Abstract
Obesity, defined as body mass index greater than 30, is a leading cause of morbidity and mortality and a financial burden worldwide. Despite significant efforts in the past decade, very few drugs have been successfully developed for the treatment of obese patients. Biological differences between rodents and primates are a major hurdle for translation of anti-obesity strategies either discovered or developed in rodents into effective human therapeutics. Here, we evaluate the ligand-directed peptidomimetic CKGGRAKDC-GG-D(KLAKLAK)2 (henceforth termed adipotide) in obese Old World monkeys. Treatment with adipotide induced targeted apoptosis within blood vessels of white adipose tissue and resulted in rapid weight loss and improved insulin resistance in obese monkeys. Magnetic resonance imaging and dual-energy x-ray absorptiometry confirmed a marked reduction in white adipose tissue. At experimentally determined optimal doses, monkeys from three different species displayed predictable and reversible changes in renal proximal tubule function. Together, these data in primates establish adipotide as a prototype in a new class of candidate drugs that may be useful for treating obesity in humans.
INTRODUCTION
Unless current trends are reversed, the epidemic of human obesity and its associated comorbidities will account for a major fraction of healthcare costs worldwide (1, 2). An international collaborative prospective analysis of nearly 1 million participants concluded that obesity is associated with increased overall and cause-specific mortality in a magnitude roughly equivalent to that of smoking (3). Given that programs to promote life-style changes in diet and exercise have been insufficient to address this chronic human condition, aggressive research of anti-obesity compounds has been pursued by the pharmaceutical and biotechnology industries. However, effective drugs have proven extremely difficult to develop (4). Currently, only two Food and Drug Administration (FDA)–approved drugs for weight loss are available in the United States: the appetite suppressant phentermine and the inhibitor of fat absorption orlistat. Despite the initial popularity of these drugs, placebo-subtracted weight losses are actually quite limited, and concerns over side effects continue to limit their use (5–8).
Conventional pharmacologic treatment of obesity relies on central nervous system (CNS) and/or peripheral metabolic mechanisms to suppress appetite and elevate energy expenditure (6, 9, 10). Many of the recently developed candidate anti-obesity drugs are centrally acting and have been associated with serious adverse events that include unanticipated cardiovascular, pulmonary, and neuropsychiatric toxicity. Moreover, sibutramine has been withdrawn from the U.S. market (8, 11, 12), and the FDA has withheld the approval of three highly anticipated anti-obesity agents because of various safety concerns. Given this conservative approach of regulatory agencies to approving pharmaceutical agents directed at the CNS, it is increasingly clear that new approaches for inducing weight loss will be essential for developing drugs to successfully treat human obesity.
In pivotal work, Rupnick and colleagues demonstrated that adipose tissue mass can be regulated through the vasculature in obese mice (13). These findings provided a conceptual framework for the use of angiogenesis inhibitors as drug candidates for weight loss. Subsequently, in mouse models, functional roles for progenitor cells of white adipose tissue within the vascular niche have been found in normal fat tissue (14) and experimental tumors (15), suggesting a cellular contribution to angiogenesis in obesity.
In previous work, our group and others reported obesity reversal by targeted induction of apoptosis in blood vessels supplying white adipose tissue in obese mice (16) and rats (17). A combinatorial phage display random peptide library selection in vivo yielded a cyclic motif (sequence CKGGRAKDC) that selectively targets endothelial cell surface expression of the receptor prohibitin within the vasculature of white adipose tissue (16). This prohibitin-binding motif was chemically fused to the d-enantiomer D(KLAKLAK)2 sequence, an amphipathic peptidomimetic that disrupts mitochondrial membranes upon receptor-mediated cell internalization and causes targeted apoptosis (16–23). Specifically, the peptidomimetic CKGGRAKDC-GG-D(KLAKLAK)2, hereafter referred to as adipotide, targeted the vasculature of white adipose tissue and resulted in ~30% weight reduction in obese mice over a period of 4 weeks (16). Therefore, unlike nonspecific angiogenesis inhibitors, adipotide is systemically targeted to the endothelium of fat through a ligand-directed mechanism and disrupts the vascular supply of white adipose tissue, at least in rodent obesity models (16, 17). Moreover, an annexin A2–prohibitin receptor system targeted by adipotide has been reported recently in the white adipose tissue vasculature of human patients (24).
The failure of standard research in rodents to model human obesity has been a major hurdle to the discovery, development, and approval of new anti-obesity compounds. Indeed, biological differences limit the relevance of data derived from rodent studies that can be translated into drugs against human obesity (25). Particularly problematic research areas include the differential functions of certain adipokines (26) and peptide hormones (27), responses to neurotransmitter stimuli (28), and differences in circadian rhythms and/or feeding behaviors (29) between rodents and primates, including humans.
To address these challenges, we evaluated whether the marked response to adipotide observed in rodent models of obesity (16, 17) could be recapitulated in obese nonhuman primates. In contrast to other mammalian species (for example, mice, rats, rabbits, and dogs), humans and Old World monkeys share considerable physiological features such as the metabolic response to lipolysis in white fat adipocytes (28) and pathological obesity-related conditions such as the development of cardiovascular disease and insulin resistance or even overt type 2 diabetes mellitus (30). Here, we analyzed three species of Old World monkeys: rhesus macaques, baboons, and cynomolgus macaques.
