Abstract
Core-shell particles preserve the performance (e.g. magnetic, plasmonic or opacifying) of a core material while modifying its surface with a shell that facilitates (e.g. by blocking its reactivity) their incorporation into a host liquid or polymer matrix. Here coating of titania (core) aerosol particles with thin silica shells (films or layers) is investigated at non-isothermal conditions by a trimodal aerosol dynamics model, accounting for SiO2 generation by gas phase and surface oxidation of hexamethyldisiloxane (HMDSO) vapor, coagulation and sintering. After TiO2 particles have reached their final primary particle size (e.g. upon completion of sintering during their flame synthesis), coating starts by uniformly mixing them with HMDSO vapor that is oxidized either in the gas phase or on the particles’ surface resulting in SiO2 aerosols or deposits, respectively. Sintering of SiO2 deposited onto the core TiO2 particles takes place transforming rough into smooth coating shells depending on process conditions. The core-shell characteristics (thickness, texture and efficiency) are calculated for two limiting cases of coating shells: perfectly smooth (e.g. hermetic) and fractal-like. At constant TiO2 core particle production rate, the influence of coating weight fraction, surface oxidation and core particle size on coating shell characteristics is investigated and compared to pertinent experimental data through coating diagrams. With an optimal temperature profile for complete precursor conversion, the TiO2 aerosol and SiO2-precursor (HMDSO) vapor concentrations have the strongest influence on product coating shell characteristics.
Keywords: particle formation, hermetic layering, population balance, condensation, nanoparticle encapsulation, powder technology
1. Introduction
Particles are frequently coated to facilitate their processing (e.g. reduce agglomeration) and enhance their performance by making them compatible with solid or liquid host matrices (Egerton, 1998). With such core-shell particles, one conserves the properties of the core material (e.g. dielectric, magnetic, plasmonic, scattering or opacifying functions) while modifying its surface with a shell material. For example, pigmentary rutile TiO2 particles are made by the “chloride” or “sulfate” process (Mezey, 1966). They are coated with silica, alumina and other oxides by wet impregnation processes to prevent the photocatalytic activity of TiO2 with the host solvent, die or polymer (Clark, 1975) and reduce its agglomeration in paints (Egerton, 1998).
Though wet phase coating of particles has been practiced for long and even for large scale manufacture of commodities, it is a demanding and costly process (Fisher and Egerton, 2001). An alternative route is to coat particles by gas phase processes that do not involve liquid by-products, offer easier particle collection and can reduce the multiple steps of wet processes (Pratsinis and Mastrangelo, 1989). Despite its advantages, coating of particles in the gas phase is challenging, as particle growth is much faster than in liquids. As a result, it is quite difficult to control and develop a scalable gas phase coating process. So, even commercial particles made by aerosol routes (e.g. TiO2 made by the “chloride” process) are always coated by wet processes.
Despite this industrial “reality”, significant effort has been made in understanding the fundamentals of aerosol particle coating in academic laboratories and developing such processes industrially (Subramanian et al., 2006). Hung and Katz (1992) investigated the formation of mixed TiO2-SiO2 powders in a counter-flow diffusion burner and identified conditions for synthesis of such core-shell particles by varying the inlet Si/Ti from 0.15 to 3. Akhtar et al. (1992) made TiO2-SiO2 particles by co-oxidation of their chloride precursors in a hot-wall reactor and found silica films on TiO2 by X-ray photoelectron spectroscopy at inlet Si/Ti = 0.04 - 0.20. Fotou et al. (1994) made and simulated flame-coating of suspended fibers with smooth or rough layers by controlling the sintering rate of flame-made silica coating particles. Powell et al. (1997a,b) deposited silica or alumina coatings on TiO2 particles made in a hot-wall reactor at inlet SiO2/TiO2 = 0.007 - 0.18. Increasing the flow rate of the coating precursor (SiCl4 or AlCl3) led to rougher coatings by formation of separate silica or alumina particles. Improving the mixing of the coating precursor with the core particles led to smoother coating shells. A moment model was developed for this process at isothermal conditions by Jain et al. (1997) that was in qualitative agreement with Powell et al. (1997a). Ehrman et al. (1998) made SiO2/TiO2 particles including coated ones in a premixed flame reactor at inlet Si/Ti = 1. Teleki et al. (2005) observed smooth SiO2 coatings on TiO2 particles by rapid quenching of the flame with a critical flow nozzle (Wegner et al., 2002). Rough and smooth coating shells were made at inlet Si/Ti = 0.33, otherwise segregated ones (Janus particles) were formed. King et al. (2008) have used atomic layer deposition to smoothly coat TiO2 particles with SiO2 layer-by-layer without increasing their aggregation in a fluidized bed. Sheen et al. (2009) made coated composite particles in a sliding co-flow diffusion flame at inlet Si/Ti = 4 - 57. Recently, Teleki et al. (2008) hermetically coated flame-made TiO2 particles with nanothin SiO2 (mole Si/Ti = 0.066 - 0.4) shells by injecting the SiO2 precursor vapor into an enclosed flame reactor at a location where TiO2 primary particle growth had been completed. The coating quality was determined by Raman, FT-IR, microscopy and photocatalysis of isopropanol in suspensions of these particles. Teleki et al. (2009a) showed also that improving the mixing between core aerosol and coating precursor vapor increases the fraction of coated core particles and the quality of coating, experimentally and by computational fluid dynamics.
Today, there is a reasonable understanding of what is needed to coat particles. For example, minimal coating thickness and high efficiency are required in coating TiO2 or Fe2O3 particles to minimize the costs of coating material or maximize the bulk properties of the core particle like magnetization performance (Teleki et al., 2009b). Smooth coatings give consistent optical performance while rough ones facilitate minimization of agglomeration (Egerton, 1998). Furthermore there is a reasonable understanding on how to control coating of aerosol-made particles in various laboratory scale flame, hot-wall or fluidized-bed aerosol reactors.
Here, emphasis is placed on the quantitative understanding of aerosol coating processes. Gas phase synthesis of nanothin coating shells on aerosol core primary particles is investigated theoretically accounting for monodisperse core (Kruis et al., 1993) and bimodal coating particle dynamics (Jeong and Choi, 2005) by coagulation, sintering and surface growth. Emphasis is placed on understanding the effect of aerosol process parameters and formation pathways, gas phase or surface reaction, on the efficiency of the process, texture of the coating shells (smooth or rough) and the equivalent thickness of the coating shell. The results of the simulations are compared with experimental data.
2. Theory
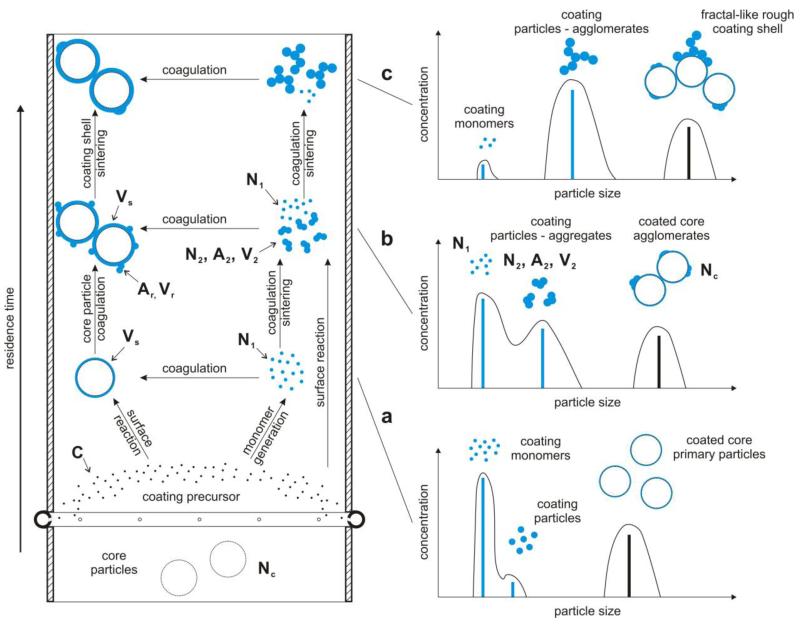
Figure 1 shows a schematic of the coating process (left hand side) and the evolution of the trimodal particle size distribution (right hand side). Core aerosol particles (e.g. TiO2, white) that no longer sinter enter the coating unit with number concentration Nc well-mixed with coating precursor vapor (e.g. HMDSO, black dots) of concentration C. Coating monomers (e.g. SiO2, blue dots) with concentration N1 are generated by precursor oxidation (Ulrich, 1971) and coagulate either with core particles creating immediately smooth shells of volume concentration Vs or among themselves forming fractal-like or spherical coating particles of concentration N2 with surface area and volume concentrations A2 and V2, respectively. These coating particles result in rough shells of area and volume concentration Ar and Vr, respectively, by coagulation with core particles. Rough shells are smoothed out by sintering, depending on their (primary) particle diameter, dpr, and temperature. Oxidation of coating vapor (e.g. HMSDO) may take place also on the surface of core or coating particles leading to smooth shells or bigger coating particles, respectively. Core particles coagulate to form agglomerates but not aggregates as their coating takes place when their sintering has ended (Tsantilis and Pratsinis, 2004). So their number concentration Nc changes but the total number of core primary particles is constant.
