1 Introduction
Approximately 40% of the world’s 6 billion people remain at risk of contracting malaria. Greater than 300 million people, primarily in sub-Saharan Africa, are afflicted by the disease resulting in more than 1 million deaths per year. These staggering numbers are compounded by unbearable social and economic losses, primarily in endemic countries.
It has been over 100 years since the work of Ronald Ross and Giovanni Grassi first demonstrated the role of the mosquito in the transmission of malaria. Since then, a great deal of knowledge has been obtained about the complex life cycle of the malaria parasite (Genus: Plasmodium) as it grows and differentiates in the mosquito vector and human hosts. Although the disease-causing forms of the parasite exist only in human blood stages, the mosquito is the obligatory vector for transmission. Here, we focus on the mosquito stages of the parasite life cycle, from its initial uptake as gametocytes to the infective salivary gland sporozoites that initiate infection of a new vertebrate host. Through this complex developmental progression, the parasite must overcome many roadblocks and barriers to ensure transmission. Whereas much of our knowledge has been obtained with the murine malaria model due to the ease of handling in the laboratory, recent work has begun to emphasize the more relevant P. falciparum human malaria model. Here we review current knowledge of Plasmodium–mosquito interactions and discuss research questions that may lead to the development of disease prevention strategies.
2 The Plasmodium life cycle in the mosquito
2.1 GAMETOGENESIS
Egg production by female mosquitoes requires a blood meal. In principle, the process of feeding and reproduction can be repeated every 3–4 days for the duration of the female mosquito’s lifespan. Plasmodium utilizes this cyclic feeding behaviour for its transmission from one vertebrate host to the next.
The vast majority of the circulating parasites in an infected human are asexually dividing merozoites. These parasites play no role in transmission and die after ingestion by the mosquito. However, a small proportion of the circulating parasites enter a terminal differentiation pathway that culminates with the production of male and female gametocytes. These non-dividing sexual forms are solely responsible for establishing the parasite life cycle in the mosquito vector and ultimately the transmission to a new vertebrate host.
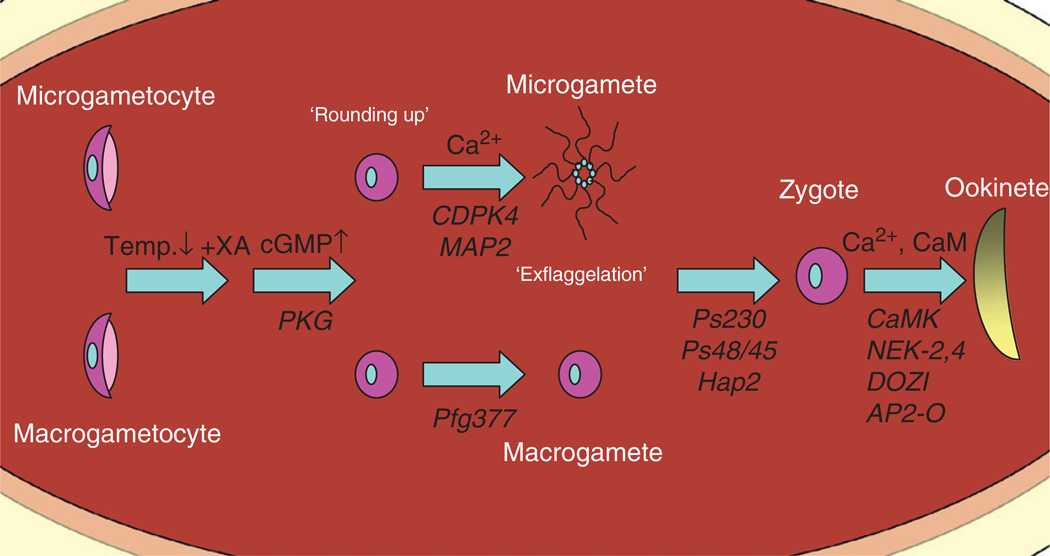
After a mosquito feeds on an infected host, ingested gametocytes undergo rapid differentiation within the mosquito midgut lumen (outlined in Fig. 1). Within minutes, gametocytes egress from their host erythrocytes to initiate gametogenesis (differentiation of gametocytes into gametes). Female gametocytes (macrogametocytes) emerge to produce a single non-motile spherical female gamete, while male gametocytes (microgametocytes) undergo ‘exflagellation’, a process originally described by Laveran, resulting in the production of eight motile male gametes. These initial steps are triggered in part by the drop in temperature from ~37 °C in the vertebrate body to ambient temperature and can be stimulated in vitro through an increase in pH from 7.4 to 8.0–8.2 (Nijhout and Carter, 1978). However, the change in pH that accompanies the mosquito blood meal is minimal and gametogenesis in vivo requires the presence of a mosquito-derived factor originally defined as the gametocyte-activating factor (GAF) (Nijhout, 1979; Sinden et al., 1996). Identified as xanthurenic acid (XA) (Billker et al., 1998; Garcia et al., 1998), an intermediate product involved in tryptophan metabolism, this small molecule was shown to stimulate exflagellation at neutral pH in vitro. As a conserved component of the eye pigmentation pathway of insects, mosquito eye colour mutants may possess reduced concentrations of GAF activity (Billker et al., 1998; Arai et al., 2001). Subsequently, it has been suggested that variation in XA production by different mosquito species may influence the rate of exflagellation and determine the specificity of vector–parasite interactions (Siden-Kiamos and Louis, 2004). An unidentified serum-derived factor may also have GAF activity, suggesting that a component of gamete activation may also be derived from the host blood (Arai et al., 2001).
FIG. 1.
Plasmodium sexual development in the mosquito midgut. Sexual maturation and fertilization are triggered by specific stimuli such as drop in temperature and exposure to xanthurenic acid (XA) when the parasites arrive in the mosquito midgut. A signalling cascade triggered by increased levels of cGMP and Ca2+ induce gametocyte rounding up (in some parasite species) and egress from the erythrocyte in a process known as gametogenesis. Resultant male and female gametes fertilize to form a zygote and subsequently a motile ookinete. Factors known to participate in this developmental progression are displayed above the arrows and the affected genes involved in the transition between each stage below the arrows. Gene identification and function at each stage of gametogenesis are described in the text.
2.1.1 Male gametogenesis
Male gametogenesis occurs in the mosquito midgut, and involves three rapid rounds of DNA replication to generate eight microgametes in a process known as exflagellation (Janse et al., 1986). The mechanisms by which these dramatic changes are accomplished within the span of 10 min are poorly understood. The influx of second messengers such as Ca2+, cGMP, and inositol (1,4,5) triphosphate (IP3) are known requirements to initiate male gametogenesis (Kawamoto et al., 1990, 1993; Martin et al., 1994), presumably through a signalling cascade in response to an external stimulus.
As previously mentioned, XA is essential for the induction of gametogenesis. XA triggers a rapid increase in the concentration of cytosolic calcium through an unknown mechanism (Billker et al., 2004). A calcium-dependent protein kinase, CDPK4, has been shown to translate this Ca2+ signal into the activation of the cell cycle and initiate DNA replication in the male gametocyte (Billker et al., 2004). Elevated levels of guanylyl cyclase and cGMP-dependent protein kinase (PKG) activity have also been implicated in the initiation of gametogenesis by XA (Muhia et al., 2001; McRobert et al., 2008). In fact, the precise timing and regulation of cGMP levels is critical in gametocyte activation and is maintained by the cGMP-phosphodiesterase (PDEd) prior to gametogenesis in P. falciparum (Taylor et al., 2008). Once activated, cGMP and Ca2+ signalling appear to also influence gamete morphology. Evidence suggests that PKG activation triggers gametocyte rounding, while intracellular Ca2+ signalling functions downstream in activating cell cycle progression and exflagellation (McRobert et al., 2008).
2.1.2 Female gametogenesis
The regulation of female gametogenesis is not well understood. Upon entry into the mosquito midgut, macrogametocytes egress from their host red blood cells but undergo only minimal morphological changes. While evidence suggests that the signalling cascades leading to the induction of gametogenesis may be the same as in the male, there appears to be some degree of sex-specificity as demonstrated by the male-specific requirement of the putative mitogen-activated protein (MAP) kinase 2 to produce functional male gametes. Inactivation of the Map-2 gene blocks microgametocyte cytokinesis and gamete formation while female gametogenesis is unaffected (Khan et al., 2005; Tewari et al., 2005a).
Distinct morphological characteristics can distinguish female gametocytes from their male counterparts. An abundance of mitochondria, ribosomes, and Golgi-derived osmiophilic bodies are present within the female gametocyte suggesting a role of these organelles in the production and storage of proteins required for later stages of development (Sinden, 1982). The function of osmiophilic bodies is not well understood, but it was observed that pfg377 gene disruption leads to a dramatic decrease in the occurrence of osmiophilic bodies and macrogametocyte emergence from the erythrocyte (Severini et al., 1999; de Koning-Ward et al., 2008).
2.2 FERTILIZATION AND FUSION OF GAMETES
Upon completion of gametogenesis in the mosquito midgut, the exflagellating motile male gametes form exflagellation centres. This clustering effect between nearby RBCs and an exflagellating male gamete are produced shortly after emergence and are likely caused by the interaction between sialic acid on the erythrocyte surface and the microgametes (Templeton et al., 1998).
Recognition, attachment and fusion (fertilization) of macro- and microgametes leading to the formation of the zygote all occur within 1 h of blood uptake by the mosquito. Several proteins on the extracellular surface of the macro- and microgametes have been implicated in these processes. Among these proteins are Pfs230 and Pfs48/45, members of a highly conserved family containing a characteristic pattern of cysteine residues (Carter et al., 1995; Templeton and Kaslow, 1999). Pfs230 is secreted from the gametocyte and later interacts with the glycosylphosphatidylinositol (GPI)-anchored Pfs48/45 present on the surface of male and female gametes of P. falciparum (Kumar, 1987; Kumar and Wizel, 1992). The presence of Pfs230 on the male gamete surface is necessary for the formation of exflagellation centres (Eksi et al., 2006), while Pfs48/45 is required by the microgamete for the attachment and fertilization of the macrogamete (van Dijk et al., 2001).
Recently, two groups independently determined that HAP2 (or glutamylcysteine synthetase 1 (GCS1)), a highly conserved component of the gamete fusion apparatus shared between Plasmodium, green algae, and plants, is required for fertilization and zygote formation (Hirai et al., 2008; Liu et al., 2008). Exposed on the surface of the male gamete, deletion of HAP2 prevents gamete fusion while the adherence between gametes remains uncompromised (Hirai et al., 2008; Liu et al., 2008). HAP2 immune sera drastically inhibited ookinete differentiation (presumably by inhibition of gamete fertilization), suggesting its application as an anti-malaria transmission-blocking vaccine (Blagborough and Sinden, 2009).
2.3 ZYGOTE TO OOKINETE PROGRESSION
Zygote formation is followed by nuclear fusion, genome replication, and meiosis. During this developmental stage, the nuclear envelope remains intact following meiosis. Nuclear division does not occur, resulting in the formation of a tetraploid zygote nucleus. During this period, members of the NIMA-related protein kinase family behave as important regulators of cell cycle progression and are essential for the differentiation into an ookinete. Expressed in the female macrogamete, parasites lacking nek-4 are able to fertilize, but are unable to initiate DNA replication within the newly formed zygote (Khan et al., 2005; Reininger et al., 2005). A similar phenotype was determined for parasites lacking nek-2, yet evidence suggests that these enzymes perform non-redundant functions during DNA replication in meiosis (Reininger et al., 2009).