RESULTS
Therapeutic dose-finding study of adipotide in a nonhuman primate model of obesity
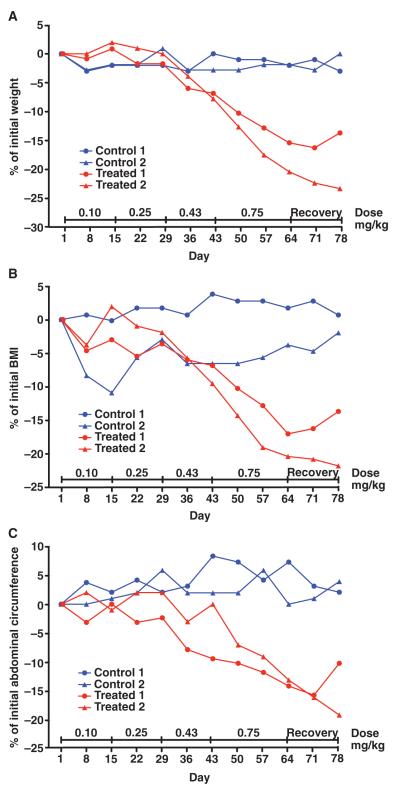
We first used spontaneously obese rhesus macaques (Macaca mulatta), one of the most relevant primate models for human obesity and its associated comorbidities. In an initial dose-finding study, female rhesus macaques (n = 4) aged 9 to 13 years were selected for study entry on the basis of a history of spontaneous obesity, increased food consumption, and failure to maintain a high level of activity. All animals were defined as obese by a body mass index (BMI) ranging from 34 to 45 according to the equivalent formula for rhesus macaques (31). Body weights ranged from 10.0 to 11.7 kg, and the heaviest monkey had a morbidly obese body habitus (BMI = 45). All monkeys had the most clinically abundant abdominal fat in a central pattern relative to that of a large cohort of adult females (n = 350) in the breeding colony. Individual monkeys were euglycemic, with fasting serum glucose levels ranging from 65 to 71 mg/dl (normal range, <80 mg/dl) and serum insulin levels ranging from 21 to 52 μU/ml (normal range, <41 μU/ml). Adipotide was prepared under Good Manufacturing Practice (GMP) to our specifications by PolyPeptide Laboratories. Daily subcutaneous treatment with increasing doses of adipotide resulted in a dose-dependent decrease in body weight, BMI, and abdominal circumference relative to that of negative control monkeys receiving saline (Fig. 1, A to C). After dosing for 9 weeks, the body weight of the two treated monkeys in this initial cohort decreased by 1.8 kg (15.4%) and 2.1 kg (20.4%; Fig. 1A). Moreover, the BMI decreased from 45.0 to 37.3 (17.1%) in the morbidly obese monkey and from 38.8 to 30.9 (20.4%) in the second treated monkey (Fig. 1B). Finally, abdominal circumferences were reduced by 6.5 cm (13.1%) and 9.0 cm (14.2%) from baseline (pretreatment) measurements (Fig. 1C). During the same time period, the body weight, BMI, and abdominal circumference of the obese control monkeys receiving only saline did not change.
Fig. 1.
Anthropometric assessment of four obese rhesus monkeys treated with increasing doses of adipotide (0.10, 0.25, 0.43, and 0.75 mg/kg). (A to C) Obese rhesus monkeys displayed improved anthropometric measurements in a time- and dose-dependent manner. The percentage change from baseline for body weight (A), BMI (B), and abdominal circumference (C) was calculated weekly throughout dosing and recovery. Dose-dependent decreases occurred for these parameters in obese monkeys receiving adipotide; in contrast, no changes were observed in monkeys receiving saline. After a 2-week recovery period, the decrease in body weight, abdominal circumference, and BMI began to reverse.
At the baseline evaluation, the morbidly obese monkey was deemed insulin-resistant on the basis of an intravenous glucose tolerance test (IVGTT); 60 min after the initial administration of glucose, the serum glucose level remained mildly elevated at 100 mg/dl (fig. S1A). The other three obese monkeys displayed relatively normal serum insulin responses to glucose administration (fig. S1, C, E, and G). A second IVGTT was performed 3 days after the final administration of adipotide. The area under the curve (AUC) for insulin decreased 61.4% and 63.5% in the two monkeys treated with adipotide (fig. S1 and table S1). These findings indicated a considerable decrease in insulin resistance based on the reduced level of insulin required to respond to acute doses of intravenous glucose.
Efficacy of adipotide at the therapeutic dose in a nonhuman primate model of obesity
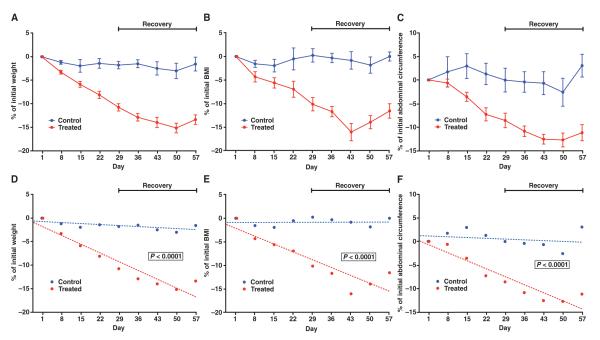
Based on the dose-finding study, we determined the optimal subcutaneous dose of adipotide (0.43 mg/kg). Subsequently, we performed a second study on a larger cohort (n = 15 obese rhesus macaques; 5 controls and 10 treated) to evaluate the effect of the optimal fixed dose of adipotide daily for 4 weeks, followed by a 4-week recovery period. At the end of the treatment interval, the weight of the control monkeys had changed from +1.0 to −3.5% from pretreatment body weight, whereas the monkeys receiving the therapy displayed marked weight loss that ranged from −7.4 to −14.7% of pretreatment body weight (Fig. 2A). The BMI of the monkeys in the treatment group decreased at the end of treatment compared to pretreatment by −3.7 to −17.3%, whereas the control group exhibited a −3.5 to +3.3% BMI change (Fig. 2B). At the end of the treatment period, the abdominal circumference had decreased by −6.5 to −14.3% in 9 of 10 monkeys in the treated group; a single monkey displayed a 2% increase in abdominal circumference. In comparison, the change in abdominal circumference of the control group ranged from −6.3 to +6.9% (Fig. 2C). Body weight, BMI, and abdominal circumference continued to decrease for an additional 3 weeks after cessation of adipotide treatment. The change in these three variables over time from the start of treatment through the end of the recovery period was statistically significant [mixed-effects model (32), P < 0.0001 for each variable] between the treated and the control groups (Fig. 2, D to F).
Fig. 2.
Anthropometric assessment of obese rhesus monkeys in a fixed-dose (0.43 mg/kg, subcutaneous daily) study of adipotide. (A to C) The average percentage change from baseline for body weight, abdominal circumference, and BMI was calculated weekly throughout the treatment (28 days) and recovery (28 days) intervals for each animal that received adipotide or saline. In the treatment group, we observed marked decreases in (A) the average body weight (10.6%), (B) BMI (10.0%), and (C) abdominal circumference (8.4%) relative to the baseline measurements. Error bars indicate SEM (control, n = 5; treated, n = 10). (D to F) These findings were statistically significant (mixed-effects model, P < 0.0001 for each variable). During a 4-week recovery period, the decrease in body weight, abdominal circumference, and BMI began to slowly reverse.