Figure 1.
Schematic of the coating process (left) and the corresponding evolution of the trimodal particle size distribution (right): a) formation of coating monomers and particles as well as smooth shells on the core particles from conversion of the corresponding precursor vapor, b) end of coating precursor vapor conversion and growth of coating particles into aggregates and rough and smooth shells, c) formation of coating agglomerates and fractal-like coating shells.
During HMDSO vapor oxidation in the gas phase, coating (SiO2) monomers are formed that grow into large coating particles by coagulation or deposit onto core particles forming smooth shells (Fig. 1a). Also HMDSO can decompose onto the particles forming SiO2 by surface reaction. As the HMDSO oxidation ceases, coating monomers are depleted while coating particles start to deposit onto the core particles to form rough shells and a typical bimodal coating monomer/particle distribution develops (Fig. 1b). As coating monomers disappear, coating aggregates and agglomerates form a unimodal distribution and deposit onto the core particles while rough shells are smoothed out by sintering (Fig. 1c).
2.1 Oxidation of coating precursor
The coating precursor vapor (e.g. HMDSO) can react with oxygen either in the gas phase, generating coating monomers or on particle surfaces, leading to surface growth. So the overall oxidation rate of coating precursor vapor is:
| (1) |
The first right hand side (RHS) term accounts for the overall (gas phase and surface) oxidation of HMDSO:
with k = 4×1017exp(−3.7×105/(8.314×T)) s−1 (Ehrman et al., 1998). The second RHS term accounts for the change in gas volume and composition by cooling and reaction. The gas flow rate, Q, is calculated by ideal gas law accounting for the concentration of each gaseous species including the reaction above and changes in temperature (Heine and Pratsinis, 2006).
The overall surface oxidation rate of HMDSO is not known., but its impact on coating can be estimated here considering that it encompasses two steps: a) transport of HMDSO molecules to the particle surface and b) their oxidation there. The rate of HMDSO transport to coating and core particles is determined by their respective collision frequencies, βp,2 and βp,c. When there is no information about the second step, the oxidation rate of the colliding molecules on the particle surface (e.g. surface oxidation of HMDSO), the overall surface oxidation rate constant, ks, for transport and reaction can be written as:
| (2) |
where βp,2N2 and βp,cNc describe the rate of transport of HMDSO molecules to the surface of coating and core particles, respectively. The α2 and αc describe the fraction of arriving HMDSO molecules that are oxidized on the particle surface (Friedlander, 2000), also called efficiency of collisions. Assuming for simplicity α = α2 = αc, two limiting cases then are distinguished: for instantaneous surface reaction α = 1 and no surface reaction α = 0. Molecules that have not been oxidized on the particle surface (e.g. the fraction 1-α) return into the gas phase immediately after collision.
For a finite overall surface reaction rate (α nonzero) with reaction order equal to that of the overall gas phase reaction (e.g. TiCl4 (Pratsinis and Spicer, 1998)) the gas phase oxidation rate constant, kg, is defined for an overall surface oxidation rate smaller than the overall oxidation rate (ks < k) as the difference between the two oxidation rate constants (Pratsinis and Spicer, 1998; Tsantilis et al., 2002):
| (3a) |
| (3b) |
where αmax is the maximum value which usually has to be determined experimentally (Friedlander, 2000). When the aerosol surface area increases so much that the estimated overall surface oxidation rate becomes higher than the overall oxidation rate (ks > k), surface reaction dominates and gas phase reaction stops. In the model the gas phase oxidation rate constant and the fraction of successful collisions are defined then as (Tsantilis et al., 2002):
| (4a) |
| (4b) |
So the surface oxidation rate of the coating precursor vapor does not exceed the experimentally determined k and the gas-phase generation of coating monomers stops (kg = 0) while coating precursor vapor is oxidized only on particle surfaces. Here calculations are carried out for the two limiting cases αmax = 1 and αmax = 0.
2.2 Coating particle dynamics
The evolution of coating monomers (index 1) and particles (index 2) is described with a bimodal model (Jeong and Choi, 2005) extended for coagulation with core particles (index c) but without surface growth on monomers as here the coating precursor (HMDSO) generates two monomers per molecule (nm = 2). The coating monomers represent the smallest possible coating particle (Ulrich, 1971) corresponding to SiO2 molecules with a diameter of 0.4 nm (Xiong and Pratsinis, 1991).
The rate of change of the coating monomer number concentration, N1, is:
| (5) |
where nm = 2 for HMDSO. The collision frequencies, β, are calculated with the Fuchs interpolation function (Kruis et al., 1993). The size ratio r = v2/v1 weighs monomer-monomer collisions and preserves the particle number and volume concentration (Jeong and Choi, 2003). The first RHS term describes formation of coating monomers by gas phase oxidation. The second and third RHS terms account for the loss of coating monomers by coagulation with monomers and coating particles, respectively. The fourth accounts for the loss of coating monomers by coagulation with core particles (forming smooth coating shells) and the fifth for the changing gas flow rate.
The rate of change of coating particle number concentration, N2, is:
| (6) |
The first RHS term describes the gain by coagulation of coating monomers, the second and third RHS terms account for the loss of coating particles by coagulation among themselves and with core particles (forming rough coating shells). The fourth RHS term accounts for the changing gas flow rate.
The rate of change of surface area concentration of coating particles, A2, is similar to equation 6 and defined as:
| (7) |
The fourth RHS term describes the loss of A2 by sintering of coating aggregates (Friedlander and Wu, 1994; Koch and Friedlander, 1990) and the fifth RHS term the gain by surface oxidation of coating precursor vapor (Jeong and Choi, 2005). The sintering time for SiO2 has been calculated with the equation of Tsantilis et. al (2001) with dp,min = 1 nm. Similarly, the rate of change of volume concentration of coating particles, V2, is:
| (8) |
The collision diameter of coating particles for the calculation of β (Kruis et al., 1993) is defined as (Heine and Pratsinis, 2006):
| (9) |
The coating particles grow as aggregates or agglomerates with constant fractal dimension Df = 1.8 (Schaefer and Hurd, 1990).
2.3 Core particle dynamics
Coating of core particles is optimal when core primary particle growth (and their sintering) has stopped (Teleki et al., 2008). As a result, during coating the initial core primary particle diameter, dpc, and the total number of core primary particles, Ncnpc, remains constant. So the evolution of the number concentration of core agglomerate particles, Nc, can be described by a monodisperse coagulation model:
| (10) |
where the coagulation rate of core agglomerates is given by Kruis et al. (1993) with the core collision diameter from section 2.3.2 accounting for the coating shell.
2.3.1 Coating of core particles
The coating shells on the core primary particles are distinguished into rough and smooth and described by their surface area and volume concentrations. So, the rate of change of rough coating shell surface area concentration, Ar, is:
| (11) |
where dpr is the primary particle diameter of the coating aggregates making up the rough coating shells: dpr = 6Vr/Ar (Fotou et al., 1994). The first RHS term describes the surface area increase by coagulation of coating and core particles, the second RHS term accounts for the loss Ar by sintering of rough into smooth coating shell lamellae that have negligible surface area (Appendix). Similarly, the rate of change of rough coating shell volume concentration, Vr, is given by:
| (12) |
The rate of change of smooth coating shell volume concentration, Vs, is given by:
| (13) |
where the first RHS term accounts for coagulation of coating monomers with core particles, the second RHS term for the gain by sintering of rough into smooth coating shells and the third RHS term for the gain by surface oxidation of coating precursor vapor. The surface area concentration of smooth coating shells, As, is calculated as the area of spheres with the volume of the core particles and smooth coating shells.