Many of the genes required for the differentiation of the zygote into an ookinete are translationally repressed in the female gametocyte by a DEAD-box RNA helicase, DOZI. Loss of DOZI causes the destabilization and subsequent degradation of a discrete subset of female mRNAs, thus impairing further zygote development (Mair et al., 2006). DOZI-dependent translational repression of specific transcripts (including P25, P28, and AP2-O) is released shortly after zygote formation, resulting in the precise temporal regulation of protein production (Mair et al., 2006; Yuda et al., 2009). Through its DOZI-dependent regulation, the transcription factor AP2-O is translated shortly after zygote formation to trigger the induction of additional genes required for ookinete development and midgut invasion (Yuda et al., 2009).
After this switch in development, the spherical zygote transitions into an intermediate retort form and then into the invasive, banana-shaped ookinete. These dramatic morphological changes rely on calcium-dependent signalling pathways similar to those that regulate the onset of gametogenesis. Both intracellular calcium and calmodulin (CaM) are required for the zygote to ookinete transition, presumably through signalling mediated by the CaM-dependent protein kinase (CaMK) (Silva-Neto et al., 2002). However, the molecular basis for the complex set of events leading to the differentiation of the zygote into an ookinete remains largely unknown.
2.4 OOKINETE INVASION OF THE MOSQUITO MIDGUT
2.4.1 The role of the mosquito peritrophic matrix
Distension of the mosquito midgut following a blood meal triggers the secretion by the midgut epithelial cells of an extracellular chitinous layer known as the peritrophic matrix (PM). Initially soft and fragile, the PM gradually thickens and polymerizes as it surrounds the ingested food bolus (Devenport and Jacobs-Lorena, 2005). The PM is composed of proteins, glycoproteins, proteoglycans, and chitin and represents a physical barrier to the ookinete (Billingsley and Rudin, 1992).
Approximately 20 h after ingestion of an infected blood meal, mature ookinetes begin their escape from the food bolus. It is unclear if the movement of the ookinete is random or directed by environmental cues that guide its movements to the PM. Upon contact with the PM, the ookinete secretes a chitinase from its micronemes (apical secretory vesicles) to locally disrupt the chitinous PM, thus facilitating traversal of this physical barrier. Inactivation of the chitinase genes dramatically reduces the ability of the ookinete to traverse the PM (Dessens et al., 2001; Tsai et al., 2001). P. gallinaceum (but not P. falciparum or P. berghei) ookinetes secrete chitinase as a zymogen that is then activated by mosquito midgut proteases (Shahabuddin et al., 1993; Shahabuddin and Kaslow, 1994), demonstrating that the parasite has adapted to the protease-rich environment of the mosquito midgut to facilitate its own development.
2.4.2 Interactions with the mosquito midgut
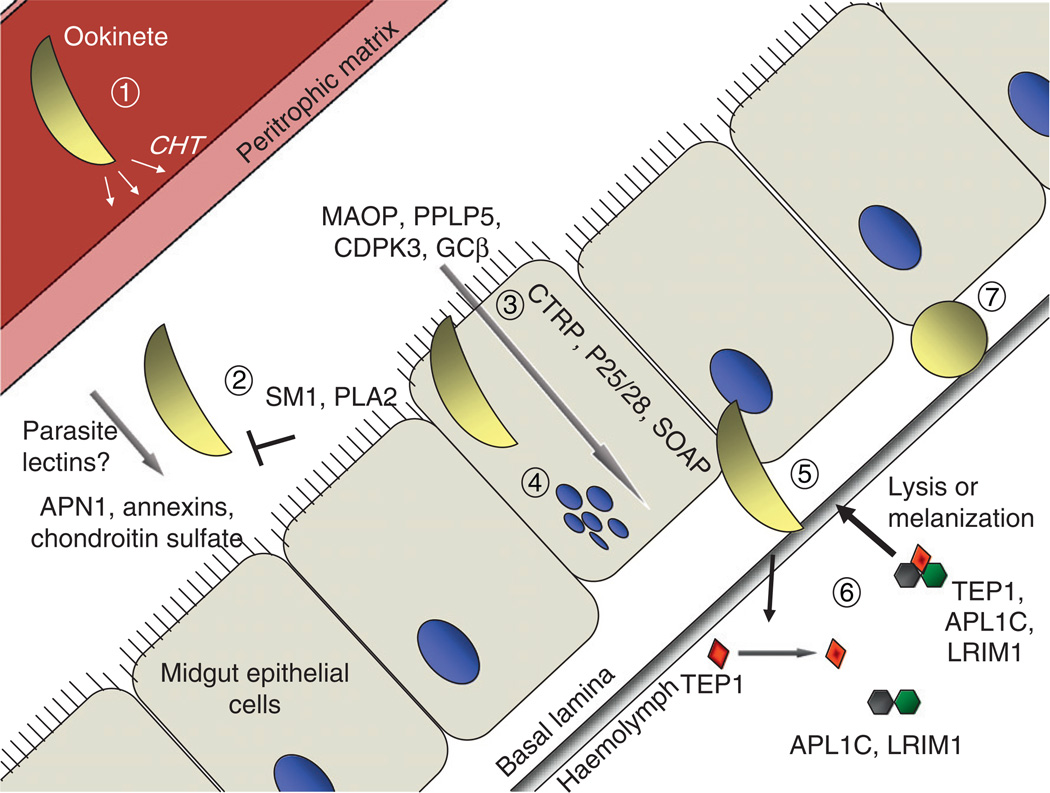
Following traversal of the PM, the ookinete invades the midgut epithelium (outlined in Fig. 2). Based on in vitro observations, it is believed that ookinetes display extensive gliding motility along the lumenal surface of the midgut epithelium that may be important to initiate midgut invasion (Zieler and Dvorak, 2000). The lumenal surface of the midgut is coated with a glycocalyx composed of an intricate mixture of at least 28 glycoproteins displaying complex glycosylation patterns, with which the ookinetes first interact (Shen et al., 1999; Wilkins and Billingsley, 2001; Dinglasan and Jacobs-Lorena, 2005; Dinglasan et al., 2007a).
FIG. 2.
Ookinete invasion of the midgut epithelium. As the ookinete moves to the periphery of the blood bolus, it secretes chitinase (CHT) to penetrate the peritrophic matrix (1). The ookinete then interacts with several components of the mosquito midgut to initiate attachment to the midgut epithelium (2). This interaction can be inhibited by SM1 and PLA2 through an unknown mechanism. Following irreversible attachment, the ookinete penetrates and invades the epithelial cell and then traverses the cytoplasm to egress at the basal end (3). Invasion triggers a series of events that result in cell death and the extrusion into the midgut lumen (4). After emerging from the midgut epithelium, ookinetes interact with the basal lamina (5) where they become exposed to circulating components of the mosquito innate immune response (6). Surviving ookinetes differentiate into oocysts leading to the formation of thousands of sporozoites (7). Further details are described in the text.
Sugar-binding proteins, known as lectins, bind specifically to the lumenal surface of midgut epithelial cells (Rudin and Hecker, 1989), suggesting that carbohydrate moieties may play a role in ookinete binding to the midgut (Ramasamy et al., 1997). This idea was reinforced by a dramatic reduction of ookinete binding in vitro following the removal of carbohydrates present on the midgut lumen upon periodate treatment (Zieler et al., 1999). Certain lectins were also identified that impair ookinete binding, suggesting that ookinetes interact with specific carbohydrate ligands (Rudin and Hecker, 1989; Zieler et al., 2000). Experimental feeding studies demonstrate that an antibody (MG96) that recognizes midgut oligosaccharides completely blocked Plasmodium development (Dinglasan et al., 2003), providing further evidence of an interaction between mosquito sugars and parasite lectins to establish invasion. Chondroitin sulphate proteoglycans displayed on the apical microvilli appear to play an essential role for ookinete midgut invasion. Repression of glycosaminoglycan biosynthesis using RNA interference diminished chondroitin sulphate abundance on the adult midgut surface and substantially inhibited ookinete development (Dinglasan et al., 2007a).
Based upon previous observations that the lectin jacalin significantly inhibits ookinete attachment to the midgut microvilli (Zieler et al., 2000), Dinglasan et al. (2007b) used a jacalin affinity column to determine that a leucine aminopeptidase (APN1) is the major glycoprotein recognized by this lectin. Antibodies against APN1 strongly inhibited the formation of oocysts (presumably interfering with ookinete invasion), identifying a possible mosquito-based transmission-blocking antigen.
In an attempt to identify midgut receptors for ookinete invasion, Ghosh et al. (2001) screened a phage display library for peptides that bind specifically to the lumenal side (the one invaded by the parasite) of the midgut epithelium. Identified as a result of this screen, the salivary gland and midgut peptide 1 (SM1) dodecapeptide strongly inhibits P. berghei ookinete invasion, presumably through competitive binding to a mosquito midgut receptor. These findings led to the creation of a transgenic mosquito that secretes SM1 into the midgut lumen every time it feeds on a blood meal, making it refractory to malaria parasite transmission (Ito et al., 2002).
Other factors mediating ookinete attachment and invasion have also been suggested, including the role of annexins to facilitate ookinete invasion (Kotsyfakis et al., 2005). In contrast, phospholipase A2 (PLA2) interferes with ookinete attachment presumably through association with the midgut lumen (Zieler et al., 2001) and transgenic mosquitoes expressing PLA2 are impaired in Plasmodium transmission (Moreira et al., 2002; Rodrigues et al., 2008). The mechanism of PLA2 inhibition is unknown, but is independent of its enzymatic activity (Zieler et al., 2001).
It appears that the invading ookinete interacts with a wide range of sugar, lipid, and protein moieties on the lumenal surface of the midgut epithelium and its surrounding extracellular matrix. We are just beginning to understand these complex interactions and the characterization of these interacting molecules remain a focus for future research.
2.4.3 Ookinete invasion
After a period of gliding along the midgut epithelium (Zieler and Dvorak, 2000), the ookinete commits towards the process of invasion. Using the P. gallinaceum/Ae. aegypti model, Shahabuddin and Pimenta (1998) suggested that a specific cell type (‘Ross cells’) rich in vesicular ATPase and containing few microvilli may serve as targets for midgut invasion. However, further studies challenged this concept, suggesting that invasion occurs at random and that phenotypic differences between neighbouring cells are the result of ookinete invasion (Han et al., 2000; Zieler and Dvorak, 2000).
After adherence, ookinetes produce a localized invagination that surrounds the ookinete (Kadota et al., 2004). The Plasmodium membrane attack ookinete protein (MAOP) plays an essential role in the process of invasion. Detailed electron microscopy demonstrated that MAOP is required for the rupture of the midgut epithelium on the lumenal surface through an interaction with the apical tip of the ookinete, suggesting that MAOP is involved in producing a poreforming complex necessary for entry into the cytoplasm of midgut epithelial cells (Kadota et al., 2004). MAOP-disruptant ookinetes are incapable of invasion but display a tight and irreversible attachment to the lumenal surface of the midgut epithelium, implying that midgut invasion is preceded by adherence to the midgut surface (Kadota et al., 2004). A perforin-like protein (PPLP5) containing a putative pore-forming MACPF-like domain similar to MAOP exhibits a similar function. PPLP5 disruptant parasites remain localized to the apical surface and do not invade the midgut epithelial cells. This virtually identical phenotype to MAOP-disruptant parasites has led Ecker et al. (2007) to propose that these two proteins (MAOP and PPLP5) may interact to form an ookinete invasion complex.