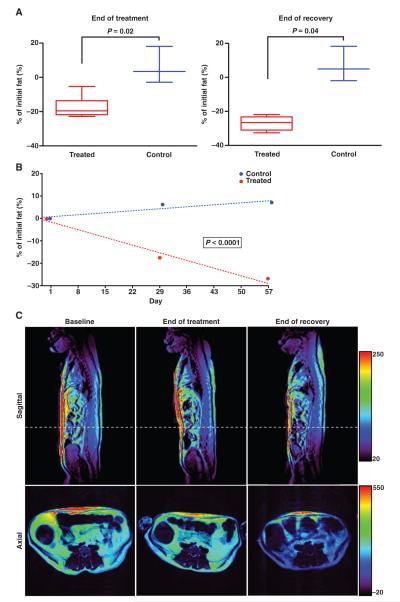
Adipotide-treated animals displayed a specific reduction in total body fat on the basis of dual-energy x-ray absorptiometry (DEXA) scans, performed weekly. Total body fat decreased throughout the treatment and recovery periods. At the end of the study, the average percentage decrease for the treated monkeys was 38.7% compared to 14.8% for the control animals (Fig. 3A). This change over time was statistically significant (mixed-effects model, P < 0.0001) between the treatment and the control groups (Fig. 3B). The average volume of white adipose tissue in the abdominal region was also determined using axial T1-weighted magnetic resonance imaging (MRI), which showed a decrease from baseline to the end of the treatment (17.5%) and recovery period (27.0%) for animals receiving adipotide (Fig. 4A). A comparison of the percentage change between the treated and the control groups was statistically significant (Mann-Whitney-Wilcoxon test; end of treatment, P = 0.02; end of recovery, P = 0.04, Fig. 4A), as was the change over time between the groups (mixed-effects model, P < 0.0001; Fig. 4B). A decrease in the color window level for both the axial and the sagittal MRI sections delineated the loss of both visceral and subcutaneous white adipose tissue in the abdominal region (Fig. 4C).
Fig. 3.
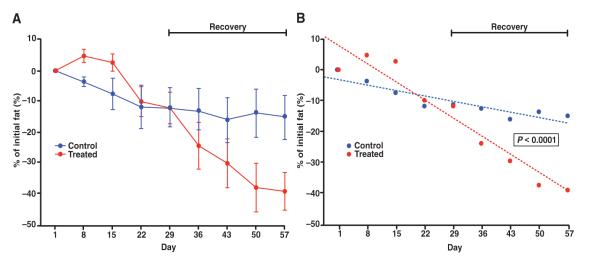
DEXA imaging confirms a loss in total body fat percentage. Whole-body DEXA scans were obtained weekly throughout dosing and recovery for a subset of animals in the control (n = 3) and treatment groups (n = 6). (A) From the start of the study to the end of the recovery period, the percentage of total body fat decreased by 38.7 and 14.8% in animals receiving adipotide and saline, respectively. Error bars indicate SEM. (B) This change over time was statistically significant (mixed-effects model, P < 0.0001).
Fig. 4.
MRI confirms that weight loss results from a marked decrease in the volume of white adipose tissue. (A) The percentage change in fat volume was determined by quantifying the volume with axial T1-weighted MR images. The change is represented as percentage change from baseline (day 1) and is significantly decreased at the end of treatment and at the end of recovery (Mann-Whitney-Wilcoxon test, P = 0.02 and P = 0.04, respectively). Error bars indicate SEM (control, n = 3; treated, n = 6). (B) A mixed-effects model of the data over time indicates significance in the decreasing fat percentage for the treated versus control groups (P < 0.0001). (C) Representative sagittal and axial T1-weighted images from one of the treated animals. The window level range is indicated by the color bar on the right. Axial images are taken at the cross section indicated by the white dashed line in the sagittal image. A decrease in fat content is represented by a decrease in window level (that is, intensity of the image display).
Baseline and post-treatment IVGTT were also performed on this cohort to individually assess the metabolic status of each animal before the start of the study. Fasting blood glucose was normal, and the post-glucose load glucose response was also normal for monkeys in both control and treatment groups (fig. S2), indicating that the obese monkeys did not have overt diabetes. A repeat IVGTT in the treated and control monkeys revealed minimal change in fasting blood glucose or the post-glucose load glucose response in either group (fig. S2). Basal insulin was elevated before treatment in both groups (fig. S2). Treatment with adipotide resulted in a reduced insulin response (fig. S2, A to J), whereas control monkeys did not display a change in their insulin profile (fig. S2, K to O). As in the dose-finding study, monkeys treated with adipotide showed a considerable decrease in the AUC for insulin at the end of the treatment interval (Fig. 5A). The average AUC from baseline to end of treatment decreased by 36.2% in monkeys receiving adipotide compared to control animals (Fig. 5A and table S2). This change was significant (Mann-Whitney-Wilcoxon, P = 0.019) and indicated an improvement in insulin sensitivity.
Fig. 5.
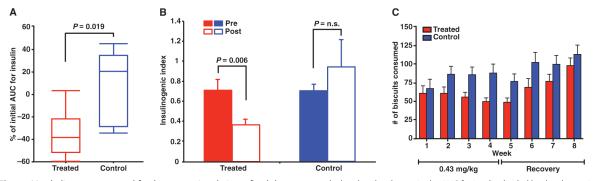
Metabolic assessment and food consumption during a fixed-dose study of adipotide. (A) The average AUC for insulin was calculated from an IVGTT performed within 7 days of the initial dose (0.43 mg/kg) and 24 hours after the final dose (0.43 mg/kg) of adipotide. Animals receiving adipotide showed a statistically significant decrease in AUC compared to monkeys in the control groups (Mann-Whitney-Wilcoxon, P = 0.019). Error bars indicate SEM (control, n = 5; treated, n = 10). (B) The insulinogenic index from an IVGTT was calculated as the change in the AUC for insulin divided by the change in AUC for glucose (paired t test, P = 0.006). Error bars indicate SEM (fixed-dose study: control, n = 5; treated, n = 10; n.s., not significant). (C) Relationship between biscuit consumption and dosing. Consumption decreased with continued dosing and appeared to increase after cessation of drug administration in the treated groups; consumption remained relatively constant throughout the studies in the control groups (control, n = 5; treated, n = 10).
Insulin resistance is an elevated insulin level in the presence of normal blood glucose, which can be quantified by the insulinogenic index (33). In the fixed-dose study, we found that the baseline insulinogenic index was equally elevated in both groups. Adipotide treatment lowered the average insulinogenic index by 48.5%, whereas the average index for the control group increased by 33.8%. This finding indicated a significant improvement in insulin resistance in the treated animals (Fig. 5B; paired t test, P = 0.006).
Monkeys receiving adipotide remained bright and alert throughout both experiments. They maintained a consistent level of activity, interacted with their caretakers appropriately, and presented no overt clinical signs of nausea, vomiting, or food aversion. Monkeys were fed a specified amount of commercially produced primate biscuits and enrichment supplements, and the amount of each food type consumed was documented daily. All treated and control monkeys consumed nearly all of their enrichment supplements throughout the treatment interval; in contrast, the number of monkey chow biscuits consumed by treated monkeys decreased in an inversely proportional manner with the dose of adipotide administered and/or the amount of weight lost over time (Fig. 5C and fig. S3).
To investigate the possibility of dyslipidemia, we determined serum levels of cholesterol, triglyceride, high-density lipoprotein, and low-density lipoprotein at weekly intervals throughout the treatment and recovery periods for obese rhesus monkeys in the fixed-dose study. All animals receiving either adipotide or saline control had normal lipid profiles that remained unchanged throughout the entire study period (table S3). Furthermore, a comprehensive profile of serum-free fatty acids (table S4) was completed for selected monkeys in the treated (n = 6) and control (n = 3) groups. Serum samples were collected at baseline and then every 2 weeks until the end of the recovery period. Free fatty acid levels showed a decreasing trend throughout the treatment interval that reversed during the recovery period (fig. S4A). This change over time was statistically significant (mixed-effects model, P = 0.05; fig. S4B).