2.3.2 Collision diameter of core agglomerates with smooth or rough shells
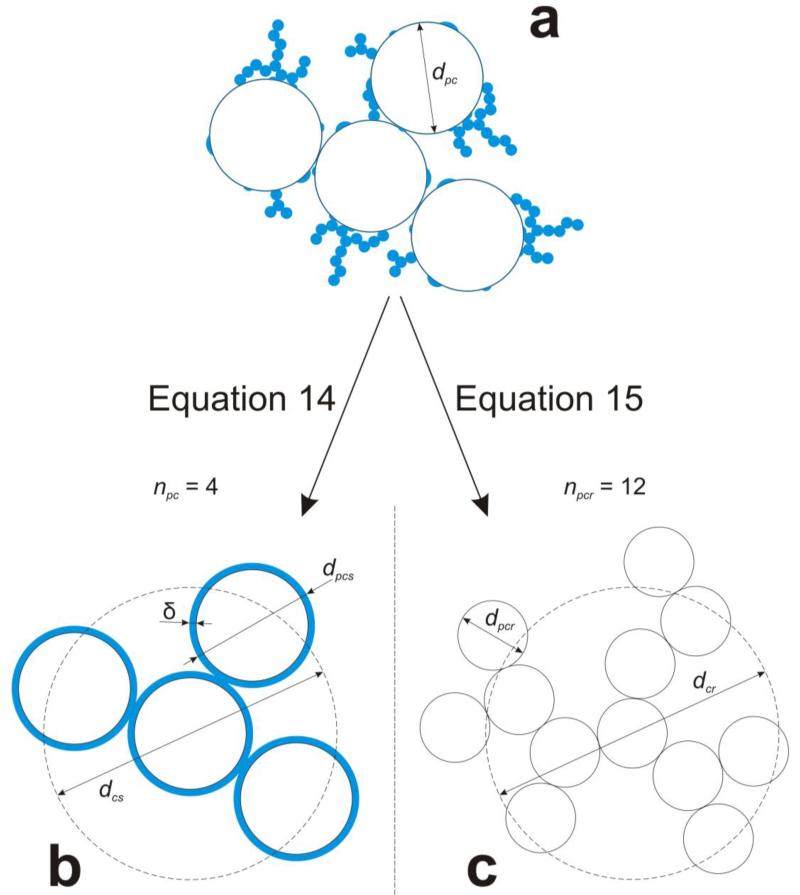
The coating particles (section 2.2) form fractal-like structures upon deposition on core agglomerates as shown exemplarily in Figure 2a. There the core agglomerate consists of four core primary particles with diameter dpc each having spherical smooth and fractal-like rough coating shells. The collision diameter of these core agglomerates dc, is calculated at two extreme limiting cases: a) an evenly distributed, spherical coating shell on the core particle (Fig. 2b) or b) a fractal-like particle composed of monodisperse primary particles with equal area and volume.
Figure 2.
Schematic of two limiting cases for calculation of the collision diameter of a fractal-like coated core agglomerate (a) having original core primary particle diameter dpc: b) dcs when accounting for the deposited coating as evenly distributed smooth shells with total thickness δ. So, its new primary particle diameter is dpcs = dpc + 2δ. c) dcr when describing the core agglomerate and fractal-like coating as one agglomerate with equal surface area and volume consisting of npcr equivalent monodisperse primary particles of diameter dpcr.
-
Assuming that smooth and rough shells are evenly and smoothly distributed over the core primary particles, one can calculate by mass balance the enlarged primary particle diameter as the diameter of a sphere with the volume of the initial core primary particle plus its total coating volume of thickness δ (Fig. 2b):
(14a) The number of core primary particles per core agglomerate, npc, is calculated by dividing the constant initial number of core primary particles, Nc0npc0, by the number of core agglomerates, Nc, accounting for the change in gas volume by cooling:
The smooth shell coated core agglomerate collision diameter, dcs, is (eq. 9):(14b) (14c) -
The fractal-like coated core agglomerate (Fig. 2a) consists of monodisperse primary particles with the same surface area and solid volume as the constituent core particles and coating shells (Fig. 2c). Core primary particles encapsulated with a smooth coating shell are accounted as in eq. 14a for as primary particles with diameter dpcδ, as the diameter of a sphere with the volume of the initial core primary particle plus the smooth coating volume per core primary particle:
(15a) The monodisperse primary particle diameter, dpcr, of that agglomerate (Fig. 2c) is:
where the brackets of the nominator represent the volume of core primary particles plus smooth and rough coating shells. The denominator is the sum of the surface area of the core primary particles encapsulated with the smooth coating shell plus the area of the fractal-like rough coating surface area. The dpcr could be smaller than the core primary particle diameter, dpc (constant), as it accounts for the fractal-like rough coating shell consisting of coating primary particles much smaller than dpc.(15b)
The number of monodisperse primary particles, npcr, is obtained by dividing the volume of core primary particles, the smooth and rough coating shells by the volume of the equivalent monodisperse primary particles dpcr:
| (15c) |
So the fractal-like shell coated core agglomerate collision diameter is:
| (15d) |
Here, calculations are carried out for both dcs and dcr to bracket the limits of aerosol coating morphology and dynamics.
2.4 Coating shell characteristics
The total coating thickness, δ, is defined as the thickness of a perfectly smooth and dense coating shell with volume concentration Vs + Vr:
| (16) |
Similarly the thickness of the smooth coating shell, δs, is:
| (17) |
The texture of the coating shell is characterized by the fraction of smooth coating volume concentration of the total coating shell volume concentration, Fsc:
| (18) |
The coating efficiency, ε, is the fraction of coating precursor vapor that has formed coating shells on core particles:
| (19) |
2.5 Simulation conditions
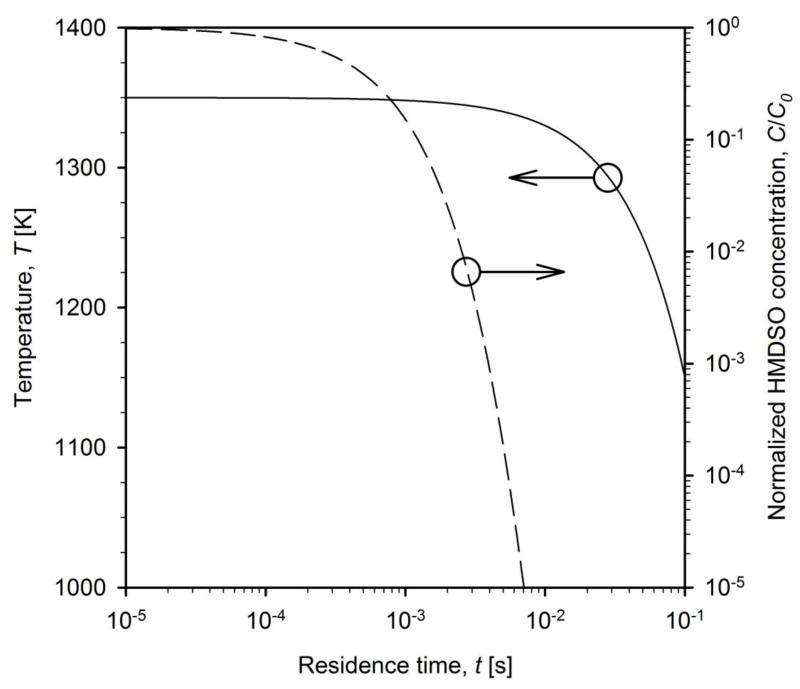
The initial temperature of the mixture of core TiO2 aerosol and HMDSO vapor is T = 1350 K (Figure 3, solid line). The temperature decreases with cooling rate CR = 2×103 K/s to account for heat losses of the coating reactor (Teleki et al., 2009a). Then the oxidation of HMDSO is completed within t = 3×10−3 s (Fig. 3).
Figure 3.
The evolution of temperature (solid line) and normalized HMDSO concentration (dashed line) as function of residence time inside the coating reactor. The initial temperature T = 1350 K decreases with cooling rate CR = 2×103 K/s (Teleki et al. 2009a).
