Abstract
Previous data suggests that the adiposity signal leptin reduces food intake in part by enhancing sensitivity to short-term signals that promote meal termination, including glucagon-like peptide 1 (GLP-1). We hypothesized that maintenance on a high-fat (HF) diet, which causes resistance to leptin, would impair GLP-1′s ability to reduce food intake. To test this hypothesis, we examined the anorexic responses to intraperitoneal injection of 100 μg/kg GLP-1 and 1 μg/kg exendin-4 (Ex-4), the potent, degradation resistant GLP-1 receptor agonist, in Wistar rats maintained on a low-fat (10%; LF) or HF (60%) diet for 4-6 weeks. Rats maintained on each of these diets were tested twice, once while consuming LF food and once while consuming HF food, to distinguish between effects of acute vs. chronic consumption of HF food. LF-maintained rats tested on LF diet reduced 60-min dark phase intake in response to GLP-1, but HF-maintained rats failed to respond to GLP-1 whether they were tested on HF or LF diet. LF-maintained rats tested on HF diet also showed no response, suggesting that even brief exposure to HF diet can impair sensitivity to GLP-1 receptor activation. Both LF- and HF-maintained rats showed significant anorexic responses to Ex4 at 4 h post-treatment, but only LF-maintained rats had significantly reduced intake and body weight 24 h after injections. To determine whether the ability of endogenous GLP-1 to promote satiation is impaired by HF maintenance, we examined the response to exendin 3 (9-39) (Ex9), a GLP-1 receptor antagonist. In LF-maintained rats, Ex9 increased intake significantly, but HF-maintained rats reduced food intake in response to Ex9. These data support the suggestion that maintenance on HF diet reduces the anorexic effects of GLP-1 receptor activation, and this phenomenon may contribute to overconsumption of high-fat foods.
Keywords: glucagon-like peptide 1, exendin-4, satiation, high-fat diet, obesity
1. Introduction
Glucagon-like peptide 1 (GLP-1), an incretin hormone secreted by intestinal L-cells in response to ingested nutrients, is widely considered to play a physiological role in the control of food intake (1). Several laboratories have shown that peripheral administration of GLP-1 and GLP-1 receptor (GLP-1-R) agonists reduce food intake (2), and peripheral administration of the GLP-1-R antagonist exendin (9-39) (Ex9) increases food intake under some conditions (3). Intraperitoneal injection of Ex9 also attenuates nutrient preload-induced satiety, suggesting that endogenous GLP-1-R stimulation plays a role in the intake-suppressive effects of nutrients delivered into the gastrointestinal tract (3). The development of diet-induced obesity (DIO) involves a deficit in satiation signaling, whereby animals maintained on a high-fat (HF) diet take larger meals than low-fat (LF)-fed controls (4, 5). We hypothesized that maintenance on a HF diet impairs the ability of GLP-1 to promote satiation, an effect that could contribute to overconsumption during DIO.
There are several reasons to believe that defective GLP-1 function could play a role in the development of obesity. It has recently been reported that the potent GLP-1-R agonist exendin-4 (Ex4) has a reduced anorexic effect in DIO-prone Osbourne-Mendel rats compared with obesity-resistant S5B/Pl rats (6). The known interactions between GLP-1 and the adiposity hormone leptin also support this hypothesis. HF-DIO rats show leptin resistance, a state in which circulating leptin levels are high but leptin receptor signaling is impaired (7). In lean rats, fasting (which decreases leptin levels) attenuates the anorexic response to GLP-1 and Ex4, and this effect is reversed by physiologic-dose leptin replacement (8). Moreover, rats that lack functional leptin receptors (fak/fak rats) failed to respond to GLP-1 at doses that significantly decrease intake in wild-type controls (8). These data suggest that reduced leptin levels or lack of leptin receptor signaling can impair sensitivity to GLP-1, and may contribute to the overconsumption and weight gain observed in leptin- and leptin-receptor deficient models. We hypothesized that HF diet-induced leptin resistance could blunt the response to GLP-1-R activation in a similar manner.