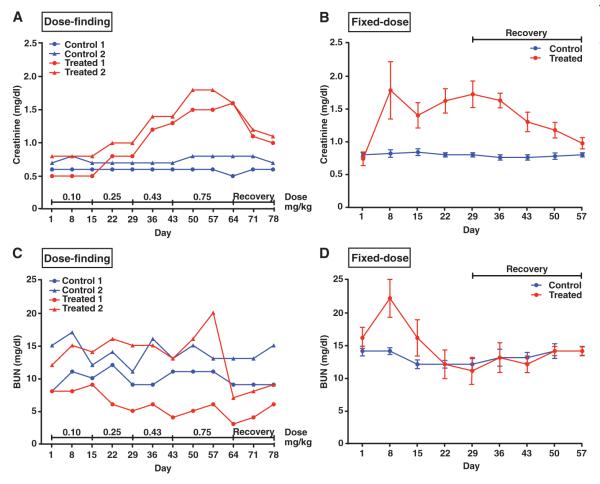
Clinical observations, serum chemistry, and urinalysis data from these studies are detailed in the Supplementary Material (tables S5 to S7). Adipotide-treated monkeys produced increased quantities of urine, and mild dehydration was noted at the highest dose levels (table S5). Similar clinicopathological alterations were identified in rhesus monkeys receiving adipotide for both the initial dose-finding study and the subsequent fixed-dose efficacy study. Slight-to-moderate dose-dependent elevations in serum creatinine were observed during subcutaneous administration of doses greater than 0.25 mg/kg (Fig. 6, A and B). Notably, concurrent elevations in blood urea nitrogen (BUN) throughout dosing were not observed in the same animals (Fig. 6, C and D). Serum phosphorus and serum potassium displayed mild-to-moderate time- and dose-dependent decreases. Consistent dose-dependent changes in urine included mild-to-marked glucosuria, mild-to-moderate proteinuria, and a slight-to-mild increase in transitional/renal epithelial cells. Most alterations in serum and urine were reversed within 28 days upon discontinuation of treatment with adipotide. After the predetermined recovery period in each experiment, clinicopathological changes were generally not noted, except for a slight residual elevation in serum creatinine and urinary glucose in one treated monkey from each study at the end of the recovery period.
Fig. 6.
Renal dysfunction is mild, dose-dependent, and reversible. (A) Serum creatinine was measured in four rhesus macaques used for the initial dose-finding study. There was a dose-dependent increase in serum creatinine in monkeys receiving adipotide that reversed after discontinuation of adipotide. (B) In the fixed-dose study, serum creatinine remained slightly to moderately elevated throughout the dosing interval and gradually returned to baseline during the recovery period (control, n = 5; treated, n = 10). (C) In contrast, BUN remained unchanged or decreased throughout dosing for the four monkeys receiving adipotide in the initial dose-finding study. (D) In the fixed-dose study, an initial elevation in BUN on day 8 was followed by a steady decline to a level below baseline by day 22 and an eventual return to baseline by the end of the recovery period. Creatinine and BUN remained unchanged throughout both studies in the control groups. Error bars indicate SEM (fixed-dose study: control, n = 5; treated, n = 10). Using a mixed-effects model to compare the change in BUN and creatinine over time (from day 1 to day 57) between the control and treated group, we found that only BUN reached significance (P = 0.001).
Toxicology studies of adipotide in lean rhesus monkeys
In addition to the information obtained in the above efficacy studies in obese rhesus monkeys, we also evaluated the safety profile of adipotide in a formal Good Laboratory Practices (GLP) study in lean rhesus monkeys. A cohort of monkeys (n = 15) received three dose levels of adipotide (0.25, 0.43, and 0.75 mg/kg) daily for 28 days. Lean rhesus monkeys receiving adipotide (0.25 and 0.43 mg/kg) did not lose weight (fig. S5) Monkeys in the highest dose group either maintained their prestudy weight or displayed mild weight loss (fig. S5). A slight-to-moderate elevation in creatinine, which increased throughout the dosing interval, was seen in the high-dose (0.75 mg/kg) but not the low-dose (0.25 mg/kg) group. Most of the monkeys receiving the therapeutic dose (0.43 mg/kg) maintained normal creatinine concentrations, whereas slight-to-moderate elevations were noted sporadically in two animals (fig. S6A). Creatinine was significantly different between both the low- and the middle-dose groups and the high-dose group only on day 22 (Kruskal-Wallis, post hoc multiple comparisons, Bonferroni correction, P < 0.05). In the high-dose group, serum creatinine levels peaked at the end of the treatment interval, decreased steadily throughout the recovery period, and remained slightly elevated at the end of the recovery period (fig. S6A). Notably, concurrent elevations in BUN from baseline were not observed, even in the high-dose adipotide–treated group (fig. S6B) Alterations in serum phosphorus, serum potassium, and the urine were similar to those described for the initial efficacy studies.
In monkeys necropsied 24 hours after the final dose of adipotide, lesions associated with the kidney were observed and were found to be dose-dependent; such lesions were not present in the control group (Fig. 7A). The observed lesions were scored minimal to mild in the low-dose group, minimal to mild in most of the middle-dose monkeys, and minimal to moderate in the high-dose group. The primary lesions were classified as degenerative/necrotic (single-cell necrosis) and reactive/regenerative (Fig. 7B). In monkeys necropsied at the end of the recovery period, minimal tubular degeneration with few degenerate cells was observed in one monkey in the middle-dose group and in two monkeys in the high-dose group. Tubular regeneration and tubular necrosis (single cell with few necrotic cells) were minimal in all monkeys after recovery (Fig. 7C). Thus, the primary side effect of adipotide is relatively mild, predictable, and reversible renal injury and altered tubular function. Abnormal lipid accumulation (including hepatic steatosis) was not noted in any of the monkeys that received adipotide.
Fig. 7.
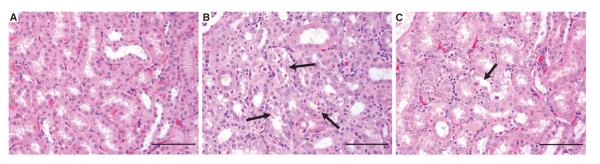
Representative kidney images from control and high-dose monkeys from the 28-day repeat-dose safety study of adipotide. (A) Histological evaluation of kidney sections indicated normal kidney tubules in the control animal. (B) Twenty-four hours after the final high dose (0.75 mg/kg) of adipotide, renal tubules frequently display mild tubular degeneration, moderate regeneration, and single-cell necrosis (arrows). (C) At the end of the high-dose recovery period, minimal to mild tubular degeneration and regeneration were infrequently noted (arrow). Scale bars, 200 μm.