The initial composition of the gas flow is determined by combining the TiO2 core aerosol, for complete conversion of its precursor, and the HMDSO-laden flow for the standard experiments of Teleki et al. (2008). That way the initial composition of the gas flow is 32.5 l/min O2, 11 l/min CO2 and 9.7 l/min H2O and 15.8 l/min HMDSO-laden N2 at T = 293 K. This corresponds to a residence time of t = 0.1 s in a tubular reactor with diameter 4.5 cm and a length of 30 cm (Teleki et al., 2009a).
The TiO2 core particle production rate is always 23.5 g/h and results in an initial single core primary particle (npc0 = 1) concentration of Nc0 = 4.23×1016 #/m3 at T = 293 K for a primary particle size of dpc = 40 nm and density ρTiO2 = 4000 kg/m3. The influence of core particle size at constant production rate is discussed also for dpc = 100 nm and 400 nm corresponding to initial number concentrations of Nc0 = 2.71×1015 and 4.23×1013 #/m3 at T = 293 K, respectively.
The amount of coating is defined by its weight fraction, WF, in the total mass of product (coating and core) particles. With density ρSiO2 = 2200 kg/m3, WF = 20 wt% SiO2 corresponds to an initial number concentration of coating precursor vapor of C0 = 6.63×1021#/m3 at T = 293 K (HMDSO, nm = 2).
3. Results and discussion
3.1 Coating particle dynamics
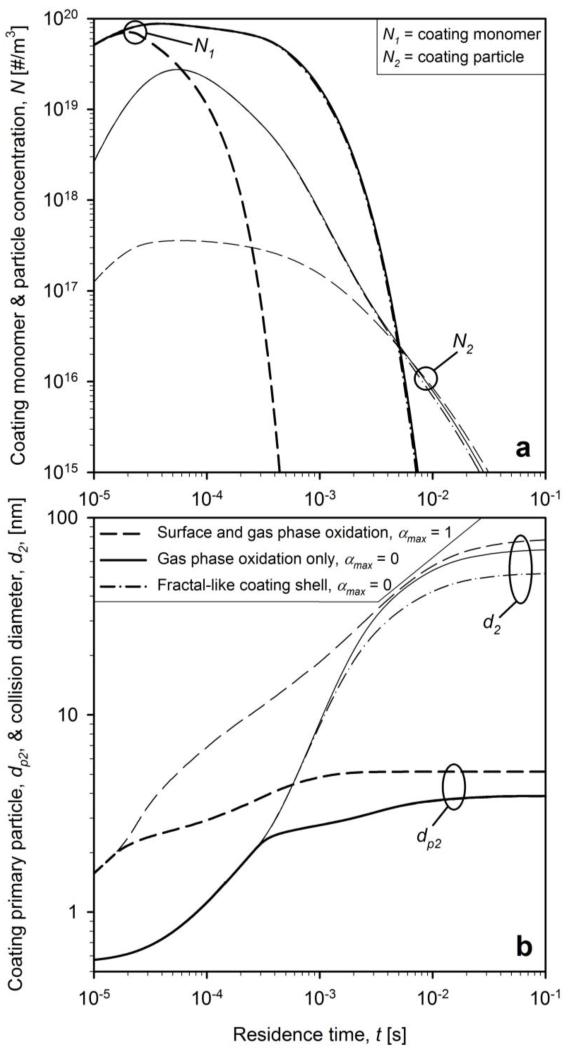
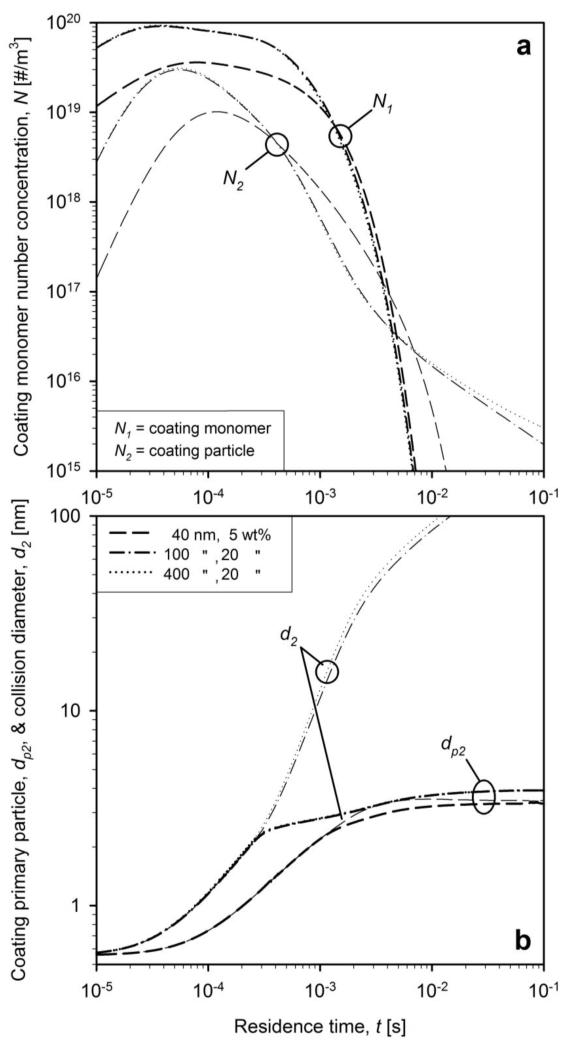
Figure 4 shows the evolution of coating aerosol: a) monomer (N1, bold lines) and particle (N2, thin lines) number concentrations and b) primary particle (dp2, bold lines) and collision (d2, thin lines) diameters for the standard conditions of Teleki et al. (2008) dpc = 40 nm and WF = 20 wt%. Results are shown for neglecting (solid and dash-dot lines, αmax = 0) and accounting for surface oxidation (dashed lines, αmax = 1). The coating thickness was accounted for assuming an evenly distributed smooth shell (eq. 14, solid and dashed lines) and compared with the fractal-like rough shell (eq. 15, dash-dot lines).
Figure 4.
The evolution of a) number concentration of coating monomers (bold lines) and particles (thin lines) and b) primary particle (bold lines) and collision (thin lines) diameter of coating particles for dpc = 40 nm and WF = 20 wt% for neglecting (solid and dash-dot lines) and accounting for HMDSO surface oxidation (dashed lines). Collision diameters are calculated with assuming smooth shells (solid and dashed lines) and fractal-like rough shells (dash-dot lines).
When neglecting HMDSO surface oxidation, the monomer concentration decreases slowly until about 0.001 s (Fig. 4a, bold solid line) as monomer generation and loss by coagulation nearly balance each other. Later on (after t = 0.003 s), N1 decreases quite fast as monomer generation ceases since HMDSO is fully oxidized (Fig. 3, dashed line) and coating monomers grow to particles (N2) or form smooth shells on the core particles. The N2 decreases also steadily (Fig. 4a, thin solid line) but slower than N1 by coagulation with coating particles or core particles to form rough shells.
Accounting for surface oxidation (dashed lines) leads to a faster reduction of N1 as the gas phase monomer generation competes with oxidation on the surface of core and coating particles (Pratsinis and Spicer, 1998: Fig. 1b). This fast reduction of coating monomer concentration (thick dashed lines) reduces also N2 (thin dashed lines). The fractal-like texture of the coating shell (dash-dotted line) hardly makes any difference in N1 and N2 evolution when surface oxidation is neglected (Fig. 4). It is worth noting, however, that coating particle concentrations converge within about 0.005 s regardless of accounting or not for surface oxidation and the fractal-like structure of the resulting shells on the core particles. Coagulation completely masks all these effects.
Figure 4b shows that when neglecting surface oxidation, the onset of aggregate formation takes place at t = 3×10−4 s and that of agglomeration at t = 0.02 s when dp2 levels off and sintering stops (Tsantilis and Pratsinis, 2004). The coating primary particles grow faster when accounting for surface oxidation (Pratsinis and Spicer, 1998: Fig. 1a) and reach the onsets of aggregate and agglomerate formation an order of magnitude faster. Nevertheless the coating primary particle size at the onset of aggregate formation is not much influenced by surface oxidation as well as the final coating primary and collision diameters. This indicates that a more accurate description of the surface reaction may not change considerably the predicted particle sizes at the employed process conditions (e.g. temperature and concentrations). The final primary particle diameter is 4 nm in coating agglomerates with a collision diameter of 65 nm. Accounting for fractal-like rough shells (eq. 15, dash-dotted line) makes hardly any difference in coating primary particles size and decreases slightly the coating agglomerate size. Apparently accounting for the fractal shells on the core particles leads to smaller coating agglomerates by more effective scavenging of coating particles with core particles, accelerating the increase of coating efficiency as it will be shown next.