Ookinete motility is critical for midgut invasion and for its intracellular journey through the cytoplasm. Gliding motility involves an actomyosin motor (Siden-Kiamos et al., 2006b) and several other Plasmodium proteins whose exact role is not yet understood. Knockout of a conserved membraneassociated guanylate cyclase (GCβ) severely impairs ookinete motility and fails to produce oocysts (Hirai et al., 2006; Taylor et al., 2008). This effect can likely be attributed to the failure to produce cGMP, which in turn may activate downstream signalling pathways leading to ookinete motility (Hirai et al., 2006). Another second messenger, calcium, may also be important for ookinete motility. Deletion of a calcium-dependent protein kinase (CDPK3) leads to the inhibition of oocyst formation most likely via inhibition of midgut invasion (Ishino et al., 2006; Siden-Kiamos et al., 2006a). In vitro experiments have shown that upon contact with insect cells, ookinete intracellular calcium concentration is greatly reduced (Siden-Kiamos and Louis, 2008). While this suggests a regulatory role of calcium in ookinete motility, it is unclear how this correlates with modulation of CDPK3 function (Siden-Kiamos and Louis, 2008). The role of second messengers in the expression and/or secretion of proteins from the micronemes that are involved in ookinete motility and invasion remains to be investigated.
During the process of invasion, the ookinete relies on several surface proteins to migrate through the midgut epithelium. One component, CTRP, is localized within the apical secretory organelles called micronemes, suggesting that the protein is secreted during invasion (Limviroj et al., 2002). CTRP knockout parasites are non-motile, and are unable to invade the midgut epithelium (Dessens et al., 1999; Yuda et al., 1999; Templeton et al., 2000). Other proteins present on the ookinete surface and required for midgut traversal include a pair of highly abundant GPI-anchored proteins (P25 and P28) and the secreted ookinete adhesive protein (SOAP). However, their role in invasion is presently unknown (Tomas et al., 2001; Dessens et al., 2003).
2.4.4 The ookinete-induced ‘time bomb’
Ookinete invasion induces dramatic changes of the invaded cell cytoskeleton (Han et al., 2000) including formation of a contractile ring around the basal membrane, resulting in the ‘pinching’ of the cell and its extrusion from the midgut epithelium (Han et al., 2000). As a result, the adjacent epithelial cells then converge to fill the gap left by the extruded cell (Han et al., 2000; Gupta et al., 2005).
Invasion also induces epithelial cell expression of nitric oxide synthase (NOS) (Luckhart et al., 1998) leading to the formation of nitrites and peroxides that in turn trigger apoptosis (Herrera-Ortiz et al., 2004; Kumar et al., 2004). Presumably, these highly unstable and toxic compounds create a cellular environment harmful for the subsistence of the ookinete and present a narrow period of time during which the ookinete must escape the cell to ensure its own survival. A series of physiological changes occur within invaded cells that includes the loss of microvilli, DNA fragmentation and cell death (Han et al., 2000). Evasion from this ‘time bomb’ by the ookinete is thought to occur either by invading a neighbouring naïve cell or by escaping the invaded cell to reach its final extracellular destination between the epithelium and the basal lamina facing the haemocoel. While it remains unclear how ookinete invasion triggers programmed cell death, this is likely a general response to remove damaged epithelial cells and may not be specific to parasite invasion (Okuda et al., 2002, 2007; Baton and Ranford-Cartwright, 2005). To what extent this response limits parasite development is unknown.
2.5 DIFFERENTIATION INTO AN OOCYST
Ookinetes that have successfully traversed the midgut epithelium emerge within the space between the midgut epithelium and the acellular basal lamina. Within this setting, components of the basal lamina (e.g. collagen, laminin) may serve as a trigger for oocyst differentiation (Sinden, 2002). Each of the aforementioned ookinete surface proteins (CTRP, P25, P28, and SOAP) interacts with laminin and may participate in the adhesion and attachment to the basal lamina to initiate oocyst progression (Vlachou et al., 2001; Limviroj et al., 2002; Dessens et al., 2003). After emerging from the midgut epithelium, the ookinete rounds up and begins a sessile stage of development in which a single ookinete differentiates into an oocyst containing thousands of sporozoites.
Through a period of extensive cell division known as sporogony, the oocyst grows in size as the parasite undergoes multiple rounds of endomitosis from the original tetraploid nucleus. Over a variable incubation period according to the species of the parasite, 2000–8000 sporozoites are formed within a single oocyst (Sinden, 2002). Once fully differentiated, the oocyst occupies approximately 1000 times its original volume and protrudes into the mosquito haemocoel.
Very little information is available about the molecular signals that initiate oocyst development, yet one recent paper has identified a factor involved in the ookinete–oocyst transition. Gene disruption experiments demonstrate that a formin-like protein, MISFIT, presumably regulates DNA replication or chromosomal segregation during zygote development (Bushell et al., 2009). Mutant ookinetes have reduced DNA content and display aberrant microneme development, yet are uncompromised in their ability to invade the midgut (Bushell et al., 2009). Mutant oocysts are impaired in growth, develop in reduced numbers, and are gradually cleared by the mosquito (Bushell et al., 2009). The precise role of MISFIT is unclear, but represents the first identified factor that mediates the differentiation and development of oocysts from ookinetes.
The oocyst is surrounded by a protective capsule that is thought to perform two vital functions. One is to allow the flux of nutrients and metabolites into and out of the developing oocyst to sustain its massive growth. Secondly, the capsule may interact with mosquito proteins to provide a ‘masking’ effect to prevent the detection of the oocyst by the mosquito immune system (Adini and Warburg, 1999). A parasite-derived transglutaminase was identified that is hypothesized to crosslink parasite- and mosquito-derived proteins, including laminin that may protect the oocyst from an immune response (Adini et al., 2001; Nacer et al., 2008). The molecular composition of the capsule is largely unknown, and only recently, the first apicomplexan capsule protein was identified as PbCAP380 (Srinivasan et al., 2008). PbCAP380 is a large protein of about 380 kDa that is synthesized soon after emergence of the ookinete into the haemocoel and is an essential component of oocyst development. PbCAP380-deficient parasites form oocysts in normal numbers but are gradually eliminated, presumably via the mosquito’s immune defences. In γ-GCS-deficient parasites, oocyst development is severely attenuated, implicating a critical component of the glutathione biosynthesis pathway in oocyst survival (Vega-Rodriguez et al., 2009). High levels of reactive oxygen species are produced within the mosquito midgut and haemolymph in response to a parasite-infected blood meal (Molina-Cruz et al., 2008), and it is possible that oocysts require glutathione biosynthesis to provide antioxidants for a redox defence necessary for survival.
A puzzling question remains of how the rigid capsule grows with the oocyst. As the capsule is formed during the earliest stages of oocyst development (Srinivasan et al., 2008), the oocyst diameter increases dramatically as it grows and differentiates. Perhaps, components of the capsule continuously disassemble and reassemble in a dynamic process to accommodate growth, but the details of this process are not clear.
2.6 SPOROZOITE DEVELOPMENT AND EGRESS
As the nuclei of the developing oocyst divide, the oocyst cytoplasm is partitioned into compartments termed sporoblasts. The plasma membrane then invaginates in between each nucleus to form individual sporozoites within the oocyst. Members of the Limilus coagulation factor C LCCL/lectin adhesive-like protein family have been found to play important roles in oocyst development. Referred to as LAP genes in P. berghei and as CCp in P. falciparum, the role of this family of presumed surface proteins is perplexing given that the genes are expressed only in gametocytes yet their inactivation leads to defects during sporozoite differentiation in the oocyst (Delrieu et al., 2002; Trueman et al., 2004; Raine et al., 2007; Scholz et al., 2008; Lavazec et al., 2009). It remains unclear how this protein family modulates the process of sporogony within the oocyst.
The circumsporozoite protein (CS) is the major sporozoite surface protein and is also present on the oocyst plasma membrane. Interestingly, inactivation of the CS gene arrests oocyst differentiation at an early stage and sporoblasts never form (Menard et al., 1997; Thathy et al., 2002). Further studies have demonstrated that the GPI-anchored C-terminus of CS is involved in the establishment of sporozoite budding sites and the cytokinesis within the oocyst (Wang et al., 2005b).
Upon maturation of the oocyst, the sporozoites must leave (egress) from the oocyst to invade the salivary glands. It appears that egress is not mediated by mechanical stress to the oocyst capsule, but rather requires a cysteine protease (ECP1) for rupture (Aly and Matuschewski, 2005). The substrate of ECP1 is unknown but this enzyme may function directly or indirectly to initiate the proteolytic processing of target proteins required for egress (Aly and Matuschewski, 2005). Proteolysis of CS within the oocyst plasma membrane is required for sporozoite egress, and may therefore be a possible candidate for ECP1 processing (Wang et al., 2005a).
2.7 SPOROZOITE INVASION OF THE SALIVARY GLANDS
Following release of the sporozoites from the oocyst, they must traverse the basal lamina that surrounds the entire midgut to enter circulation in the haemolymph. No information is available on how escape from the basal lamina is accomplished, but a mechanism similar to that promoting sporozoite from the oocyst may also operate to disrupt the basal lamina. Alternatively, different specialized enzymes may accomplish this task. Once in the haemolymph, it is likely that the sporozoites passively circulate with the haemolymph throughout the mosquito’s body cavity (Rodriguez and Herna´ndez-Herna´ndez, 2004). The flow of haemolymph along the dorsal vessel occurs in an anterior direction from the abdomen to the head where it is delivered in close proximity to the salivary glands thus facilitating sporozoite invasion (Hillyer et al., 2007). An alternative view is that the sporozoites respond to a chemotactic signal from the salivary glands (Akaki and Dvorak, 2005), but how this occurs is more difficult to understand given the rapid flow of the haemolymph.
Sporozoite invasion of the salivary glands is specific and occurs only at the distal lateral and medial lobes of the salivary gland (Sterling et al., 1973). Additional evidence for the specificity of sporozoite recognition of salivary glands has been provided by Rosenberg (1985) who showed that P. knowlesi sporozoites efficiently invade An. dirus but not An. freeborni salivary glands. Similar to parasite invasion of the midgut, carbohydrate residues may play a role in sporozoite recognition/invasion of the salivary glands (Perrone et al., 1986; Barreau et al., 1995). Sporozoite invasion is blocked by specific lectins that presumably compete for sporozoite binding sites on the salivary glands (Barreau et al., 1995).
Recently, new insight into the process of salivary gland invasion has been reported. A phage display library screen, similar to that previously mentioned for the midgut, was conducted to identify peptides that bind specifically to the salivary glands of anopheline mosquitoes. Surprisingly, these experiments determined that the same dodecapeptide, SM1, binds to both salivary glands and midguts, inhibiting parasite invasion of both tissues (Ghosh et al., 2001). These results imply that the peptide binds to a surface receptor that the parasite needs to recognize in order for invasion to occur. Using a double-derivatized SM1 peptide, the receptor was identified as the protein saglin (Ghosh et al., 2009). Saglin contains a signal peptide but no transmembrane domain, and is rich in glutamines, suggesting that these residues may be involved in protein– protein interactions. RNAi-mediated knock-down experiments indicated that saglin is essential for sporozoite salivary gland invasion (Ghosh et al., 2009). Since saglin is present on the salivary gland surface (SGS), anti-saglin antibodies administered to mosquitoes inhibit P. berghei (Brennan et al., 2000; Okulate et al., 2007) and P. falciparum (Ghosh et al., 2009) sporozoite invasion of salivary glands.