Studies performed in other Old World monkey species
Additional preclinical safety studies have been performed on a large cohort (n = 52) of cynomolgus macaques (Macaca fascicularis), a third species of Old World monkey, for the class of targeted D(KLAKLAK)2-containing candidate drugs (including adipotide). Although detailed single- and multiple-dose toxicology studies will be published elsewhere, here, we report that dose-dependent toxicity in monkeys receiving single doses of adipotide up to 100 mg/kg (~133-fold of the corresponding therapeutic dose) did not result in lethality.
Finally, an additional efficacy study was performed in baboons (Papio sp.), a second Old World monkey species and also an important model of human obesity (34). Preliminary data from a small baboon cohort (n = 2) in a dose-finding noncontrolled pilot study produced a similar magnitude of weight loss after 12 weeks of daily adipotide subcutaneous administration (fig. S7). Results of this study were used to assess scaling of dosage to larger primates, as discussed below.
DISCUSSION
On the basis of a combined assessment of efficacy and toxicity at the four doses tested in our initial dose-finding study, we identified a daily subcutaneous injection of adipotide (0.43 mg/kg) as the optimal therapeutic dose in spontaneously obese rhesus macaques. In translating the dosage of adipotide from previous mouse and rat studies to obese rhesus macaques and baboons, we found that the empirically determined optimal therapeutic daily dose of adipotide scaled with the changes in body surface area (BSA) between species, as expected. These results are supportive of a relative dose migration of adipotide from smaller to larger mammalian species, including humans, based on BSA (fig. S8).
The results of this dose-finding study confirmed that adipotide treatment resulted in marked weight loss in rhesus monkeys, similar to previous studies performed in mice and rats (16, 17). In addition, the monkeys showed evidence of improved metabolic status in the form of reduced insulin resistance. The hallmark of insulin resistance is an elevated insulin level in the presence of normal blood glucose, which can be quantified by the insulinogenic index (33). In the fixed-dose study, we found that the baseline insulinogenic index was equally elevated in both groups of rhesus macaques. Adipotide treatment significantly lowered the average insulinogenic index. Improved serum insulin responses without concurrent changes in blood glucose clearance are documented in people who lose body fat in response to medication or physical exercise (35, 36). Here, we show that the loss of body fat in response to adipotide treatment appears to produce a similar effect in obese, nondiabetic, insulin-resistant monkeys.
Whereas a detailed mechanism of action for adipotide treatment remains to be fully elucidated, we observed that weight loss in obese rhesus monkeys occurred concurrently with a reduction in food intake. Strikingly, lean rhesus monkeys receiving a therapeutic dose of adipotide did not lose weight (fig. S5). This finding suggests that the mechanism of action may be obesity-specific in primates. Metabolic rate could not be assessed in the current primate study, and further behavioral and metabolic studies will be required to determine whether the cause of decreased food consumption was related to improved satiety, decreased demand for nutrients adjusted for relative weight loss, or a combination of white fat endothelium–mediated mechanisms, ultimately leading to reduced food intake plus increase in energy expenditure. Although the possibility of mild nausea as a contributor to overall weight loss cannot be completely ruled out in this primate model, the selective change in food intake observed (reduction in biscuits, but most enrichment foods still consumed) supports the conclusion that any appetite reduction in response to adipotide treatment occurred because of enhanced satiety.
Previously, paired-feeding experiments in obese rodents (16, 17) also indicated decreased food intake as a possible contributor to weight loss in mice and rats treated with adipotide. It has been demonstrated (17, 37) that targeting endothelial cells of white adipose tissue in obese rats triggers a feedback signal from fat to the hypothalamus (or elsewhere in the CNS) to curb appetite; however, this presumed chemical signal along with a potential role for adipose stem cells in the vascular niche (14, 15) are still unknown. Notably, treatment with adipotide caused a resorption of established white adipose tissue but not the expected adaptive response of a decreased metabolic rate; on the contrary, the metabolic rate in treated obese rodents in previous studies was unchanged or even slightly increased (16, 17).
Adipotide treatment was generally well tolerated in all three species of nonhuman primates tested. The primary side effect, renal toxicity, was generally mild and reversible. Decreased serum phosphorus and potassium and urinary changes that included mild-to-marked glucosuria, mild-to-moderate proteinuria, and a slight-to-mild increase in transitional/renal epithelial cells were noted throughout the dosing interval. These findings, which were indicative of altered proximal tubular function and tubular injury, resolved during the recovery period. Clinical evidence of dehydration at the highest dose tested is suggestive of a decreased glomerular filtration rate (GFR); however, a reduction in GFR is usually accompanied by an elevation of both BUN and serum creatinine. Given the elevation of serum creatinine without a concurrent and commensurate increase in BUN in obese monkeys treated with adipotide, we considered other potential mechanisms such as increased loss of urea and/or decreased reabsorption of creatinine in the proximal tubule. The modulation of creatinine transport in the proximal tubule before overt renal toxicity after administration of certain drugs or imaging agents has been well established (38). Thus, one cannot rule out the possibility that cleared adipotide might serve as a substrate for the cation transport system in the proximal tubule and would competitively inhibit the active tubular secretion of creatinine. This question notwithstanding, our findings are also consistent with nephrotoxicity reports identifying renal D-amino acid oxidase as the only known mammalian enzyme that can use the D(KLAKLAK)2 moiety of adipotide as a substrate (39); thus, this reaction might be the rate-limiting catabolic step of adipotide in the kidney.
MRI and DEXA imaging confirmed that weight loss in the rhesus monkeys occurred primarily because of visceral fat loss. Given the rapid loss of adipose tissue over a short period of time, one might have expected an abnormal increase in serum-free fatty acids and possibly dyslipidemia because of rapid fat mobilization. Surprisingly, free fatty acid concentrations showed a decreasing trend throughout the treatment interval (fig. S4A), consistent with the normal metabolic processing of excess adipose tissue. Similar trends were noted for monounsaturated fatty acids as well as many polyunsaturated fatty acids. It is tempting to speculate that in addition to the loss of white adipose tissue, the decrease in circulating free fatty acids may have contributed to the amelioration in insulin resistance in animals treated with adipotide (40). However, further studies are needed to explore changes observed for each individual fatty acid moiety.
With the rapid reduction in white adipose tissue observed, the possibility of abnormal fecal elimination of lipids was considered. For drugs that inhibit intestinal absorption of fat from food, excess fecal lipids have resulted in undesirable side effects that include oily stools, flatulence, fecal incontinence, and diarrhea. Whereas fecal lipids were not directly measured in our nonhuman primate studies, the absence of these side effects was considered inconsistent with increased fecal elimination of lipids after adipotide administration.