3.2 Coating shell characteristics
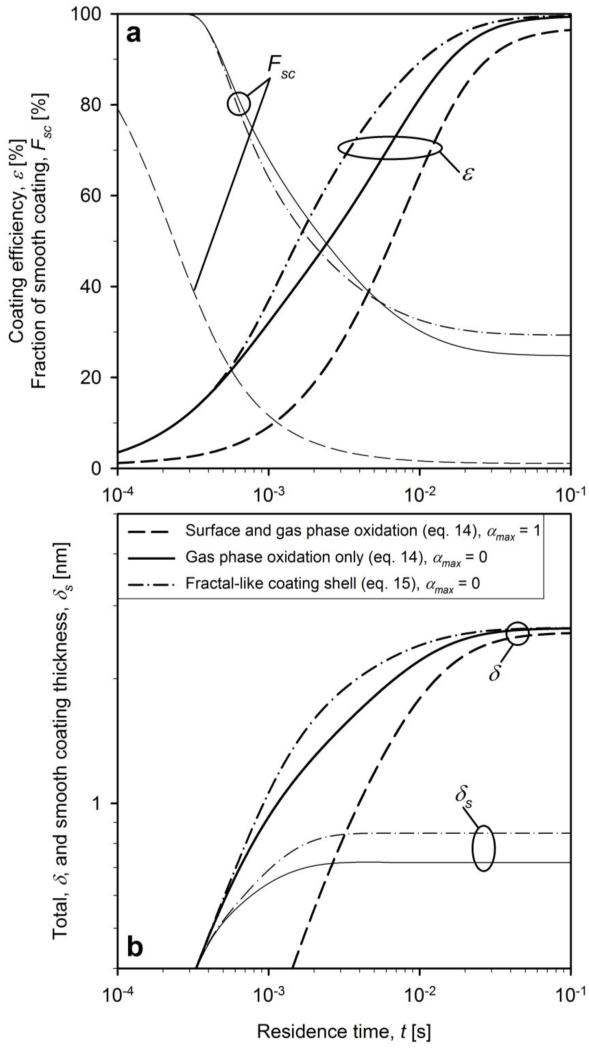
Figure 5 shows the evolution of a) coating efficiency, ε (bold lines), and fraction of smooth coating, Fsc (thin lines), and b) total, δ (bold lines), and smooth, δs (thin lines), coating thickness for the conditions of Figure 4, neglecting (solid and dash-dot lines, αmax = 0) and accounting for surface oxidation (dashed lines, αmax = 1). The coating thickness was accounted for assuming core particles with evenly distributed smooth (eq. 14, solid and dashed lines) or fractal-like rough coating shells (eq. 15, dash-dot lines).
Figure 5.
The evolution of a) coating efficiency (bold lines) and fraction of smooth coating (thin lines) and b) total (bold lines) and smooth (thin lines) coating thickness for dpc = 40 nm and WFSiO2 = 20 wt% for neglecting (solid and dash-dot lines) and accounting for HMDSO surface oxidation (dashed lines). Collision diameters are calculated assuming smooth (solid and dashed lines) or fractal-like rough shells (dash-dot lines).
As coating takes place, coating monomers and particles deposit on the surface of core particles increasing steadily the coating efficiency, ε. Early on, the fraction of smooth coating, Fsc, is 100 % as the shell is formed primarily by coating monomers and small coating particles (clusters) that sinter fast into smooth coating shells. Later on, Fsc decreases as soon as coating particles start to form aggregates (Fig. 4b) at t = 3×10−4 s (solid line). Then, sintering becomes too slow to smooth out the coating shell. Finally, Fsc approaches a constant value as soon as ε approaches 100 % and the coating process is completed. Figure 5b shows that coating thickness increases quite fast. At the onset of (coating) aggregate formation (t = 3×10−4 s, Fig. 4b), δ increases faster than δs in agreement with the decreasing fraction of smooth coating (Fig. 5a). It can be seen that the smooth coating thickness, δs, reaches the final value quite early, at t = 2×10−3 s. This happens because the concentration of coating monomers N1 (which form smooth coating shells) decreases rapidly at that time (Fig. 4a).
Accounting for surface oxidation reduces drastically the fraction of smooth coatings, Fsc, and coating efficiency, ε, which is a counterintuitive result (dashed lines). The total coating thickness increases slower but nearly to the same value as without surface oxidation. The smooth coating thickness is practically zero as only rough coatings are found. What happens? The core TiO2 particles compete for coating precursor molecules with the newly formed SiO2 coating particles. As the latter have much larger surface area than the core particles, large coating particles form resulting in aggregates quite early (t = 2×10−5 s) that stop sintering at t = 10−3 s (Fig. 4b).
Although the detailed reaction kinetics for gas phase and in particular for surface oxidation of HMDSO are unknown and had to be approximated, a clear trend emerges: if HMDSO surface oxidation competes effectively with its gas phase oxidation, there is little chance for synthesis of smooth coatings at the employed process conditions. As rather smooth coating shells were obtained experimentally at these conditions (Teleki et al., 2008), the effect of surface oxidation is neglected in subsequent calculations as the surface reaction rate of HMDSO is really unknown and perhaps rather small at the employed conditions, but not necessarily zero. For example, surface growth of HMDSO is driving growth of SiO2 nanofibers or nanowires in flame deposition of antifogging silica films on glass substrates at low HMDSO concentrations (Tricoli et al., 2009). Similarly, the SiO2 sintering rate employed here (Tsantilis et al., 2001) was compared to that by Xiong et al. (1993). The latter gave much lower coating efficiencies and rougher coating in contrast to experimental data (Teleki et al., 2008). As a result, the sintering rate of Tsantilis et al. was used in all simulations.
Accounting for the fractal-like structure of rough coating shells (dash-dot lines) accelerates the evolution of both ε (Fig. 5a) and δ (Fig. 5b) by higher coagulation rates but reach the same asymptotic values as when neglecting it (solid lines). This can be seen also by the slightly lower coating particle concentration (N2) at higher residence times in Fig. 4a (dash-dot lines). The fraction of smooth coating attains a slightly higher value than the evenly distributed coating shells (solid line). Perhaps, fractal-like structures scavenge more efficiently coating SiO2 monomers up to about t = 0.001 s (Fig. 4a,b).
3.3 Effect of core particle diameter and coating vapor concentration
Figure 6 shows the evolution of a) N1 (bold lines) and N2 (thin lines) and b) dp2 (bold lines) and d2 (thin lines) for lower coating weight fraction, WF = 5 wt% (dashed lines), with dpc = 40 nm and bigger core primary particles dpc = 100 (dash-dot lines) or 400 nm (dotted lines), but with WF = 20 wt% and neglecting surface oxidation, accounting for evenly distributed coating shells (eq. 14). Lowering the WF (dashed lines) leads to generation of less coating monomers that result in lower N1 and N2. At the same WF, increasing the core primary particle size and therefore decreasing the number concentration of core particles hardly affects N1 and N2 that are nearly identical to those of Fig. 4a (solid lines).
Figure 6.
The evolution of a) number concentration of coating monomers (bold lines) and particles (thin lines) and b) primary particle (bold lines) and collision (thin lines) diameter of coating particles for dpc = 40 nm and WF = 5 wt% (dashed lines), as well as WF = 20 wt% and dpc = 100 (dash-dot lines) and 400 nm (dotted lines).
Figure 6b shows that less coating material, WF = 5 wt%, leads to slower coating particle growth, with slightly smaller primary particles but amazingly little aggregate and agglomerate formation. This is caused by slower coagulation rates arising from lower particle concentrations (Fig. 6a). At higher WF = 20 wt%, increasing the core primary particle size (dpc) leads to coating agglomerates with slightly higher collision diameter, but identical primary particles size. Larger core primary particles offer less area for coating monomer and particle deposition. As a result, more coating material remains in the gas phase to coagulate into larger coating agglomerates.