Inhibition of sporozoite invasion by SM1 suggested the hypothesis that SM1 competes with a parasite protein for binding to the putative saglin receptor. However, the amino acid sequence of SM1 does not share homology to any predicted Plasmodium protein, suggesting that its conformation, rather than primary amino acid sequence, may resemble a sporozoite protein. Through the use of an anti-SM1 antibody, the sporozoite protein TRAP was identified as a mimotope of SM1 (TRAP is recognized by the anti-SM1 antibody) and its binding to saglin was confirmed in vitro (Ghosh et al., 2009). These data strongly argue for an essential role of saglin–TRAP interactions for the invasion of the salivary glands. However, sporozoite invasion of the salivary gland is a complex process (Pimenta et al., 1994) that depends on the successful completion of a number of other steps. Thus, the saglin–TRAP interaction should be considered as only one of many steps required for successful sporozoite invasion of the salivary gland.
The essential role of TRAP in salivary gland invasion and sporozoite motility has been previously defined (Sultan et al., 1997). The extracellular adhesive domains of TRAP interact with the cell surface, while TRAP’s cytoplasmic domain connects with the actomyosin motor via aldolase to drive sporozoite gliding motility (Jewett and Sibley, 2003). Given that TRAP-deficient sporozoites are still capable of attachment to the salivary glands, it would appear as though TRAP performs an active role during salivary gland invasion.
The CS comprises ~ 15% of total sporozoite protein (Yoshida et al., 1981) and has a functional role in motility and salivary gland invasion. Secreted from the apical end of the sporozoite, CS presumably attaches to the sporozoites outer membrane via a GPI anchor. It is translocated to the posterior end of the parasite through an apparent actin-mediated surface motor (Stewart and Vanderberg, 1991), thus propelling the sporozoite forward. Once at the posterior end, CS is cleaved leaving a trail of processed protein (Stewart and Vanderberg, 1991). While a clear association with the cytoskeletal network has not been found, it has been suggested that CS may interact with the extracellular domains of other membrane-bound proteins to function in motility (Kappe et al., 2004). Involved in sporozoite binding to the salivary glands, CS interacts specifically with the medial and distal lateral lobes, the preferred sites for sporozoite invasion (Sidjanski et al., 1997). Given the ability of CS to bind heparin sulphate in the liver, CS may likewise play a role in the recognition and attachment of specific carbohydrate residues on the basal lamina of the salivary glands (Myung et al., 2004). Possibly, as a result of this specific recognition response, the expression of P. gallinaceum CS protein in transgenic P. berghei parasites resulted in dramatically reduced numbers of salivary gland sporozoites and suggests that the protein may determine specific vector/parasite combinations (Tewari et al., 2005b).
Two sporozoite transmembrane proteins, S6 and MAEBL, have also been implicated in salivary gland recognition and invasion. S6 has been shown to function in sporozoite motility and may perform a role similar to that of TRAP during salivary gland invasion (Steinbuechel and Matuschewski, 2009). However, unlike TRAP, S6 does not appear to contain any conserved extracellular adhesive domains (Steinbuechel and Matuschewski, 2009). The secreted protein MAEBL also mediates sporozoite recognition and attachment to the salivary glands (Kariu et al., 2002). MAEBL shares structural homology with merozoite proteins involved in erythrocyte invasion and in the mosquito is presumed to play a similar role as an essential ligand during the early stages of salivary gland invasion (Saenz et al., 2008).
A family of SGS proteins has also been implicated during the process of sporozoite invasion of the salivary glands. Identified as a component of the basal lamina, SGS proteins contain heparin-binding domains that may interact with the sporozoite surface proteins in the initial stages of salivary gland invasion (Korochkina et al., 2006).
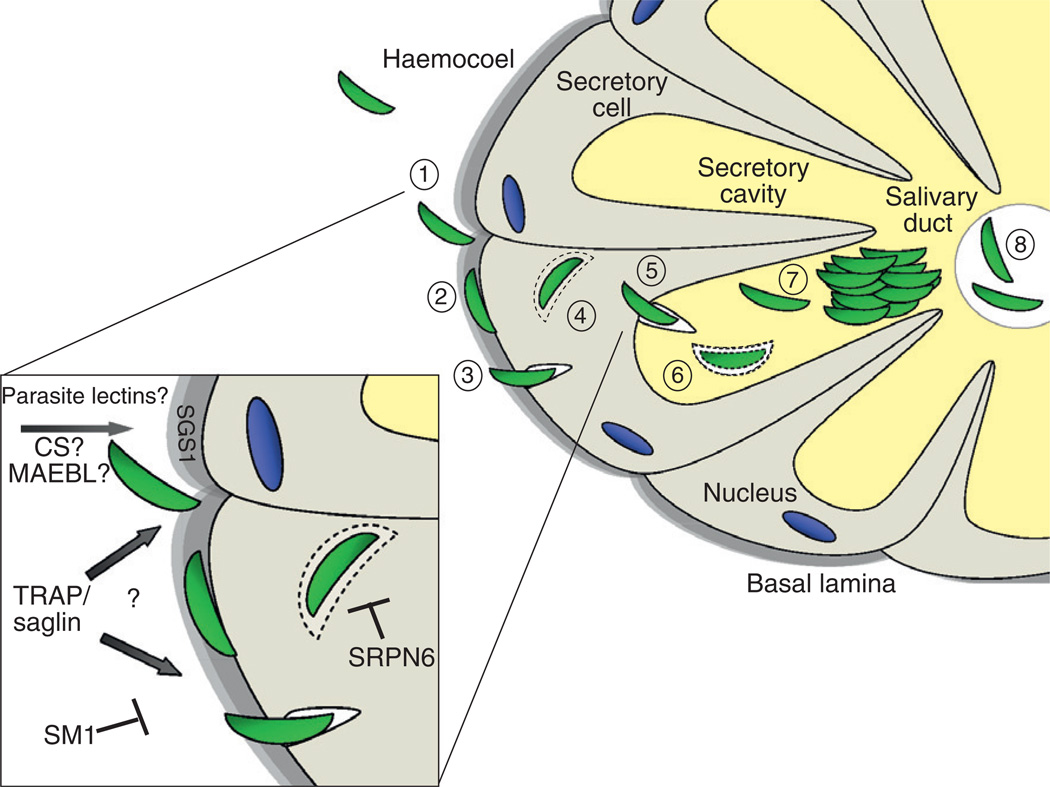
In summary, a number of components involved in salivary gland invasion have been identified, yet beyond the recent description of the saglin–TRAP interaction, the precise role they play in this process is unknown. As outlined in Fig. 3, the sporozoite initially attaches to the basal lamina of the salivary glands, possibly via CS- or MAEBL-mediated interactions (Kappe et al., 2004). Sporozoite invasion of the salivary gland epithelial cell is accompanied by the formation of a parasitophorous vacuole around the sporozoite (Pimenta et al., 1994). Once inside the host cell, this vacuole disintegrates and the sporozoites migrate to the basal side of the cell, from where it exits to the salivary gland lumen while forming again a transient parasitophorous vacuole (Pimenta et al., 1994). During each feeding cycle, a few sporozoites enter the salivary gland ducts by an unknown mechanism, from where they are delivered to the vertebrate host with the saliva (Pimenta et al., 1994). Although some damage may occur to the plasma membrane as a result of sporozoite invasion, the infected cells only display some localized swelling and protein reorganization within the affected tissues (Maier et al., 1987). Based upon these mild effects, the formation of a parasitophorous vacuole may limit the ability of invaded cells to recognize the invading pathogen and elicit a physiological response, a direct contrast to ookinete invasion of the midgut.
FIG. 3.
Sporozoite invasion of the salivary gland. Sporozoites circulating in the haemolymph recognize and attach to the basal lamina of the salivary gland (1). This is followed by the traversal through the space between the basal lamina and the salivary gland epithelium (2) until it begins the process of invasion of the plasma membrane through the formation of a parasitophorous vacuole (3). Within the cytoplasm of the secretory cell, the vacuole is degraded (4) and the sporozoite forms a new vacuole as it invades the secretory cavity (5). Once the sporozoite passes into the secretory cavity, the vacuole is again degraded (6) and sporozoites begin to assemble into large bundles within the secretory cavity (7). A small subset of these sporozoites enter the salivary duct (8) from where they are then delivered along with saliva components to a vertebrate host upon probing and biting. Salivary gland invasion is examined at the molecular level in the inset. Initial attachment of the sporozoite may be mediated by interactions of carbohydrate residues on the basal lamina with a parasite lectin, CS, MAEBL, or SGS1. Although the TRAP/saglin interaction is an important component of salivary gland invasion, it is unclear whether this occurs at the junction of the basal lamina or the salivary gland epithelium. This association can be competitively inhibited by the SM1 peptide. As a component of the mosquito immune response, SRPN6 presumably acts upon sporozoites once they invade the cytoplasm of the secretory cell to limit sporozoite numbers. The figure was adapted from Pimenta et al. (1994) and Rodriguez and Herna´ndez-Herna´ndez (2004).
2.8 SPOROZOITE REPROGRAMMING AFTER SALIVARY GLAND INVASION
Sporozoite invasion of the salivary gland triggers extensive reprogramming of gene expression. ‘Midgut sporozoites’ are virtually non-infectious to the vertebrate host, in contrast to the high infectivity of ‘salivary gland sporozoites’ (Vanderberg, 1975). Phenotypically displayed as an increased circular gliding motility after invasion of salivary glands (Vanderberg, 1975), this maturation coincides with a significant reprogramming of gene expression (Matuschewski et al., 2002; Mikolajczak et al., 2008). Microarray analysis indicates expression changes in approximately 10% of sporozoite genes (Mikolajczak et al., 2008). The maturation of salivary gland sporozoites also coincides with the redistribution of MAEBL on the sporozoite surface, and the expression of previously translationally repressed AMA-1 transcripts (Srinivasan et al., 2004). Sporozoite maturation seems to correlate with changes in expression of genes required for the recognition, invasion, and survival in vertebrate tissues. Once inside salivary glands, sporozoites are unable to re-invade salivary glands (Touray et al., 1992), supporting the hypothesis that salivary gland invasion triggers the reprogramming for transmission to the vertebrate host. The precise trigger that initiates this reprogramming remains unclear.
The accumulation of sporozoites within the secretory cavity alters salivary gland function and mosquito behaviour. Mature sporozoites may interfere with the secretion of saliva during probing and feeding as measured by reduced levels of apyrase activity found in infected mosquitoes (Rossignol et al., 1984). Thus, by interfering with the secretion of saliva, sporozoites may promote an increase in mosquito probing behaviour to increase their chances of delivery to the vertebrate host (Rossignol et al., 1984).
3 Mosquito immune response to Plasmodium
In addition to the physical barriers (PM, midgut epithelium, salivary gland epithelium), the innate immune system poses a significant challenge for parasite development in the mosquito. Efforts to understand the mosquito immune responses to Plasmodium is a rapidly developing field (see Christophides et al. (2004) and Blandin et al. (2008) for excellent reviews). Here we address mosquito immunity in general terms and focus only on a few selected aspects.