Here, we asked whether Wistar rats maintained on HF diet show a reduced anorexic response to GLP-1 and Ex4 treatment compared with rats maintained on a low fat (LF) diet. We examined the rats' feeding responses to GLP-1 and Ex4 under conditions that would allow us to distinguish between effects of chronic HF consumption vs. acute HF diet exposure. All rats were tested on their maintenance diet and also tested while consuming the non-maintenance diet (i.e., LF-maintained rats tested while consuming HF food; HF-maintained rats tested while consuming LF food). Our results provide clear evidence that maintenance on HF diet impairs the anorexic response to GLP-1-R activation. We then asked whether the role of endogenous GLP-1 in satiation is affected by HF diet maintenance. It has been reported that maintenance on HF diet for 8 weeks reduces baseline plasma active GLP-1 levels as well as the magnitude of the postprandial GLP-1 response (9). We evaluated plasma GLP-1 levels after 4 weeks on HF or LF diet, to determine whether such changes occur early in the course of HF-DIO. Such an effect would support the suggestion that circulating GLP-1 levels have less of an impact on feeding in HF-maintained rats. We directly assessed this by examining the response of HF- and LF-maintained rats to GLP-1-R blockade with Ex9. If HF-maintained rats release less GLP-1 and/or are less sensitive to endogenous activation of GLP-1-R than LF rats, then the orexigenic effect of Ex9 should be impaired in HF rats.
2. Materials and Methods
2.1. Subjects
Naïve male Wistar rats (Charles River, Wilmington, MA) were individually housed in standard Plexiglass cages with food hoppers. The room was temperature controlled and maintained on a 12 h light:12 h dark cycle. Distilled water was available ad libitum. Rats were maintained on standard rat chow (Purina, St. Louis, MO) with ad libitum access until they were switched to LF- or HF diets (D12450B and D12492, respectively, from Research Diets, New Brunswick, NJ) as described below (see Table 1 for details of diet composition). All subjects were handled daily and habituated to ip injections of saline before the experiments began. Body weight was measured daily and food intake was measured continuously. All experimental procedures were approved by the Florida State University institutional animal care and use committee, and conform to the standards of the Guide for the Care and Use of Laboratory Animals (National Research Council 2010).
Table 1.
Composition and energy density of low-fat (LF) and high-fat (HF) diets used in these studies.
| % kcal from: | ||||
|---|---|---|---|---|
| Diet | Protein | Carbohydrate | Fat | kcal/g |
| LF | 20 | 70 | 10 | 3.85 |
| HF | 20 | 20 | 60 | 5.24 |
2.2. Drugs
GLP-1 (7-36) and amylin were purchased from Bachem (Torrence, CA). Ex4 and Ex9 were purchased from California Peptide Research (Napa, CA). Each was dissolved in sterile 0.9% saline for injections. The doses used here (100 μg/kg GLP-1, 1 μg/kg Ex4, and 100 μg/kg Ex9) were chosen to be low, near-threshold doses based on our own published (8) and unpublished dose response studies, as well as those of other research groups (10).
2.3. Experimental Procedures
2.3.1. Experiment 1: Effect of HF diet on the anorexic response to GLP-1
Rats (n = 14) were first tested for responsiveness to GLP-1 while maintained on standard chow diet, in a within-subjects design. On test days, food was removed 3 hrs prior to onset of the dark cycle. Each rat received either a 100 μg/kg dose of GLP-1 or 1 ml saline, administered ip 20 minutes before the onset of the dark cycle. Pre-weighed food was returned to the subjects immediately prior to onset of dark. Food was weighed 60 min after injections. The animals' treatments were counterbalanced with at least 48 h between injections. Next, we divided the rats into 2 weight- and intake-matched groups: low-fat (LF, 10%) or high-fat (HF, 60%) diet (See Table 1).