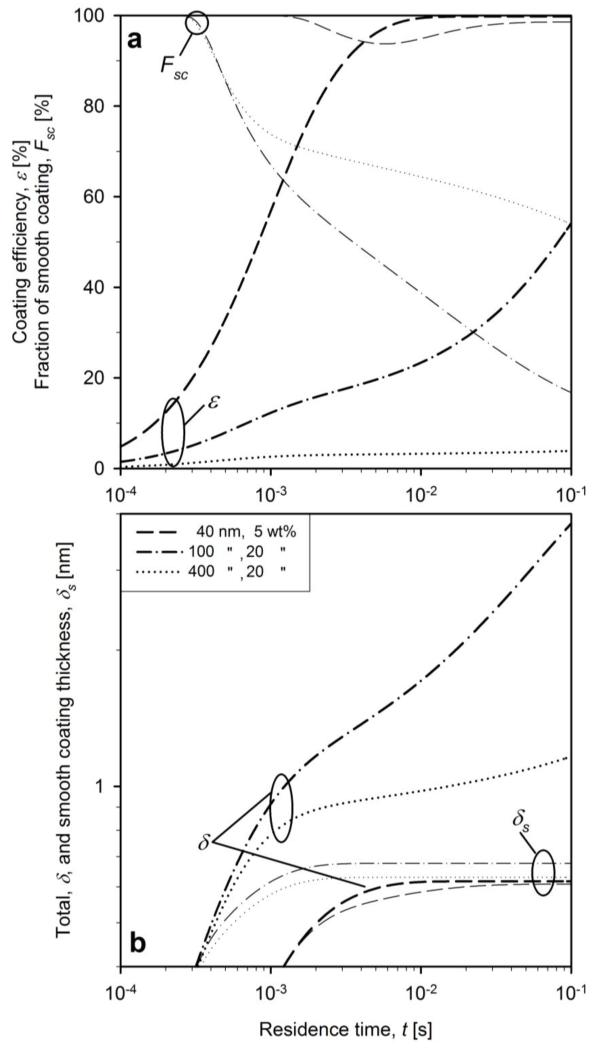
Figure 7 shows the evolution of coating characteristics for WF = 5 wt% for dpc = 40 nm (dashed lines) and WF = 20 wt% for dpc = 100 (dash-dot lines) and 400 nm (dotted lines) neglecting surface oxidation, all accounting for evenly distributed coating shells (eq. 14). Decreasing the WF to 5 wt% for dpc = 40 nm accelerates the attainment of maximum of coating efficiency (Fig. 7a). Increasing the core particle size to 100 and 400 nm reduces the coating efficiency below that of 40 nm core primary particles (Fig. 7a) by the lower core particle number and area concentrations for deposition. The fraction of smooth coating decreases as soon as coating particles start to form aggregates at t = 3×10−4 s for dpc = 100 and 400 nm (Fig. 7). For WF = 5 wt% only a small amount of intermediate rough coating shells is formed decreasing the fraction of smooth coating to 95 % between t = 10−3 - 10−2 s in agreement with the short duration of aggregate formation (Fig 6b).
Figure 7.
The evolution of a) the coating efficiency (bold lines) and fraction of smooth coating (thin lines) and b) the evolution of total (bold lines) and smooth (thin lines) coating thickness for dpc = 40 nm and WF = 5 wt% (dashed lines), as well as WF = 20 wt% and dpc = 100 (dash-dot lines) and 400 nm (dotted lines).
Figure 7b shows that for WF = 5 wt% the total and smooth coating thickness are identical at about 0.6 nm, corresponding to a coating monolayer and consistent with the limited formation of aggregates (Fig. 6b). At constant core particle production rate, increasing the core particle diameter slightly increases the smooth coating thickness, but most of the coating texture is quite rough with low coating efficiency as most coating material has formed SiO2 particles rather than shells onto the core TiO2 particles. It is worth noting, however, that at WF = 20 wt% for dpc = 400 nm the Fsc is higher (Fig. 7a, dotted line) than that for dpc = 100 nm (Fig. 7a, das-dotted line) and even higher than that for 40 nm (Fig. 5a, solid line) at t = 0.1 s. This paradox is attributed to formation of large coating particles that hardly reach the core particles, resulting in a really low coating efficiency (Fig. 7a, dotted line). As a result, one may obtain a higher fraction of smooth shells but at reduced and probably unacceptable process yield (coating efficiency, ε).
3.4 Coating diagrams and comparison with experimental data
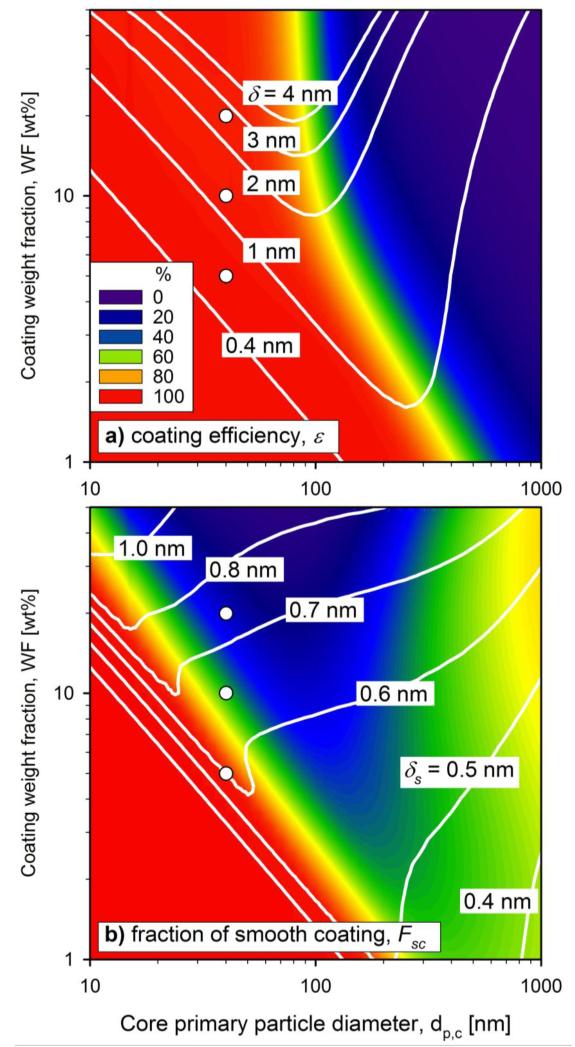
Figure 8 shows a) coating efficiency and b) fraction of smooth coating in the parameter space of core primary particle diameter, dpc, and coating weight fraction, WF, at constant core particle production rate and coating reactor residence time t = 0.1 s. Following the analysis above, only evenly distributed coating shells (eq. 14) have been considered and surface oxidation of HMDSO has been neglected as these assumptions do not alter the product characteristics considerably. The white contours represent the total, δ (Fig. 8a), and smooth coating thickness, δs (Fig. 8b). The WF ranges from 1 to 50 wt% corresponding to a mole Si/Ti = 0.013 - 0.66 that experimentally seems to give good coating characteristics (Akhtar et al., 1992; Hung and Katz, 1992; Powell et al., 1997a,b; Teleki et al., 2005, 2008, 2009b).
Figure 8.
The influence of core primary particle diameter and coating weight fraction on a) coating efficiency and b) fraction of smooth coating at the outlet of the coating unit (t = 0.1 s). The white contour lines correspond to a) total and b) smooth coating thickness. The white circles correspond to the data of Teleki et al. (2008a).
Increasing the core particle diameter dpc at constant core particle production rate does not affect coating efficiency (Fig. 8a) up to dpc = 100 nm. At these conditions, coagulation of coating particles is fast enough to let all coating material deposit onto the core particles, resulting in perfect coating efficiencies (ε = 100 %, red). Larger dpc, however, lower the ε (blue) because the coagulation rate between core and coating particles is reduced by the low concentration of core particles. The coating weight fraction, WF, weakly influences ε in the investigated range (Fig. 8a). This weak influence can be explained by the corresponding small variation in coating precursor vapor concentration compared to the variation in Nc by changing dpc. Increasing dpc by a factor of 10 reduces Nc0 by 1000 at constant core particle production rate and therefore lowers the core particle surface area concentration by a factor of 10. For low WF the range for dpc to achieve high coating efficiencies is slightly increased.