The signals that operate in the activation of the mosquito innate immune system in response to Plasmodium are largely unknown, yet the mosquito appears to sense the presence of the parasite in an infected blood meal (Dong et al., 2006). Although some activation may occur before ookinete invasion of the midgut, a major induction of the innate immune response is triggered by the physical contact between the ookinete and midgut epithelial cells (Dong et al., 2006). Localized immune responses by infected cells may trigger a systemic response to initiate the production and release of mosquito immune factors from the distally located fat body and haemocytes. Moreover, antimicrobial peptides produced by the Toll and IMD immune pathways provide strong anti-Plasmodium defences (Garver et al., 2009). Other factors, including nitric oxide (Luckhart et al., 1998) and the predicted serine protease inhibitor, SRPN6 (Abraham et al., 2005), have been shown to impede the success of midgut invasion. On the other hand, the parasite has also evolved means to escape the mosquito defences and increase its survival. Mosquito factors such as CTL4 and CTLMA2 provide protection to the ookinete from mosquito immune responses. When expression of these genes is inhibited, the success of the parasite development is significantly reduced (Osta et al., 2004).
The identification of mosquito strains resistant to parasite development has provided insights into the mechanisms of parasite killing. The laboratory selection of Plasmodium-resistant mosquito strains has led to the identification of two possible killing mechanisms. The ookinete can be lysed while traversing the midgut epithelial cell (Vernick et al., 1995), or alternatively, the ookinete can undergo melanotic encapsulation in the basal epithelium as it emerges from the invaded midgut cell (Collins et al., 1986). Natural variations in susceptibility to Plasmodium infection have also been detected within field isolates of An. gambiae. By genetic mapping, a major locus was identified that confers natural resistance to P. falciparum (Niaré et al., 2002). RNAi-mediated knock-down experiments identified APL1C as a candidate resistance gene against P. berghei within this locus (Riehle et al., 2006, 2008). Evidence now suggests that APL1C only confers protection against P. berghei, while another APL1 gene family member, APL1A, confers the response against P. falciparum (Mitri et al., 2009).
After several years of research, it now appears as though the mosquito factors TEP1, LRIM1, and APL1C (or presumably APL1A against P. falciparum) form a complex that circulates within the haemolymph and targets invading parasites for destruction (Fraiture et al., 2009; Povelones et al., 2009). Upon immune activation, the cleavage of TEP1 by an unknown protease produces an active form that binds directly to the surface of the ookinete. TEP1 binding then initiates a complement-like cascade resulting in the killing of the parasite (Blandin et al., 2004; Fraiture et al., 2009). The other components of the complex, LRIM1 and the aforementioned APL1C, have been previously characterized for their involvement in a strong anti-parasitic response (Osta et al., 2004; Riehle et al., 2006) and are required for stabilizing the activated TEP1 to ensure parasite killing (Fraiture et al., 2009; Povelones et al., 2009).
Although the majority of immunity studies have focused on midgut invasion, the mosquito innate immune system also acts in limiting the success of sporozoite invasion of the salivary glands. After sporozoite release from mature oocysts into the haemolymph, they are exposed to the circulating components of the mosquito immune system. Within minutes of their release into the haemocoel, the majority of sporozoites are degraded (Hillyer et al., 2007) and only ~20% of the released sporozoites reach the salivary gland lumen (Rosenberg and Rungsiwongse, 1991; Barreau et al., 1995). It is unclear what mechanism is responsible for this dramatic attenuation.
Initial evidence of immune activation within the salivary glands was provided by the analysis of a subset of immune genes induced at the time of invasion (Dimopoulos et al., 1998). More recently, 37 immunity-related genes were identified using serial analysis of gene expression (SAGE) analysis in response to salivary gland infection (Rosinski-Chupin et al., 2007). Among the genes recognized by this study, the previously characterized serine protease inhibitor SRPN6 was identified, and independently shown to impair sporozoite development in the salivary glands (Pinto et al., 2008). With a similar inhibitory role within the midgut in response to ookinete infection (Abraham et al., 2005), there may be a similar mechanism of SRPN6 activation in the salivary gland. These findings suggest an overlap between the immune activation within the midgut and salivary gland in response to parasite invasion.
4 The role of commensal bacteria on Plasmodium midgut invasion
Like most metazoans, mosquitoes contain microbiota in their midgut. Shortly after blood ingestion, the resident microbiota undergoes rapid proliferation that peaks roughly 24 h later (Pumpuni et al., 1996). This is also the approximate time of ookinete invasion, and bacteria proliferation is likely to result in an immune response that is independent of the parasite.
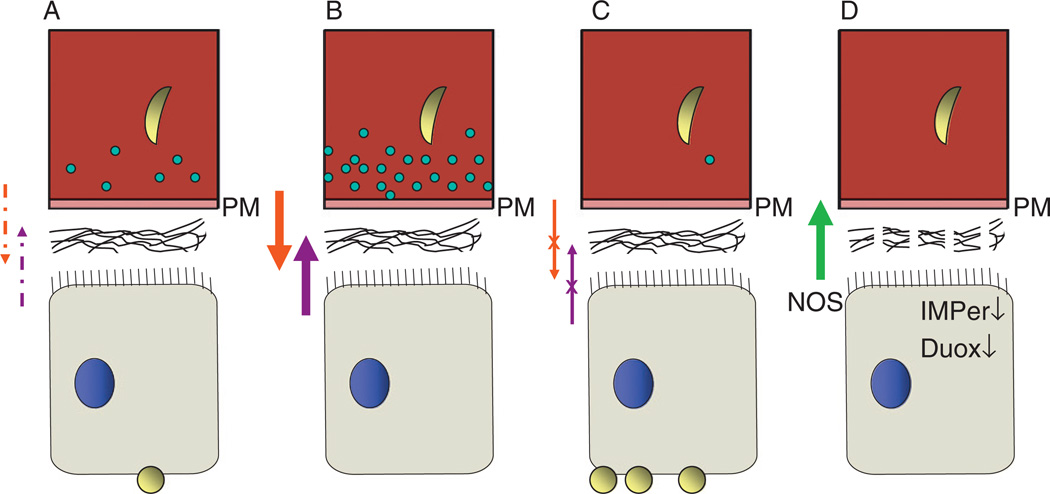
Co-feeding experiments in which bacteria are fed at the same time as an infectious blood meal reduce the survival of P. falciparum parasites within the midgut (Dong et al., 2009). Conversely, antibiotic treatment to remove the endogenous microbiota increased the levels of parasite infection (Dong et al., 2009). Taken together, this suggests that the endogenous microbiota primes an immune response independent of parasite invasion that impacts the success of parasite survival (summarized in Fig. 4A – C).
FIG. 4.
Proposed model of the interactions between commensal bacteria on Plasmodium development. Under normal circumstances (A), there is a delicate interplay between the mosquito immune system to regulate the abundance of endogenous microbiota. Basal levels of immune activation (dashed upward purple arrow) are regulated by the Duox/IMPer system that limits the amount of immune elicitors (dashed downward red arrow) allowed to pass through the constitutive extracellular matrix (different from the peritrophic matrix) lining the midgut epithelium. As a result, the immune response limits the ability of the parasite to successfully invade the midgut epithelium and transition into an oocyst. In bacterial co-feeding experiments (B), the dramatically increased number of bacteria elicits a much stronger immune response (bold red and purple arrows) resulting in further reduction of the success of parasite invasion. Upon pre-treatment with antibiotics to remove the endogenous gut microbiota (C), bacterial priming of the immune response does not occur (broken red arrow) and therefore immune activation is limited (broken purple arrow). As a result, parasite survival is significantly increased. By removing components of the Duox/IMPer system by RNAi (D), increased permeability of the extracellular matrix covering the midgut epithelium produces a strong NOS response (bold green arrow) that drastically reduces the number of commensal bacteria and parasite survival. These concepts are based on data from Dong et al. (2009) and Kumar et al. (2010).
A delicate balance exists between the commensal gut microbiota and its mosquito host to limit bacterial overproliferation and the subsequent mosquito immune response. Kumar et al. (2010) have recently demonstrated that this tight regulation is at least in part mediated by dual oxidase (Duox) and an immunomodulatory peroxidase (IMPer). RNAi-mediated knock-down of both genes results in increased permeability of the midgut, suggesting that the Duox/IMPer system mediates the cross-linking of proteins within the extracellular matrix on the lumenal side of the midgut epithelium (Fig. 4D). As a result, this limits the diffusion of immune elicitors from bacteria (or Plasmodium) within the midgut to maintain the natural balance of commensal bacteria. While ensuring the survival of the commensal bacteria, this manner of regulation allows for precious time for Plasmodium to evade detection, thus making the mosquito more susceptible to parasite infection.
While many questions remain as to the interaction with the endogenous microbiota and Plasmodium, efforts to capitalize upon this interaction with the mosquito immune response may lead to novel malaria intervention strategies.
5 Vector–parasite co-evolution
Successful completion of the Plasmodium cycle in the mosquito is an absolute requirement for transmission to occur and for parasite survival in nature. Thus, there has been enormous pressure on the parasite to evolve means to escape mosquito immune defences. Some examples illustrate this point. In the study by Collins et al. (1986), the selected refractory An. gambiae strain displayed variability to melanize and destroy P. falciparum depending on its geographical origin. African parasites were significantly more proficient in evading the melanization response than those of New World or Asian origins. Although the molecular basis for these differences remains unclear, it is striking that the refractory African An. gambiae mosquitoes are the least effective in destroying the co-indigenous African malaria parasites as compared with the parasites originating from other continents (Collins et al., 1986).
A second example is the comparison of the immune response of An. gambiae towards the human parasite P. falciparum (a natural vector–parasite combination) and the rodent parasite P. berghei (a combination that does not occur in nature). Dong et al. (2006) conducted a microarray analysis comparing the mosquito transcriptional responses between the two parasites. Although some universal immune responses were detected across Plasmodium species, many more were species specific. Only limited gene activation was observed in the natural An. gambiae–P. falciparum combination, while a much broader and extensive transcriptional activation was observed for the artificial An. gambiae–P. berghei combination.
Both examples are suggestive of parasite strategies to evade the mosquito’s immune defences. However, parasite–mosquito interactions are complex in that even a small number of parasites decrease mosquito fitness, while mosquito activation of immune responses against the parasite have the same fitness effect (Hurd et al., 2005; Voordouw et al., 2009). From the parasite’s perspective, low numbers are sufficient, since one oocyst can generate enough sporozoites to render the mosquito infective for life. From the mosquito’s perspective, permissiveness to the parasite is perhaps advantageous because of the high cost of mounting an immune defence. In nature, parasite prevalence is low even in high transmission areas, and infected mosquitoes carry low parasite numbers (Niaré et al., 2002).
6 Concluding remarks
The past decade has witnessed enormous strides in our understanding of how Plasmodium develops in the mosquito, yet many questions remain. The sequencing of multiple genomes of the malaria parasite and the mosquito host facilitates the application of powerful genomic and proteomic approaches to these questions. Analysis of gene expression with microarrays has provided important new insights on vector–parasite interactions (Dong et al., 2006). Technical advances in proteomic approaches have allowed researchers to dissect the composition of specific vector structures (Dinglasan et al., 2009) or collect information from specific stages of parasite development (Hall et al., 2005; Khan et al., 2005). Genetic transformation technologies have become routine for Plasmodium, and the advent of RNAi has had a profound influence on mosquito genetics where germ line transformation technologies remain cumbersome.