In summary, we demonstrate through anthropometric measurements and imaging that adipotide rapidly induces weight loss that is attributable to a marked reduction in the volume of white adipose tissue in obese nonhuman primates. The weight loss is also accompanied by a modest reduction in serum-free fatty acids and an improvement in insulin resistance. The primary side effect is relatively mild, predictable, and reversible renal injury and altered tubular function, demonstrating that the efficacy of adipotide in highly relevant primate models of human obesity suggests that it is a promising candidate drug for translation into clinical applications. Moreover, given the potential for direct discovery of new ligand peptides homing to the vasculature of white adipose tissue in patients (41) and improved mitochondria-disrupting peptides (42) or delivery systems (43), other targeted anti-obesity drugs from the adipotide class can be designed, including those with a broader therapeutic index, on the basis of the discovery of a new human ligand receptor in white adipose tissue (24). These approaches may facilitate the clinical development of adipotide for treating human obesity.
MATERIALS AND METHODS
Subjects
Male and female adult rhesus macaques (n = 39) were housed at the Michale E. Keeling Center for Comparative Medicine and Research (KCCMR), an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)–accredited veterinary facility at the University of Texas M. D. Anderson Cancer Center (UTMDACC). In caring for the monkeys, the trained animal handlers followed the National Research Council’s Guide for the Care and Use of Laboratory Animals (44). The monkeys were singly housed in a temperature- and humidity-controlled environment with fixed 12-hour light cycles.
The monkeys were enrolled in the standard environmental enrichment program at KCCMR. Obese rhesus monkeys had body weight ranging from 8.0 to 16.7 kg and BMI ranging from 30.2 to 53.3 kg/m2. The animals were fed a commercial diet consisting of 28% protein, 12% fat, and 59% carbohydrate (LabDiet, Purina Mills), and water was available ad libitum. In addition, the animals received fresh produce and food enrichment (for example, assorted vegetables, fruits, and nuts) in accordance with the nutritional program at KCCMR. All rhesus monkeys were negative for simian retrovirus, simian immunodeficiency virus, simian T cell lymphotropic virus, and tuberculosis.
Two obese female baboons (age >8 but <17 years old) were selected from a large breeding colony at the Southwest Foundation for Biomedical Research (SFBR) for a 6-week acclimation period in single cages. Baseline body weights were 24.1 and 27.6 kg. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee either at UTMDACC or at the SFBR.
Study design
Three species of Old World nonhuman primates were used to evaluate the efficacy and safety profile of adipotide: rhesus macaques, baboons, and cynomolgus monkeys. After the initial proof-of-concept study in two obese baboons, a dose-finding study in obese rhesus macaques was initiated to determine the therapeutic dose of adipotide in obese rhesus macaques followed by a 2-week recovery period. Two animals each were randomly assigned to a control and treatment group that received either saline or adipotide produced under GMP. A standard formula was used to estimate the therapeutic window of adipotide for obese monkeys based on the established dose in rodents (45). From a mathematical conversion, 0.10, 0.25, 0.43, and 0.75 mg/kg were used in the initial dose-finding study of adipotide; doses were increased incrementally every 2 weeks, with the final dose level administered for 3 weeks (that is, daily subcutaneous administration for 63 consecutive days). Throughout the treatment and recovery period, body weights, BMI, and a complete panel of anthropometric measurements that included crown-rump length, abdominal circumference, chest circumference, upper and lower abdominal skin fold thickness, thigh circumference, triceps skin fold and scapular skin fold thickness, and upper arm circumference were determined weekly. In addition, blood for a complete blood count, chemistry profile and coagulation panel, and urine for a urinalysis were collected weekly throughout the treatment and recovery period.
For the fixed-dose study, a cohort of 15 animals was randomized into control (n = 5) or treatment (n = 10) groups. The BMI of the selected monkeys ranged from 30 to 52. Treated animals received adipotide (0.43 mg/kg) for 28 consecutive days followed by a 4-week recovery period; control animals received saline. Animals were not anesthetized for the dosing procedure, and subcutaneous injection sites were rotated among four distinct trunk locations to avoid or minimize skin irritation. To keep the volumes relatively constant (that is, 150 to 300 μl), we progressively adjusted the concentration of adipotide in the treatment interval accordingly. Body weight, crown-rump length, and abdominal circumference were determined, and blood for a complete blood count, chemistry profile, coagulation panel, and a fatty acid panel, and urine for a urinalysis were collected weekly throughout the dosing and recovery period. In addition, a DEXA scan was performed weekly and an MRI was performed immediately before treatment, 24 hours after the final dose, and at the end of the recovery period. During the recovery period, a single animal in the treatment group became obstipated because of overgrooming plus trichophagia. Although the condition was medically resolved, the food intake decreased and mild dehydration ensued. Because the treating veterinarians deemed this condition as not a direct effect of the test article, the animal was removed from study for the duration of the recovery period and no data were included in the analysis.
To assess the safety of adipotide in nonhuman primates, we conducted a multidose toxicity study in rhesus macaques (n = 20) in accordance with the Code of Federal Regulations Title 21 Part 58 Good Laboratory Practice for Non-Clinical Laboratory Studies (46). Briefly, the study consisted of four dose groups each containing five monkeys: control, low (0.25 mg/kg), middle (0.43 mg/kg), and high (0.75 mg/kg) dose. All monkeys received 28 consecutive daily injections of adipotide or saline control. Three monkeys in each group were humanely euthanized 24 hours after the final administration and two were humanely euthanized after a 4-week recovery period.
MRI and DEXA
Animals were sedated with a combination of tiletamine plus zolazepam (3 to 5 mg/kg, intramuscular) or with ketamine (5 to 10 mg/kg, intramuscular) alone for the imaging procedures. If necessary, diazepam (5 mg/kg, intravenous) was used to reduce movement. In both scanners, animals were positioned in custom-made foam holders that resembled the normal shape of a rhesus monkey. The purpose of the holder was to maintain a consistent body position for the MRI scanners and to provide thermal support within the unit. Heart rate and oxygen saturation were monitored with pulse oximetry. All procedures were performed under the direction of a clinical veterinarian.
Body composition estimates were performed by DEXA on a GE Lunar Prodigy Pro (GE Lunar Corporation). Encore software (version 12.20.023) provided fat tissue and lean tissue estimates both in total grams and as a percentage of body weight. The Prodigy Pro is a narrow-angle (4.5°) transversely scanning fan beam densitometer that uses a cadmium–zinc telluride detector to directly convert x-rays into an electronic signal. The DEXA machine was calibrated against a standard calibration block supplied by the manufacturer. The instrument was set to a standard scan depth based on the thickness of the rhesus monkeys. Total body scans were performed on the monkey positioned supine with the midline of the body centered on the table within the foam holder with elapsed scan times of 2 to 3 min.