Increasing WF leads to proportionally increasing total coating thickness (white contours), depending on coating efficiency. This is consistent with Boies et al. (2009) who found that the coating precursor flow rate and nitrogen purge gas dilution influenced the coating thickness the most in their reactor. Up to dpc = 100 nm, the total coating thickness increases with dpc as the surface area concentration of core particles is decreasing with increasing dpc and ε = 100 % (red). Above 100 nm, δ decreases with the decreasing coating efficiency, ε.
The fraction of smooth coating, Fsc, reaches up to 100 % (red) for small dpc and low WF (Fig. 8b) where most of the shell is deposited by the coating monomers and small clusters at the high coagulation rates. The apparent minimum of δ and Fsc (blue) is attributed to the formation of aggregates and the decreasing coagulation rates of core particles with coating particles as discussed in Figure 7. Higher WF lead to more coating particles while larger dpc decrease the coagulation rate of coating with core particles, both promoting the formation of coating aggregates that finally lead to rough shells (Fig. 4b and 5a). For the largest dpc barely any shells are deposited by coagulation with coating particles within the reactor residence time while coagulation with coating monomers early in the process still leads to thin smooth shells. The thickness of these smooth shells shows a weaker dependence on dpc than the total thickness. This faster decrease of the deposited amount of rough shells than that of smooth shells leads to an increase of Fsc after the minimum for increasing dpc. In Figure 8a it can be seen that these smoother shells at the largest dpc come, however, at fairly low coating efficiencies, which means low and perhaps unacceptable process yields.
The circles in Figure 8 correspond to the experimental conditions of Teleki et al. (2008) where spherical TiO2 core particles with a diameter of 40 nm were coated with WF = 5, 10 and 20 wt% of SiO2 and high ε were reported (few separate SiO2 particles in the product), which is consistent with the above diagrams. The total and smooth coating thickness are increasing and the fraction of smooth coating shells is decreasing for increasing WF consistent with Teleki et al. (2008). More specifically, for WF = 20 wt% the simulation results in total and smooth coating thicknesses between 2 to 3 nm and 0.7 to 0.8 nm, respectively and coating shells with Fsc = 30 %. This is in agreement with Teleki et al. (2008) who reported a total coating thickness of 2 nm with rather rough surface texture at these conditions. This dependency of Fsc on WF is consistent also with Hung and Katz (1992), who reported that increasing the precursor concentration ratio (increasing Si/Ti or WF) led to less uniform coating thickness distribution on the same core particle. For low concentrations of SiO2 precursor vapor, they observed discrete spots of SiO2 on TiO2 core particles. The diagram is also consistent with Powell et al. (1997a,b) who found that increasing the coating precursor vapor concentration led to rougher coatings.
The lower WF = 5 wt % leads to thinner coating shells with a thickness a little above 0.4 nm corresponding to a monolayer of coating monomers, too thin to be detected by microscopy and without any rough coatings! The presence of such a thin SiO2 shell is consistent again with Teleki et al. (2008) who observed 50% reduction of photo-oxidation of isopropanol to acetone and attributed this to partially-coated particles. The thickness and completeness of these totally smooth coating shells could be increased by multiple injection of precursor vapor of low WF in series building up coating shells layer-by-layer along the aerosol reactor similar to atomic layer deposition processes (King et al., 2008).
4. Conclusions
A trimodal model for high temperature aerosol coating was developed accounting for gas phase oxidation, coagulation, sintering and surface growth. Coating of TiO2 aerosol nanoparticles with nanothin SiO2 shells by hexamethyldisiloxane (HMDSO) vapor oxidation was investigated predicting the coating thickness, texture and efficiency in terms of design diagrams that were consistent with pertinent experimental data.
Smooth coatings were formed primarily by deposition of freshly-formed SiO2 monomers and sintering of small SiO2 clusters and to a much lesser extent by surface oxidation of HMDSO on the TiO2 core particles. Accounting for surface oxidation by a two-step model in the absence of an overall reaction rate, resulted in rough coating shells at the employed process conditions. This was in contrast to pertinent experimental data indicating the minimal significance of surface reaction here. Accounting also for the structure of shells (rough fractal-like or smooth) had a little influence on the design calculations at the present process conditions.
The concentrations of core aerosol and coating precursor vapor (here HMDSO) had the strongest influence on coating efficiency and shell texture in agreement with the literature. Bigger core particles at constant production rate lead to lower coating efficiency. Increasing the coating weight fraction hardly influences the coating efficiency and leads to proportionally higher total coating thickness but also to rougher coating shells. Low concentrations of coating precursor vapor and high concentrations of core particles lead to high fractions of smooth shells and high coating efficiency. The thickness of these smooth shells could be increased by multiple injections of coating precursor vapor at low concentrations in series, building up smooth coating shells layer-by-layer.
Acknowledgments
Financial support from Swiss National Science Foundation (SNF) grant # 200021-119946/1 and European Research Council is gratefully acknowledged.
Appendix
Sintering reduces the rough coating shell surface area, Ar, and volume, Vr, concentration. For a perfectly smooth and fully coalesced coating no rough coating exists and therefore Ar and Vr, converge to zero. As a result the entire rough coating film area concentration is the driving power for sintering, while the kinetics are given by the characteristic sintering time (Tsantilis et al., 2001), τ(dpr):
| (A1) |
As rough coating shells become smooth by sintering, its volume concentration decreases as follows:
| (A2) |
The gain in smooth coating volume is given by the overall volume balance:
| (A3) |
6. Nomenclature
| Ar | surface area concentration of rough coating shell | [m2 m−3] |
| As | surface area concentration of smooth coating shell | [m2 m−3] |
| A2 | surface area concentration of coating particles | [m2 m−3] |
| a1 | surface area of coating monomer | [m2] |
| a2 | surface area of coating particle | [m2] |
| a2f | surface area of fully-coalesced (spherical) coating particle | [m2] |
| C | coating precursor vapor concentration | [# m−3] |
| C0 | initial coating precursor vapor concentration | [# m−3] |
| CR | cooling rate | [K s−1] |
| Df | fractal dimension | [-] |
| d2 | collision diameter of coating particle | [m] |
| dcs | collision diameter of core particle (smooth) | [m] |
| dcr | collision diameter of core particle (fractal-like) | [m] |
| dpc | primary particle diameter of core particle | [m] |
| dpcr | equivalent primary particle diameter of core agglomerate coated with rough shells | [m] |
| dpcs | diameter of core primary particle (smooth) | [m] |
| dpcδ | diameter of core primary particle with smooth coating shell (fractal-like) |
[m] |
| dp,min | minimum sintering primary particle diameter | [m] |
| dpr | primary particle diameter of rough coating shell | [m] |
| dp2 | primary particle diameter of coating particle | [m] |
| Fsc | fraction of smooth coating shells | [%] |
| k | overall oxidation rate constant | [s−1] |
| kg | gas phase oxidation rate constant | [s−1] |
| ks | overall surface oxidation rate constant | [s−1] |
| N1 | number concentration of coating monomers | [# m−3] |
| N2 | number concentration of coating particles | [# m−3] |