Through the use of anti-Plasmodium effector genes, proof-of-concept experiments to utilize genetically modified mosquitoes to interfere with parasite transmission have shown promise (Ito et al., 2002; Moreira et al., 2002; Marrelli et al., 2007). Yet, a major challenge is to devise safe and effective means to drive these interfering (effector) genes into mosquito populations in the field.
Identification of proteins involved in parasite development in the mosquito has revealed new targets for transmission-blocking vaccines. Moreover, vaccines that target conserved vector proteins required for ookinete invasion may lead to the development of transmission-blocking strategies that are effective across multiple Plasmodium species (Dinglasan and Jacobs-Lorena, 2008).
Given the complexity of the Plasmodium life cycle, it is perhaps surprising how successful this parasite has been in overcoming the numerous roadblocks imposed as it develops in its insect (and vertebrate) host. On the other hand, targeting the resultant developmental bottlenecks presents an opportunity for intervention and reduction of vector competence.
References
- Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, Kafatos FC, Jacobs-Lorena M, Michel K. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc. Natl. Acad. Sci. USA. 2005;102:16327–16332. doi: 10.1073/pnas.0508335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adini A, Warburg A. Interaction of Plasmodium gallinaceum ookinetes and oocysts with extracellular matrix proteins. Parasitology. 1999;119(Pt 4):331–336. doi: 10.1017/s0031182099004874. [DOI] [PubMed] [Google Scholar]
- Adini A, Krugliak M, Ginsburg H, Li L, Lavie L, Warburg A. Transglutaminase in Plasmodium parasites: activity and putative role in oocysts and blood stages. Mol. Biochem. Parasitol. 2001;117:161–168. doi: 10.1016/s0166-6851(01)00345-0. [DOI] [PubMed] [Google Scholar]
- Akaki M, Dvorak JA. A chemotactic response facilitates mosquito salivary gland infection by malaria sporozoites. J. Exp. Biol. 2005;208:3211–3218. doi: 10.1242/jeb.01756. [DOI] [PubMed] [Google Scholar]
- Aly AS, Matuschewski K. A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts. J. Exp. Med. 2005;202:225–230. doi: 10.1084/jem.20050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Billker O, Morris HR, Panico M, Delcroix M, Dixon D, Ley SV, Sinden RE. Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol. Biochem. Parasitol. 2001;116:17–24. doi: 10.1016/s0166-6851(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Barreau C, Touray M, Pimenta PF, Miller LH, Vernick KD. Plasmodium gallinaceum: sporozoite invasion of Aedes aegypti salivary glands is inhibited by anti-gland antibodies and by lectins. Exp. Parasitol. 1995;81:332–343. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21:573–580. doi: 10.1016/j.pt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Billingsley PF, Rudin W. The role of the mosquito peritrophic membrane in bloodmeal digestion and infectivity of Plasmodium species. J. Parasitol. 1992;78:430–440. [PubMed] [Google Scholar]
- Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- Blagborough AM, Sinden RE. Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine. 2009;27:5187–5194. doi: 10.1016/j.vaccine.2009.06.069. [DOI] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Blandin SA, Marois E, Levashina EA. Antimalarial responses in Anopheles gambiae: from a complement-like protein to a complement-like pathway. Cell Host Microbe. 2008;3:364–374. doi: 10.1016/j.chom.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Brennan JD, Kent M, Dhar R, Fujioka H, Kumar N. Anopheles gambiae salivary gland proteins as putative targets for blocking transmission of malaria parasites. Proc. Natl. Acad. Sci. USA. 2000;97:13859–13864. doi: 10.1073/pnas.250472597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell ES, Ecker A, Schlegelmilch T, Goulding D, Dougan G, Sinden RE, Christophides GK, Kafatos FC, Vlachou D. Paternal effect of the nuclear formin-like protein MISFIT on Plasmodium development in the mosquito vector. PLoS Pathog. 2009;5:e1000539. doi: 10.1371/journal.ppat.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R, Coulson A, Bhatti S, Taylor BJ, Elliott JF. Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Mol. Biochem. Parasitol. 1995;71:203–210. doi: 10.1016/0166-6851(94)00054-q. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol. Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, Collins WE, Campbell CC, Gwadz RW. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- de Koning-Ward TF, Olivieri A, Bertuccini L, Hood A, Silvestrini F, Charvalias K, Berzosa Diaz P, Camarda G, McElwain TF, Papenfuss T, et al. The role of osmiophilic bodies and Pfg377 expression in female gametocyte emergence and mosquito infectivity in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 2008;67:278–290. doi: 10.1111/j.1365-2958.2007.06039.x. [DOI] [PubMed] [Google Scholar]
- Delrieu I, Waller CC, Mota MM, Grainger M, Langhorne J, Holder AA. PSLAP, a protein with multiple adhesive motifs, is expressed in Plasmodium falciparum gametocytes. Mol. Biochem. Parasitol. 2002;121:11–20. doi: 10.1016/s0166-6851(02)00016-6. [DOI] [PubMed] [Google Scholar]
- Dessens JT, Beetsma AL, Dimopoulos G, Wengelnik K, Crisanti A, Kafatos FC, Sinden RE. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999;18:6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens JT, Mendoza J, Claudianos C, Vinetz JM, Khater E, Hassard S, Ranawaka GR, Sinden RE. Knockout of the rodent malaria parasite chitinase pbCHT1 reduces infectivity to mosquitoes. Infect. Immun. 2001;69:4041–4047. doi: 10.1128/IAI.69.6.4041-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens JT, Siden-Kiamos I, Mendoza J, Mahairaki V, Khater E, Vlachou D, Xu XJ, Kafatos FC, Louis C, Dimopoulos G, Sinden RE. SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Mol. Microbiol. 2003;49:319–329. doi: 10.1046/j.1365-2958.2003.03566.x. [DOI] [PubMed] [Google Scholar]
- Devenport M, Jacobs-Lorena M. The peritrophic matrix of hematophagous insects. In: Marquardt WC, editor. Biology of Disease Vectors. Elsevier Academic Press; Amsterdam; 2005. [Google Scholar]
- Dimopoulos G, Seeley D, Wolf A, Kafatos FC. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO J. 1998;17:6115–6123. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinglasan RR, Jacobs-Lorena M. Insight into a conserved lifestyle: protein-carbohydrate adhesion strategies of vector-borne pathogens. Infect. Immun. 2005;73:7797–7807. doi: 10.1128/IAI.73.12.7797-7807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinglasan RR, Jacobs-Lorena M. Flipping the paradigm on malaria transmission-blocking vaccines. Trends Parasitol. 2008;24:364–370. doi: 10.1016/j.pt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinglasan RR, Fields I, Shahabuddin M, Azad AF, Sacci JB., Jr. Monoclonal antibody MG96 completely blocks Plasmodium yoelii development in Anopheles stephensi . Infect. Immun. 2003;71:6995–7001. doi: 10.1128/IAI.71.12.6995-7001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinglasan RR, Alaganan A, Ghosh AK, Saito A, van Kuppevelt TH, Jacobs-Lorena M. Plasmodium falciparum ookinetes require mosquito midgut chondroitin sulfate proteoglycans for cell invasion. Proc. Natl. Acad. Sci. USA. 2007a;104:15882–15887. doi: 10.1073/pnas.0706340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinglasan RR, Kalume DE, Kanzok SM, Ghosh AK, Muratova O, Pandey A, Jacobs-Lorena M. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc. Natl. Acad. Sci. USA. 2007b;104:13461–13466. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinglasan RR, Devenport M, Florens L, Johnson JR, McHugh CA, Donnelly-Doman M, Carucci DJ, Yates JR3rd, Jacobs-Lorena M. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem. Mol. Biol. 2009;39:125–134. doi: 10.1016/j.ibmb.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker A, Pinto SB, Baker KW, Kafatos FC, Sinden RE. Plasmodium berghei: Plasmodium perforin-like protein 5 is required for mosquito midgut invasion in Anopheles stephensi . Exp. Parasitol. 2007;116:504–508. doi: 10.1016/j.exppara.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksi S, Czesny B, van Gemert GJ, Sauerwein RW, Eling W, Williamson KC. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol. 2006;61:991–998. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, Hoffmann JA, Blandin SA, Levashina EA. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium . Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Wirtz RA, Barr JR, Woolfitt A, Rosenberg R. Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. J. Biol. Chem. 1998;273:12003–12005. doi: 10.1074/jbc.273.20.12003. [DOI] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Ribolla PE, Jacobs-Lorena M. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proc. Natl. Acad. Sci. USA. 2001;98:13278–13281. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Devenport M, Jethwaney D, Kalume DE, Pandey A, Anderson VE, Sultan AA, Kumar N, Jacobs-Lorena M. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. 2009;5:e1000265. doi: 10.1371/journal.ppat.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta L, Kumar S, Han YS, Pimenta PF, Barillas-Mury C. Midgut epithelial responses of different mosquito-Plasmodium combinations: the actin cone zipper repair mechanism in Aedes aegypti . Proc. Natl. Acad. Sci. USA. 2005;102:4010–4015. doi: 10.1073/pnas.0409642102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N, Karras M, Raine JD, Carlton JM, Kooij TWA, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, James K, Rutherford K, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Ortiz A, Lanz-Mendoza H, Martinez-Barnetche J, Hernandez-Martinez S, Villarreal-Trevino C, Aguilar-Marcelino L, Rodriguez MH. Plasmodium berghei ookinetes induce nitric oxide production in Anopheles pseudopunctipennis midguts cultured in vitro. Insect Biochem. Mol. Biol. 2004;34:893–901. doi: 10.1016/j.ibmb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Barreau C, Vernick KD. Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito hemocoel. Int. J. Parasitol. 2007;37:673–681. doi: 10.1016/j.ijpara.2006.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Arai M, Kawai S, Matsuoka H. PbGCβ is essential for Plasmodium ookinete motility to invade midgut cell and for successful completion of parasite life cycle in mosquitoes. J. Biochem. 2006;140:747–757. doi: 10.1093/jb/mvj205. [DOI] [PubMed] [Google Scholar]
- Hirai M, Arai M, Mori T, Miyagishima SY, Kawai S, Kita K, Kuroiwa T, Terenius O, Matsuoka H. Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr. Biol. 2008;18:607–613. doi: 10.1016/j.cub.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Hurd H, Taylor PJ, Adams D, Underhill A, Eggleston P. Evaluating the costs of mosquito resistance to malaria parasites. Evolution. 2005;59:2560–2572. [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol. Microbiol. 2006;59:1175–1184. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Van der Klooster PF, Van der Kaay HJ, Van der Ploeg , Overdulve JP. Rapid repeated DNA replication during microgametogenesis and DNA synthesis in young zygotes of Plasmodium berghei . Trans. R. Soc. Trop. Med. Hyg. 1986;80:154–157. doi: 10.1016/0035-9203(86)90219-1. [DOI] [PubMed] [Google Scholar]
