radiology Extra. 2010;74(3):e55–e57. [Google Scholar]
- 7.Vanlangenhove P, Defreyne L, Kunnen M. Spontaneous thrombosis of a pseudoaneurysm complicating pancreatitis. Abdominal Imaging. 1999;24(5):491–493. doi: 10.1007/s002619900546. [DOI] [PubMed] [Google Scholar]
- 8.Vege SS. Clinical manifestations and diagnosis of acute pancreatitis [webpage on the Internet] Waltham, MA: UpToDate; 2012. [Accessed November 20, 2012]. Available from: http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-acute-pancreatitis. [Google Scholar]
- 9.Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol. 2007;188(4):992–999. doi: 10.2214/AJR.06.0794. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Ishida H, Komatsuda T, et al. Pseudoaneurysm of the gastroduodenal artery ruptured into the superior mesenteric vein in a patient with chronic pancreatitis. J Clin Ultrasound. 2003;31(5):278–282. doi: 10.1002/jcu.10170. [DOI] [PubMed] [Google Scholar]
- 11.Balsarkar DJ, Joshi MA. Rupture of splenic artery pseudoaneurysm presenting with massive upper gastrointestinal bleed. Am J Surg. 2002;183(2):197–198. doi: 10.1016/s0002-9610(01)00872-8. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hourani S, Al-Bdour MN, Rashaideh MA, et al. Pseudo aneurysm complicates pancreatic pseudo cyst: importance of early detection and management. Journal of Surgery Pakistan (International) 2008;13(1):42–45. [Google Scholar]
- 13.Ballinas-Oseguera GA, Martínez-Ordaz JL, Sinco-Nájera TG, Caballero-Luengas C, Arellano-Sotelo J, Blanco-Benavides R. Management of pseudoaneurysm of the splenic artery: report of two cases. Cir Cir. 2011;79(3):246–251. [PubMed] [Google Scholar]
- 14.Yattoo GN, Khuroo MS, Wani NA, Wani KA, Bhat FA. Haemosuccus pancreaticus: a clinical challenge. J Gastroenterol Hepatol. 1999;14(2):172–175. doi: 10.1046/j.1440-1746.1999.01834.x. [DOI] [PubMed] [Google Scholar]
- 15.Udd M, Leppäniemi AK, Bidel S, Keto P, Roth WD, Haapiainen RK. Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg. 2007;31(3):504–510. doi: 10.1007/s00268-006-0209-z. [DOI] [PubMed] [Google Scholar]
- 16.Ammori BJ, Madan M, Alexander DJ. Haemorrhagic complications of pancreatitis: presentation, diagnosis and management. Ann R Coll Surg Engl. 1998;80(5):316–325. [PMC free article] [PubMed] [Google Scholar]
- 17.Piffaretti G, Tozzi M, Lomazzi C, et al. Splenic artery aneurysms: postembolization syndrome and surgical complications. Am J Surg. 2007;193(2):166–170. doi: 10.1016/j.amjsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.De Ronde T, Van Beers B, de Cannière L, Trigaux JP, Melange M. Thrombosis of splenic artery pseudoaneurysm complicating pancreatitis. Gut. 1993;34(9):1271–1273. doi: 10.1136/gut.34.9.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]