| Nc | number concentration of core particles | [# m−3] |
| Nc0 | initial number concentration of core particles | [# m−3] |
| nm | number of monomers per precursor molecule | [-] |
| np2 | number of primary particles per coating particle | [-] |
| npc | number of core primary particles per core particle | [-] |
| npc0 | initial number of core primary particles per core particle | [-] |
| npcr | number of equivalent primary particles per core agglomerate coated with rough shells | [-] |
| Q | gas flow rate | [m3 s−1] |
| Q0 | initial gas flow rate | [m3 s−1] |
| r | volume ratio, r = v2/v1 | [-] |
| T | temperature | [K] |
| t | residence time | [s] |
| Vr | volume concentration of rough coating shells | [m3 m−3] |
| Vs | volume concentration of smooth coating shells | [m3 m−3] |
| V2 | volume concentration of coating particles | [m3 m−3] |
| v1 | volume of coating monomer | [m3] |
| v2 | volume of coating particle | [m3] |
| WF | weight fraction of coating material | [%] |
6.1 Greek Letters
| α | fraction of collisions leading to surface oxidation | [-] |
| β | collision frequency | [m3 s−1] |
| δ | total coating thickness | [m] |
| δs | smooth coating thickness | [m] |
| ε | coating efficiency | [%] |
| ρSiO2 | density of SiO2 | [kg m−3] |
| ρTiO2 | density of TiO2 | [kg m−3] |
| τ | characteristic sintering time | [s] |
References
- Akhtar MK, Pratsinis SE, Mastrangelo SVR. Dopants in Vapor-Phase Synthesis of Titania Powders. J. Am. Ceram. Soc. 1992;75:3408–3416. [Google Scholar]
- Boies AM, Roberts JT, Girshick SL, Zhang B, Nakamura T, Mochizuki A. SiO2 coating of silver nanoparticles by photoinduced chemical vapor deposition. Nanotechnology. 2009;20:295604. doi: 10.1088/0957-4484/20/29/295604. [DOI] [PubMed] [Google Scholar]
- Clark HB, Long JS. Titanium dioxide pigments. In: Myers PR, editor. Treatise on Coatings, 3, Pigments Part I. Marcel Dekker; New York: 1975. pp. 479–532. [Google Scholar]
- Egerton TA. The Modification of Fine Powders by Inorganic Coatings. KONA Powder and Particles. 1998:16. [Google Scholar]
- Ehrman SH, Friedlander SK, Zachariah MR. Characteristics of SiO2/TiO2 nanocomposite particles formed in a premixed flat flame. J. Aerosol Sci. 1998;29:687–706. [Google Scholar]
- Fisher J, Egerton TA. Titanium compounds, Inorganic, Kirk-Othmer Encyclopedia of Chemical Technology (Electronic Edition) John Wiley & Sons, Inc. 2001 [Google Scholar]
- Fotou GP, Pratsinis SE, Baron PA. Coating of silica fibers by ultrafine particles in a flame reactor. Chem. Eng. Sci. 1994;49:1651–1662. [Google Scholar]
- Friedlander SK. second ed Oxford University Press; New York: 2000. Smoke, Dust and Haze: Fundamentals of Aerosol Dynamics. [Google Scholar]
- Friedlander SK, Wu MK. Linear rate law for the decay of the excess surface area of a coalescing solid particle. Physical Review B. 1994;49:3622. doi: 10.1103/physrevb.49.3622. [DOI] [PubMed] [Google Scholar]
- Heine MC, Pratsinis SE. High concentration agglomerate dynamics at high temperatures. Langmuir. 200622:10238–10245. doi: 10.1021/la062022q. [DOI] [PubMed] [Google Scholar]
- Hung CH, Katz JL. Formation of mixed-oxide powders in flames .1. TiO2-SiO2. J. Mater. Res. 1992;7:1861–1869. [Google Scholar]
- Jain S, Fotou GP, Kodas TT. A theoretical study on gas-phase coating of aerosol particles. J. Colloid Interface Sci. 1997;185:26–38. doi: 10.1006/jcis.1996.4558. [DOI] [PubMed] [Google Scholar]
- Jeong JI, Choi M. A simple bimodal model for the evolution of non-spherical particles undergoing nucleation, coagulation and coalescence. J. Aerosol Sci. 2003;34:965–976. [Google Scholar]
- Jeong JI, Choi M. A bimodal particle dynamics model considering coagulation, coalescence and surface growth, and its application to the growth of titania aggregates. J. Colloid Interface Sci. 2005;281:351–359. doi: 10.1016/j.jcis.2004.08.096. [DOI] [PubMed] [Google Scholar]
- King DM, Liang X, Burton BB, Akhtar MK, Weimer AW. Passivation of pigment-grade TiO2 particles by nanothick atomic layer deposited SiO2 films. Nanotechnology. 2008;19:255604. doi: 10.1088/0957-4484/19/25/255604. [DOI] [PubMed] [Google Scholar]
- Koch W, Friedlander SK. The effect of particle coalescence on the surface area of a coagulating aerosol. J. Colloid Interface Sci. 1990;140:419–427. [Google Scholar]
- Kruis FE, Kusters KA, Pratsinis SE, Scarlett B. A simple-model for the evolution of the characteristics of aggregate particles undergoing coagulation and sintering. Aerosol Science and Technology. 1993;19:514–526. [Google Scholar]
- Mezey EJ. Jr. Wiley; New York: 1966. Pigments and Reinforcing Agents. [Google Scholar]
- Powell QH, Fotou GP, Kodas TT, Anderson BM. Synthesis of alumina- and alumina/silica-coated titania particles in an aerosol flow reactor. Chem. Mater. 1997a;9:685–693. [Google Scholar]
- Powell QH, Fotou GP, Kodas TT, Anderson BM, Guo YX. Gas-phase coating of TiO2 with SiO2 in a continuous flow hot-wall aerosol reactor. J. Mater. Res. 1997b;12:552–559. [Google Scholar]
- Pratsinis SE, Mastrangelo SVR. Material Synthesis in Aerosol Reactors. Chem. Eng. Prog. 1989;85:62–66. [Google Scholar]
- Pratsinis SE, Spicer PT. Competition between gas phase and surface oxidation of TiCl4 during synthesis of TiO2 particles. Chem. Eng. Sci. 1998;53:1861–1868. [Google Scholar]
- Schaefer DW, Hurd AJ. Growth and Structure of Combustion Aerosols: Fumed Silica. Aerosol Sci. Technol. 1990;12:876–890. [Google Scholar]
- Sheen S, Yang S, Jun K, Choi M. One-step flame method for the synthesis of coated composite nanoparticles. Journal of Nanoparticle Research. 2009;11:1767–1775. [Google Scholar]
- Subramanian NS, Diemer RB, Gai PL. E. I. du Pont de Nemours and Company. Wilmington, DE, US: 2006. Process for making durable rutile titanium dioxide pigment by vapor phase deposition of surface treatment. U.S. Patent 200627303(A1) [Google Scholar]
- Teleki A, Buesser B, Heine MC, Krumeich F, Akhtar MK, Pratsinis SE. Role of Gas-Aerosol Mixing during in Situ Coating of Flame-Made Titania Particles. Industrial & Engineering Chemistry Research. 2009a;48:85–92. [Google Scholar]
- Teleki A, Heine MC, Krumeich F, Akhtar MK, Pratsinis SE. In-situ coating of flame-made TiO2 particles by nanothin SiO2 films. Langmuir. 2008;24:12553–12558. doi: 10.1021/la801630z. [DOI] [PubMed] [Google Scholar]
- Teleki A, Pratsinis SE, Wegner K, Jossen R, Krumeich F. Flame-coating of titania particles with silica. J. Mater. Res. 2005;20:1336–1347. [Google Scholar]
- Teleki A, Suter M, Kidambi PR, Ergeneman O, Krumeich F, Nelson BJ, Pratsinis SE. Hermetically Coated Superparamagnetic Fe2O3 Particles with SiO2 Nanofilms. Chemistry of Materials. 2009b;21:2094–2100. [Google Scholar]
- Tricoli A, Righettoni M, Pratsinis SE. Anti-Fogging Nanofibrous SiO2 and Nanostructured SiO2-TiO2 Films Made by Rapid Flame Deposition and In Situ Annealing. Langmuir. 2009;25:12578–12584. doi: 10.1021/la901759p. [DOI] [PubMed] [Google Scholar]
- Tsantilis S, Briesen H, Pratsinis SE. Sintering time for silica particle growth. Aerosol Sci. Technol. 2001;34:237–246. [Google Scholar]
- Tsantilis S, Kammler HK, Pratsinis SE. Population balance modeling of flame synthesis of titania nanoparticles. Chemical Engineering Science. 2002;57:2139–2156. [Google Scholar]
- Tsantilis S, Pratsinis SE. Soft- and hard-agglomerate aerosols made at high temperatures. Langmuir. 2004;20:5933–5939. doi: 10.1021/la036389w. [DOI] [PubMed] [Google Scholar]
- Ulrich GD. Theory of Particle Formation and Growth in Oxide Synthesis Flames. Combust. Sci. Technol. 1971;4:47–57. [Google Scholar]
- Wegner K, Stark WJ, Pratsinis SE. Flame-nozzle synthesis of nanoparticles with closely controlled size, morphology and crystallinity. Mater. Lett. 2002;55:318–321. [Google Scholar]
- Xiong Y, Akhtar MK, Pratsinis SE. Formation of agglomerate particles by coagulation and sintering--Part II. The evolution of the morphology of aerosol-made titania, silica and silica-doped titania powders. J. Aerosol Sci. 1993;24:301–313. [Google Scholar]
- Xiong Y, Pratsinis SE. Gas-phase production of particles in reactive turbulent flows. J. Aerosol Sci. 1991;22:637–655. [Google Scholar]