Quantitative body fat measurement was performed on a General Electric 1.5-T whole-body MRI scanner operating under the 9.1 software platform (GE Healthcare Technologies). After sedation, each animal was positioned supine, feet first, and in a plastic foam holder that was placed on top of the scanner table. A four-channel torso-phased array coil was used for signal reception without repositioning the animals. Spatial coverage from shoulder to knee was achieved in axial plane in four to five body stations (depending on the height of an animal) and in coronal plane in two body stations. For axial plane and each body station, T1-weighted images with and without water suppression were acquired with a fast spin-echo sequence and with the following scan parameters: repetition time = 500 to 550 ms, echo time = 10 ms, echo train length = 3, receiver bandwidth = ±21 kHz, acquisition matrix = 256 × 192, field of view ≈ 28 cm, slice thickness/gap = 5 mm/0 mm. The scan time for a total of 24 slices per body station was 0:52 min for acquiring images without water suppression and 1:23 min for acquiring images with water suppression. For coronal plane, only T1-weighted images with water suppression were acquired and the scan parameters were similar to those used for acquiring the corresponding axial images.
The percentage change in fat volume was determined by quantifying the volume with axial T1-weighted MR images. For each animal and time point, the five slices distal and proximal to the umbilical slice were selected and analyzed with the grow region tool as implemented in OsiriX 3.8.1 (47). The change was calculated as percentage change from baseline (day 1). Representative sagittal and axial T1-weighted images from one of the treated animals were pseudocolor with the Color Look-Up Table “Rainbow 2” as implemented in OsiriX. Scale, window levels (WLs), and window widths (WWs) were kept consistent in each image (sagittal: WL = 135, WW = 230; axial: WL = 265, WW = 570).
Supplementary Material
Acknowledgments
We thank C. R. Abee, F. I. Staquicini, and C. S. van Pelt for discussions. Additional details of the GLP toxicology studies discussed in this paper are available from the authors upon request. Funding: This work was supported by NIH, AngelWorks, the Gillson-Longenbaugh Foundation, the Kleberg Foundation, the Marcus Foundation, the Prostate Cancer Foundation, and the National Cancer Institute.
Footnotes
Author contributions: K.F.B., D.R.C., P.W.H., W.H.P.D., B.J.B., W.B.B., S.W., M.T., J.M., M.G.K., P.K.S., K.-A.D., J.F.H., J.G.G., L.C., W.A., and R.P. participated in the design and/or interpretation of the reported experiments or results. K.F.B., D.R.C., P.W.H., W.H.P.D., W.B.B., S.W., M.T., J.M., M.G.K., P.K.S., K.-A.D., J.F.H., J.G.G., L.C., W.A., and R.P. participated in the acquisition and/or analysis of data. K.F.B., D.R.C., P.W.H., W.H.P.D., W.B.B., S.W., J.M., M.G.K., P.K.S., K.-A.D., J.F.H., J.G.G., L.C., W.A., and R.P. participated in the drafting and/or revising of the manuscript. Primarily responsible for a particular specialized role: (i) clinical pathology: K.F.B.; (ii) reagent acquisition and data assembly: K.F.B., D.R.C., W.H.P.D., and J.F.H.; (iii) primate medicine: P.W.H. and B.J.B.; (iv) image acquisition and analysis: K.F.B., P.W.H., W.H.P.D., M.T., J.M., and J.G.G.; (v) pathology: W.B.B.; (vi) statistical analysis: K.F.B., D.R.C., W.H.P.D., S.W., and K.-A.D.; (vii) metabolic analysis: K.F.B., P.W.H., P.K.S., and L.C.; (viii) overall study coordination: W.A. and R.P.
SUPPLEMENTARY MATERIAL www.sciencetranslationalmedicine.org/cgi/content/full/3/108/108ra112/DC1 Materials and Methods
Competing interests UTMDACC and some of its researchers (W.A. and R.P.), as well as J.F.H., own equity stock in Ablaris Therapeutics, which is subjected to certain restrictions under university policy; the university manages and monitors the terms of these arrangements in accordance with its conflict of interest policy. W.A. and R.P. are paid consultants for Ablaris Therapeutics. M.G.K., W.A., and R.P. are coinventors on patents filed by UTMDACC that cover adipotide and are entitled to royalties that could result from commercial success of the drug. The adipose-targeting peptide compounds described in this manuscript are the subject of issued U.S. patents 7,452,964 and 7,951,362, European patent EP 1322755, and pending patent CA 2730627. The other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Withrow D, Alter DA. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011;12:131–141. doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig DS, Pollack HA. Obesity and the economy: From crisis to opportunity. JAMA. 2009;301:533–535. doi: 10.1001/jama.2009.52. [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledford H. Slim spoils for obesity drugs. Nature. 2010;468:878. doi: 10.1038/468878a. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH. Clinical practice. Nonsurgical management of obesity in adults. N. Engl. J. Med. 2008;358:1941–1950. doi: 10.1056/NEJMcp0801652. [DOI] [PubMed] [Google Scholar]
- 6.Witkamp RF. Current and future drug targets in weight management. Pharm. Res. 2011;28:1792–1818. doi: 10.1007/s11095-010-0341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell TM, Khera A. Therapeutic approaches to obesity. Curr. Treat. Options Cardiovasc. Med. 2010;12:381–395. doi: 10.1007/s11936-010-0080-y. [DOI] [PubMed] [Google Scholar]
- 8. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm228830.htm.