- Jewett TJ, Sibley LD. Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol. Cell. 2003;11:885–894. doi: 10.1016/s1097-2765(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Kadota K, Ishino T, Matsuyama T, Chinzei Y, Yuda M. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc. Natl. Acad. Sci. USA. 2004;101:16310–16315. doi: 10.1073/pnas.0406187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappe SH, Buscaglia CA, Nussenzweig V. Plasmodium sporozoite molecular cell biology. Annu. Rev. Cell Dev. Biol. 2004;20:29–59. doi: 10.1146/annurev.cellbio.20.011603.150935. [DOI] [PubMed] [Google Scholar]
- Kariu T, Yuda M, Yano K, Chinzei Y. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J. Exp. Med. 2002;195:1317–1323. doi: 10.1084/jem.20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto F, Alejo-Blanco R, Fleck SL, Kawamoto Y, Sinden RE. Possible roles of Ca2+ and cGMP as mediators of the exflagellation of Plasmodium berghei and Plasmodium falciparum. Mol. Biochem. Parasitol. 1990;42:101–108. doi: 10.1016/0166-6851(90)90117-5. [DOI] [PubMed] [Google Scholar]
- Kawamoto F, Fujioka H, Murakami R, Syafruddin Hagiwara M, Ishikawa T, Hidaka H. The roles of Ca2+/calmodulin- and cGMP-dependent pathways in gametogenesis of a rodent malaria parasite, Plasmodium berghei . Eur. J. Cell Biol. 1993;60:101–107. [PubMed] [Google Scholar]
- Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M, Waters AP. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology . Cell. 2005;121:675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Korochkina S, Barreau C, Pradel G, Jeffery E, Li J, Natarajan R, Shabanowitz J, Hunt D, Frevert U, Vernick KD. A mosquito-specific protein family includes candidate receptors for malaria sporozoite invasion of salivary glands. Cell. Microbiol. 2006;8:163–175. doi: 10.1111/j.1462-5822.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Ehret-Sabatier L, Siden-Kiamos I, Mendoza J, Sinden RE, Louis C. Plasmodium berghei ookinetes bind to Anopheles gambiae and Drosophila melanogaster annexins. Mol. Microbiol. 2005;57:171–179. doi: 10.1111/j.1365-2958.2005.04664.x. [DOI] [PubMed] [Google Scholar]
- Kumar N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 1987;9:321–335. doi: 10.1111/j.1365-3024.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Kumar N, Wizel B. Further characterization of interactions between gamete surface antigens of Plasmodium falciparum. Mol. Biochem. Parasitol. 1992;53:113–120. doi: 10.1016/0166-6851(92)90013-a. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gupta L, Han YS, Barillas-Mury C. Inducible peroxidases mediate nitration of Anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion . J. Biol. Chem. 2004;279:53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavazec C, Moreira CK, Mair GR, Waters AP, Janse CJ, Templeton TJ. Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Mol. Biochem. Parasitol. 2009;163:1–7. doi: 10.1016/j.molbiopara.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Limviroj W, Yano K, Yuda M, Ando K, Chinzei Y. Immuno-electron microscopic observation of Plasmodium berghei CTRP localization in the midgut of the vector mosquito Anopheles stephensi. J. Parasitol. 2002;88:664–672. doi: 10.1645/0022-3395(2002)088[0664:IEMOOP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, Billker O. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008;22:1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier WA, Becker-Feldman H, Seitz HM. Pathology of malaria-infected mosquitoes. Parasitol. Today. 1987;3:216–218. doi: 10.1016/0169-4758(87)90063-9. [DOI] [PubMed] [Google Scholar]
- Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, Khan SM, Dimopoulos G, Janse CJ, Waters AP. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli MT, Li C, Rasgon JL, Jacobs-Lorena M. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc. Natl. Acad. Sci. USA. 2007;104:5580–5583. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SK, Jett M, Schneider I. Correlation of phosphoinositide hydrolysis with exflagellation in the malaria microgametocyte. J. Parasitol. 1994;80:371–378. [PubMed] [Google Scholar]
- Matuschewski K, Ross J, Brown SM, Kaiser K, Nussenzweig V, Kappe SH. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J. Biol. Chem. 2002;277:41948–41953. doi: 10.1074/jbc.M207315200. [DOI] [PubMed] [Google Scholar]
- McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008;6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard R, Sultan AA, Cortes C, Altszuler R, van Dijk MR, Janse CJ, Waters AP, Nussenzweig RS, Nussenzweig V. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- Mikolajczak SA, Silva-Rivera H, Peng X, Tarun AS, Camargo N, Jacobs-Lorena V, Daly TM, Bergman LW, de la Vega P, Williams J, Aly ASI, Kappe SHI. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol. Cell. Biol. 2008;28:6196–6207. doi: 10.1128/MCB.00553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri C, Jacques JC, Thiery I, Riehle MM, Xu J, Bischoff E, Morlais I, Nsango SE, Vernick KD, Bourgouin C. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 2009;5:e1000576. doi: 10.1371/journal.ppat.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, Jaramillo-Gutierrez G, Barillas-Mury C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium . J. Biol. Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Abraham EG, Crisanti A, Nolan T, Catteruccia F, Jacobs-Lorena M. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J. Biol. Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- Muhia DK, Swales CA, Deng W, Kelly JM, Baker DA. The gametocyte-activating factor xanthurenic acid stimulates an increase in membrane-associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparuml . Mol. Microbio. 2001;42:553–560. doi: 10.1046/j.1365-2958.2001.02665.x. [DOI] [PubMed] [Google Scholar]
- Myung JM, Marshall P, Sinnis P. The Plasmodium circumsporozoite protein is involved in mosquito salivary gland invasion by sporozoites. Mol. Biochem. Parasitol. 2004;133:53–59. doi: 10.1016/j.molbiopara.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Nacer A, Walker K, Hurd H. Localisation of laminin within Plasmodium berghei oocysts and the midgut epithelial cells of Anopheles stephensi . Parasit. Vectors. 2008;1:33. doi: 10.1186/1756-3305-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaré O, Markianos K, Volz J, Oduol F, Touré A, Bagayoko M, Sangaré D, Traoré SF, Wang R, Blass C, Dolo G, Bouaré M, et al. Genetic loci affecting resistance to human malaria parasites in a West African mosquito vector population. Science. 2002;298:213–216. doi: 10.1126/science.1073420. [DOI] [PubMed] [Google Scholar]
- Nijhout MM. Plasmodium gallinaceum: exflagellation stimulated by a mosquito factor. Exp. Parasitol. 1979;48:75–80. doi: 10.1016/0014-4894(79)90056-0. [DOI] [PubMed] [Google Scholar]
- Nijhout MM, Carter R. Gamete development in malaria parasites: bicarbonate-dependent stimulation by pH in vitro. Parasitology. 1978;76:39–53. doi: 10.1017/s0031182000047375. [DOI] [PubMed] [Google Scholar]
- Okuda K, de Souza Caroci A, Ribolla PE, de Bianchi AG, Bijovsky AT. Functional morphology of adult female Culex quinquefasciatus midgut during blood digestion. Tissue Cell. 2002;34:210–219. doi: 10.1016/s0040-8166(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Okuda K, de Almeida F, Mortara RA, Krieger H, Marinotti O, Bijovsky AT. Cell death and regeneration in the midgut of the mosquito, Culex quinque-fasciatus . J. Insect Physiol. 2007;53:1307–1315. doi: 10.1016/j.jinsphys.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Okulate MA, Kalume DE, Reddy R, Kristiansen T, Bhattacharyya M, Chaerkady R, Pandey A, Kumar N. Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites. Insect Mol. Biol. 2007;16:711–722. doi: 10.1111/j.1365-2583.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- Perrone JB, DeMaio J, Spielman A. Regions of mosquito salivary glands distinguished by surface lectin-binding characteristics. Insect Biochem. 1986;16:313–318. [Google Scholar]
- Pimenta PF, Touray M, Miller L. The journey of malaria sporozoites in the mosquito salivary gland. J. Eukaryot. Microbiol. 1994;41:608–624. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Pinto SB, Kafatos FC, Michel K. The parasite invasion marker SRPN6 reduces sporozoite numbers in salivary glands of Anopheles gambiae. Cell. Microbiol. 2008;10:891–898. doi: 10.1111/j.1462-5822.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC. Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am. J. Trop. Med. Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- Raine JD, Ecker A, Mendoza J, Tewari R, Stanway RR, Sinden RE. Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathog. 2007;3:e30. doi: 10.1371/journal.ppat.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Wanniarachchi IC, Srikrishnaraj KA, Ramasamy MS. Mosquito midgut glycoproteins and recognition sites for malaria parasites. Biochim. Biophys. Acta. 1997;1361:114–122. doi: 10.1016/s0925-4439(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Reininger L, Billker O, Tewari R, Mukhopadhyay A, Fennell C, Dorin-Semblat D, Doerig C, Goldring D, Harmse L, Ranford-Cartwright L, Packer J. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J. Biol. Chem. 2005;280:31957–31964. doi: 10.1074/jbc.M504523200. [DOI] [PubMed] [Google Scholar]
- Reininger L, Tewari R, Fennell C, Holland Z, Goldring D, Ranford-Cartwright L, Billker O, Doerig C. An essential role for the Plasmodium Nek-2 Nima-related protein kinase in the sexual development of malaria parasites. J. Biol. Chem. 2009;284:20858–20868. doi: 10.1074/jbc.M109.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle MM, Markianos K, Niaré O, Xu J, Li J, Touré AM, Podiougou B, Oduol F, Diawara S, Diallo M, Coulibaly B, Ouatara A, et al. Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science. 2006;312:577–579. doi: 10.1126/science.1124153. [DOI] [PubMed] [Google Scholar]
- Riehle MM, Xu J, Lazzaro BP, Rottschaefer SM, Coulibaly B, Sacko M, Niaré O, Morlais I, Traoré SF, Vernick KD. Anopheles gambiae APL1 is a family of variable LRR proteins required for Rel1-mediated protection from the malaria parasite, Plasmodium berghei. PLoS ONE. 2008;3:e3672. doi: 10.1371/journal.pone.0003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues FG, Santos MN, de Carvalho TX, Rocha BC, Riehle MA, Pimenta PF, Abraham EG, Jacobs-Lorena M, Alves de Brito CF, Moreira LA. Expression of a mutated phospholipase A2 in transgenic Aedes fluviatilis mosquitoes impacts Plasmodium gallinaceum development. Insect Mol. Biol. 2008;17:175–183. doi: 10.1111/j.1365-2583.2008.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MH, Hernández-Hernández F, de L. Insect-malaria parasites interactions: the salivary gland. Insect Biochem. Mol. Biol. 2004;34:615–624. doi: 10.1016/j.ibmb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. Inability of Plasmodium knowlesi sporozoites to invade Anopheles freeborni salivary glands. Am. J. Trop. Med. Hyg. 1985;34:687–691. doi: 10.4269/ajtmh.1985.34.687. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Rungsiwongse J. The number of sporozoites produced by individual malaria oocysts. Am. J. Trop. Med. Hyg. 1991;45:574–577. doi: 10.4269/ajtmh.1991.45.574. [DOI] [PubMed] [Google Scholar]
- Rosinski-Chupin I, Briolay J, Brouilly P, Perrot S, Gomez SM, Chertemps T, Roth CW, Keime C, Gandrillon O, Couble P, Brey PT. SAGE analysis of mosquito salivary gland transcriptomes during Plasmodium invasion. Cell. Microbiol. 2007;9:708–724. doi: 10.1111/j.1462-5822.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- Rossignol PA, Ribeiro JM, Spielman A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am. J. Trop. Med. Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- Rudin W, Hecker H. Lectin-binding sites in the midgut of the mosquitoes Anopheles stephensi Liston and Aedes aegypti L. (Diptera: Culicidae) Parasitol. Res. 1989;75:268–279. doi: 10.1007/BF00931811. [DOI] [PubMed] [Google Scholar]