- 9.Cooke D, Bloom S. The obesity pipeline: Current strategies in the development of anti-obesity drugs. Nat. Rev. Drug Discov. 2006;5:919–931. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- 10.Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nat. Rev. Endocrinol. 2010;6:255–269. doi: 10.1038/nrendo.2010.19. [DOI] [PubMed] [Google Scholar]
- 11.Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, Hamm CW, Montalescot G, Steg PG, Pearson TA, Cohen E, Gaudin C, Job B, Murphy JH, Bhatt DL. CRESCENDO Investigators, Rimonabant for prevention of cardiovascular events (CRESCENDO): A randomised, multicentre, placebo-controlled trial. Lancet. 2010;376:517–523. doi: 10.1016/S0140-6736(10)60935-X. [DOI] [PubMed] [Google Scholar]
- 12.Idelevich E, Kirch W, Schindler C. Current pharmacotherapeutic concepts for the treatment of obesity in adults. Ther. Adv. Cardiovasc. Dis. 2009;3:75–90. doi: 10.1177/1753944708098226. [DOI] [PubMed] [Google Scholar]
- 13.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Woods SC, Seeley RJ. Peptide designed to elicit apoptosis in adipose tissue endothelium reduces food intake and body weight. Diabetes. 2010;59:907–915. doi: 10.2337/db09-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 19.Zurita AJ, Troncoso P, Cardó-Vila M, Logothetis CJ, Pasqualini R, Arap W. Combinatorial screenings in patients: The interleukin-11 receptor α as a candidate target in the progression of human prostate cancer. Cancer Res. 2004;64:435–439. doi: 10.1158/0008-5472.can-03-2675. [DOI] [PubMed] [Google Scholar]
- 20.Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Lahdenranta J, Sidman RL, Pasqualini R, Arap W. Treatment of hypoxia-induced retinopathy with targeted proapoptotic peptidomimetic in a mouse model of disease. FASEB J. 2007;21:3272–3278. doi: 10.1096/fj.07-8273com. [DOI] [PubMed] [Google Scholar]
- 22.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J. Biol. Chem. 2008;283:29447–29460. doi: 10.1074/jbc.M804595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mintz PJ, Cardó-Vila M, Ozawa MG, Hajitou A, Rangel R, Guzman-Rojas L, Christianson DR, Arap MA, Giordano RJ, Souza GR, Easley J, Salameh A, Oliviero S, Brentani RR, Koivunen E, Arap W, Pasqualini R. An unrecognized extracellular function for an intracellular adapter protein released from the cytoplasm into the tumor microenvironment. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2182–2187. doi: 10.1073/pnas.0807543105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staquicini FI, Cardó-Vila M, Kolonin MG, Trepel M, Edwards JK, Nunes DN, Sergeeva A, Efstathiou E, Sun J, Almeida NF, Tu S-M, Botz GH, Wallace MJ, O’Connel DJ, Krajewski S, Gershenwald JE, Molldrem JJ, Flamm AL, Koivunen E, Pentz RD, Dias-Neto E, Setubal JC, Cahill DJ, Troncoso P, Do K-A, Logothetis CJ, Sidman RL, Pasqualini R, Arap W. Vascular ligand-receptor mapping by direct combinatorial selection in cancer patients. Proc. Natl. Acad. Sci. U.S.A. doi: 10.1073/pnas.1114503108. 10.1073/pnas.1114503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 26.Arner P. Resistin: Yet another adipokine tells us that men are not mice. Diabetologia. 2005;48:2203–2205. doi: 10.1007/s00125-005-1956-3. [DOI] [PubMed] [Google Scholar]
- 27.Sengenès C, Zakaroff-Girard A, Moulin A, Berlan M, Bouloumié A, Lafontan M, Galitzky J. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R257–R265. doi: 10.1152/ajpregu.00453.2001. [DOI] [PubMed] [Google Scholar]
- 28.Bousquet-Mélou A, Galitzky J, Lafontan M, Berlan M. Control of lipolysis in intra-abdominal fat cells of nonhuman primates: Comparison with humans. J. Lipid Res. 1995;36:451–461. [PubMed] [Google Scholar]
- 29.Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus) Obesity. 2009;17:1499–1505. doi: 10.1038/oby.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodkin NL, Hannah JS, Ortmeyer HK, Hansen BC. Central obesity in rhesus monkeys: Association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? Int. J. Obes. Relat. Metab. Disord. 1993;17:53–61. [PubMed] [Google Scholar]
- 31.Raman A, Colman RJ, Cheng Y, Kemnitz JW, Baum ST, Weindruch R, Schoeller DA. Reference body composition in adult rhesus monkeys: Glucoregulatory and anthropometric indices. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:1518–1524. doi: 10.1093/gerona/60.12.1518. [DOI] [PubMed] [Google Scholar]
- 32.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. ed. 2 Oxford Univ. Press; New York: 2002. [Google Scholar]
- 33.Miyazaki Y, Matsuda M, DeFronzo RA. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes Care. 2002;25:517–523. doi: 10.2337/diacare.25.3.517. [DOI] [PubMed] [Google Scholar]
- 34.Chavez AO, Gastaldelli A, Guardado-Mendoza R, Lopez-Alvarenga JC, Leland MM, Tejero ME, Sorice G, Casiraghi F, Davalli A, Bastarrachea RA, Comuzzie AG, DeFronzo RA, Folli F. Predictive models of insulin resistance derived from simple morphometric and biochemical indices related to obesity and the metabolic syndrome in baboons. Cardiovasc. Diabetol. 2009;8:22. doi: 10.1186/1475-2840-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hersey WC, III, Graves JE, Pollock ML, Gingerich R, Shireman RB, Heath GW, Spierto F, McCole SD, Hagberg JM. Endurance exercise training improves body composition and plasma insulin responses in 70- to 79-year-old men and women. Metabolism. 1994;43:847–854. doi: 10.1016/0026-0495(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 36.Diamond MP, Thornton K, Connolly-Diamond M, Sherwin RS, DeFronzo RA. Reciprocal variations in insulin-stimulated glucose uptake and pancreatic insulin secretion in women with normal glucose tolerance. J. Soc. Gynecol. Investig. 1995;2:708–715. doi: 10.1016/1071-5576(95)00023-8. [DOI] [PubMed] [Google Scholar]
- 37.Reitman ML. Magic bullets melt fat. Nat. Med. 2004;10:581–582. doi: 10.1038/nm0604-581. [DOI] [PubMed] [Google Scholar]
- 38.van Acker BA, Koomen GC, Koopman MG, de Waart DR, Arisz L. Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet. 1992;340:1326–1329. doi: 10.1016/0140-6736(92)92502-7. [DOI] [PubMed] [Google Scholar]
- 39.Krug AW, Völker K, Dantzler WH, Silbernagl S. Why is d-serine nephrotoxic and α-aminoisobutyric acid protective. Am. J. Physiol. Renal Physiol. 2007;293:F382–F390. doi: 10.1152/ajprenal.00441.2006. [DOI] [PubMed] [Google Scholar]
- 40.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 41.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardó-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis CJ, Pasqualini R. Steps toward mapping the human vasculature by phage display. Nat. Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 42.Horton KL, Kelley SO. Engineered apoptosis-inducing peptides with enhanced mitochondrial localization and potency. J. Med. Chem. 2009;52:3293–3299. doi: 10.1021/jm900178n. [DOI] [PubMed] [Google Scholar]
- 43.Hossen MN, Kajimoto K, Akita H, Hyodo M, Ishitsuka T, Harashima H. Ligand-based targeted delivery of a peptide modified nanocarrier to endothelial cells in adipose tissue. J. Control Release. 2010;147:261–268. doi: 10.1016/j.jconrel.2010.07.100. [DOI] [PubMed] [Google Scholar]
- 44.Guide for the Care and Use of Laboratory Animals (National Research Council . Committee for the Update of the Guide for the Care and Use of Laboratory Animals. ed. 8 National Academies Press; Washington, DC: 2010. [Google Scholar]
- 45.Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. http://www.fda.gov/SiteIndex/default.htm.
- 46. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=58&showfr=1.
- 47. http://www.springerlink.com/content/x57mul0frg64apmh/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.