- Saenz FE, Balu B, Smith J, Mendonca SR, Adams JH. The transmembrane isoform of Plasmodium falciparum MAEBL is essential for the invasion of Anopheles salivary glands. PLoS ONE. 2008;3:e2287. doi: 10.1371/journal.pone.0002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz SM, Simon N, Lavazec C, Dude MA, Templeton TJ, Pradel G. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int. J. Parasitol. 2008;38:327–340. doi: 10.1016/j.ijpara.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Severini C, Silvestrini F, Sannella A, Barca S, Gradoni L, Alano P. The production of the osmiophilic body protein Pfg377 is associated with stage of maturation and sex in Plasmodium falciparum gametocytes. Mol. Biochem. Parasitol. 1999;100:247–252. doi: 10.1016/s0166-6851(99)00050-x. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M, Kaslow DC. Plasmodium: parasite chitinase and its role in malaria transmission. Exp. Parasitol. 1994;79:85–88. doi: 10.1006/expr.1994.1066. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M, Pimenta PF. Plasmodium gallinaceum preferentially invades vesicular ATPase-expressing cells in Aedes aegypti midgut. Proc. Natl Acad. Sci. USA. 1998;95:3385–3389. doi: 10.1073/pnas.95.7.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabuddin M, Toyoshima T, Aikawa M, Kaslow DC. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc. Natl. Acad. Sci. USA. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Dimopoulos G, Kafatos FC, Jacobs-Lorena M. A cell surface mucin specifically expressed in the midgut of the malaria mosquito Anopheles gambiae . Proc. Natl. Acad. Sci. USA. 1999;96:5610–5615. doi: 10.1073/pnas.96.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siden-Kiamos I, Louis C. Interactions between malaria parasites and their mosquito hosts in the midgut. Insect Biochem. Mol. Biol. 2004;34:679–685. doi: 10.1016/j.ibmb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Siden-Kiamos I, Louis C. Intracellular calcium levels in the Plasmodium berghei ookinete. Parasitology. 2008;135:1355–1362. doi: 10.1017/S0031182008004939. [DOI] [PubMed] [Google Scholar]
- Siden-Kiamos I, Ecker A, Nyback S, Louis C, Sinden RE, Billker O. Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Mol. Microbiol. 2006a;60:1355–1363. doi: 10.1111/j.1365-2958.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siden-Kiamos I, Pinder JC, Louis C. Involvement of actin and myosins in Plasmodium berghei ookinete motility. Mol. Biochem. Parasitol. 2006b;150:308–317. doi: 10.1016/j.molbiopara.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Sidjanski SP, Vanderberg JP, Sinnis P. Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum . Mol. Biochem. Parasitol. 1997;90:33–41. doi: 10.1016/s0166-6851(97)00124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Neto MA, Atella GC, Shahabuddin M. Inhibition of Ca2+/ calmodulin-dependent protein kinase blocks morphological differentiation of Plasmodium gallinaceum zygotes to ookinetes. J. Biol. Chem. 2002;277:14085–14091. doi: 10.1074/jbc.M107903200. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Gametocytogenesis of Plasmodium falciparum in vitro: an electron microscopic study. Parasitology. 1982;84:1–11. doi: 10.1017/s003118200005160x. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Molecular interactions between Plasmodium and its insect vectors. Cell. Microbiol. 2002;4:713–724. doi: 10.1046/j.1462-5822.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Butcher GA, Billker O, Fleck SL. Regulation of infectivity of Plasmodium to the mosquito vector. Adv. Parasitol. 1996;38:53–117. doi: 10.1016/s0065-308x(08)60033-0. [DOI] [PubMed] [Google Scholar]
- Srinivasan P, Abraham EG, Ghosh AK, Valenzuela J, Ribeiro JM, Dimopoulos G, Kafatos FC, Adams JH, Fujioka H, Jacobs-Lorena M. Analysis of the Plasmodium and Anopheles transcriptomes during oocyst differentiation. J. Biol. Chem. 2004;279:5581–5587. doi: 10.1074/jbc.M307587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P, Fujioka H, Jacobs-Lorena M. PbCap380, a novel oocyst capsule protein, is essential for malaria parasite survival in the mosquito. Cell. Microbiol. 2008;10:1304–1312. doi: 10.1111/j.1462-5822.2008.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuechel M, Matuschewski K. Role for the Plasmodium sporozoite-specific transmembrane protein S6 in parasite motility and efficient malaria transmission. Cell. Microbiol. 2009;11:279–288. doi: 10.1111/j.1462-5822.2008.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling CR, Aikawa M, Vanderberg JP. The passage of Plasmodium berghei sporozoites through the salivary glands of Anopheles stephensi: an electron microscope study. J. Parasitol. 1973;59:593–605. [PubMed] [Google Scholar]
- Stewart MJ, Vanderberg JP. Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J. Protozool. 1991;38:411–421. doi: 10.1111/j.1550-7408.1991.tb01379.x. [DOI] [PubMed] [Google Scholar]
- Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, Nussenzweig RS, Menard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, McRobert L, Baker DA. Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis. Mol. Microbiol. 2008;69:110–118. doi: 10.1111/j.1365-2958.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton TJ, Kaslow DC. Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol. Biochem. Parasitol. 1999;101:223–227. doi: 10.1016/s0166-6851(99)00066-3. [DOI] [PubMed] [Google Scholar]
- Templeton TJ, Keister DB, Muratova O, Procter JL, Kaslow DC. Adherence of erythrocytes during exflagellation of Plasmodium falciparum microgametes is dependent on erythrocyte surface sialic acid and glycophorins. J. Exp. Med. 1998;187:1599–1609. doi: 10.1084/jem.187.10.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton TJ, Kaslow DC, Fidock DA. Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol. Microbiol. 2000;36:1–9. doi: 10.1046/j.1365-2958.2000.01821.x. [DOI] [PubMed] [Google Scholar]
- Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol. Microbiol. 2005a;58:1253–1263. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- Tewari R, Rathore D, Crisanti A. Motility and infectivity of Plasmodium berghei sporozoites expressing avian Plasmodium gallinaceum circumsporozoite protein. Cell. Microbiol. 2005b;7:699–707. doi: 10.1111/j.1462-5822.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- Thathy V, Fujioka H, Gantt S, Nussenzweig R, Nussenzweig V, Menard R. Levels of circumsporozoite protein in the Plasmodium oocyst determine sporozoite morphology. EMBO J. 2002;21:1586–1596. doi: 10.1093/emboj/21.7.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas AM, Margos G, Dimopoulos G, van Lin LH, de Koning-Ward TF, Sinha R, Lupetti P, Beetsma AL, Rodriguez MC, Karras M, et al. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 2001;20:3975–3983. doi: 10.1093/emboj/20.15.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touray MG, Warburg A, Laughinghouse A, Krettli AU, Miller LH. Developmentally regulated infectivity of malaria sporozoites for mosquito salivary glands and the vertebrate host. J. Exp. Med. 1992;175:1607–1612. doi: 10.1084/jem.175.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueman HE, Raine JD, Florens L, Dessens JT, Mendoza J, Johnson J, Waller CC, Delrieu I, Holders AA, Langhorne J, et al. Functional characterization of an LCCL-lectin domain containing protein family in Plasmodium berghei . J. Parasitol. 2004;90:1062–1071. doi: 10.1645/GE-3368. [DOI] [PubMed] [Google Scholar]
- Tsai YL, Hayward RE, Langer RC, Fidock DA, Vinetz JM. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect. Immun. 2001;69:4048–4054. doi: 10.1128/IAI.69.6.4048-4054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104:153–164. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- Vanderberg JP. Development of infectivity by the Plasmodium berghei sporozoite. J. Parasitol. 1975;61:43–50. [PubMed] [Google Scholar]
- Vega-Rodriguez J, Franke-Fayard B, Dinglasan RR, Janse CJ, Pastrana-Mena R, Waters AP, Coppens I, Rodriguez-Orengo JF, Srinivasan P, Jacobs-Lorena M, Serrano AE. The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog. 2009;5:e1000302. doi: 10.1371/journal.ppat.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernick KD, Fujioka H, Seeley DC, Tandler B, Aikawa M, Miller LH. Plasmodium gallinaceum: a refractory mechanism of ookinete killing in the mosquito, Anopheles gambiae . Exp. Parasitol. 1995;80:583–595. doi: 10.1006/expr.1995.1074. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Lycett G, Siden-Kiamos I, Blass C, Sinden RE, Louis C. Anopheles gambiae laminin interacts with the P25 surface protein of Plasmodium berghei ookinetes. Mol. Biochem. Parasitol. 2001;112:229–237. doi: 10.1016/s0166-6851(00)00371-6. [DOI] [PubMed] [Google Scholar]
- Voordouw M, Anholt B, Taylor P, Hurd H. Rodent malaria-resistant strains of the mosquito, Anopheles gambiae, have slower population growth than susceptible strains. BMC Evol. Biol. 2009;9:76. doi: 10.1186/1471-2148-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Fujioka H, Nussenzweig V. Exit of Plasmodium sporozoites from oocysts is an active process that involves the circumsporozoite protein. PLoS Pathog. 2005a;1:e9. doi: 10.1371/journal.ppat.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Fujioka H, Nussenzweig V. Mutational analysis of the GPI-anchor addition sequence from the circumsporozoite protein of Plasmodium . Cell. Microbiol. 2005b;7:1616–1626. doi: 10.1111/j.1462-5822.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- Wilkins S, Billingsley PF. Partial characterization of oligosaccharides expressed on midgut microvillar glycoproteins of the mosquito, Anopheles stephensi Liston. Insect Biochem. Mol. Biol. 2001;31:937–948. doi: 10.1016/s0965-1748(01)00040-6. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Potocnjak P, Nussenzweig V, Nussenzweig RS. Biosynthesis of Pb44, the protective antigen of sporozoites of Plasmodium berghei . J. Exp. Med. 1981;154:1225–1236. doi: 10.1084/jem.154.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuda M, Sakaida H, Chinzei Y. Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J. Exp. Med. 1999;190:1711–1716. doi: 10.1084/jem.190.11.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuda M, Iwanaga S, Shigenobu S, Mair GR, Janse CJ, Waters AP, Kato T, Kaneko I. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol. Microbiol. 2009;71:1402–1414. doi: 10.1111/j.1365-2958.2009.06609.x. [DOI] [PubMed] [Google Scholar]
- Zieler H, Dvorak JA. Invasion in vitro of mosquito midgut cells by the malaria parasite proceeds by a conserved mechanism and results in death of the invaded midgut cells. Proc. Natl. Acad. Sci. USA. 2000;97:11516–11521. doi: 10.1073/pnas.97.21.11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieler H, Nawrocki JP, Shahabuddin M. Plasmodium gallinaceum ookinetes adhere specifically to the midgut epithelium of Aedes aegypti by interaction with a carbohydrate ligand. J. Exp. Biol. 1999;202:485–495. doi: 10.1242/jeb.202.5.485. [DOI] [PubMed] [Google Scholar]
- Zieler H, Garon CF, Fischer ER, Shahabuddin M. A tubular network associated with the brush-border surface of the Aedes aegypti midgut: implications for pathogen transmission by mosquitoes. J. Exp. Biol. 2000;203:1599–1611. doi: 10.1242/jeb.203.10.1599. [DOI] [PubMed] [Google Scholar]
- Zieler H, Keister DB, Dvorak JA, Ribeiro JMC. A snake venom phospholipase A2 blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J. Exp. Biol. 2001;204:4157–4167. doi: 10.1242/jeb.204.23.4157. [DOI] [PubMed] [Google Scholar]