After 4 weeks of ad libitum access to the LF or HF food, the anorexic response to GLP-1 was tested again. Rats were each tested twice, once on the maintenance diet and once on the other diet, to allow us to distinguish between any effects of longer-term maintenance on a HF diet vs. acute ingestion of HF food. Therefore, each rat received 4 experimental conditions: vehicle/HF diet; GLP-1/HF diet; vehicle/LF diet; GLP-1/LF diet. To limit food neophobia, rats were exposed to the non-maintenance diet for the first 60 min of the dark phase on 2 occasions several days before the drug conditions took place. On experimental days, food was removed 3 hrs prior to onset of the dark cycle. Each rat received either 1 ml saline or 100 μg/kg GLP-1 ip 20 minutes before the onset of the dark cycle. Pre-weighed food was returned to the subjects immediately prior to onset of dark, and then food was weighed 60 min after injections. On days when rats were tested on their non-maintenance diet, the test food was removed after the 60 min measurement and was replaced with their maintenance diet. Drug and diet conditions were counterbalanced across the four test days, with 48 - 72 h between injections.
2.3.2. Experiment 2: Effect of HF diet on the anorexic response to Ex4
Rats (n = 22) were divided into LF or HF groups, matched for body weight and chow intake. After 6 weeks on LF or HF diet, their responses to Ex4 were examined as follows.
On experimental days, food was removed 3 hrs prior to onset of the dark cycle. Each rat received either a 1 μg/kg dose of Ex4 or 1 ml saline, administered ip within 20 minutes before the onset of the dark cycle. Pre-weighed food was returned to the subjects immediately prior to onset of dark. Food was weighed at 4 and 24 h after injections, and body weight was measured before injections and 24 h later. The animals' treatments were counterbalanced with 72 h between injections.
As in Experiment 1, we assessed the effect of Ex4 on food intake while the animals consumed their maintenance diet and also on a separate occasion when they were given the other diet. Even at low doses, Ex4′s anorexic effect is more robust and longer in duration than that of GLP-1, so we used a slightly different design for this experiment. Here, rats were tested for responsiveness to Ex4 on their maintenance diets first. Next, they were given access to their non-maintenance diet exclusively for 24 h, to minimize neophobia during the subsequent test. They were then given ad lib access to their maintenance diet for 72 h before the first day of the Ex4 test, to allow for washout of any metabolic effects that may have resulted from the 1-day exposure to the non-maintenance diet. At that point, rats received either saline or Ex4 injections as described above, but were then given their non-maintenance diet as their only choice of food for the next 24 h. Food was weighed at 4 and 24 h after injections. This was done as a between-subjects test because multiple days of exposure to the non-maintenance diet could have significant gastrointestinal or metabolic effects (e.g., rats that had been maintained on HF diet may begin to lose weight when put on LF diet for several days).
2.3.3. Experiment 3: Effect of amylin on food intake in HF-maintained rats
When HF-maintained rats were tested on LF diet, intakes after vehicle injections were low compared with baselines under other conditions. This complicates the interpretation of the lack of GLP-1 and Ex4 effects in HF-maintained rats tested on LF food, because it raises the concern that we simply have a floor effect in this test situation. We therefore performed this control experiment to demonstrate that other anorexigenic agents can still suppress intake in HF-maintained rats consuming LF food. We examined the effect of amylin, a hormone produced by the pancreas, because it has been shown that HF maintenance does not impair rats' ability to respond to this treatment (11).
The HF-maintained rats from Experiment 2 were used in this experiment 1 week after the last Ex4 test. Food was removed 3 hrs prior to onset of the dark cycle. A between-subjects design was used in which each rat received either a 10 μg/kg dose of amylin or 1 ml saline administered ip within 20 minutes before the onset of the dark cycle. Pre-weighed LF food was returned to the subjects immediately prior to onset of dark. Food was weighed at 1, 4, and 24 h after injections. Body weight was measured before injections and 24 h after treatment.
2.3.4. Experiment 4: Effect of HF diet maintenance on plasma GLP-1 levels
The same subjects in Experiment 2 were used to determine whether a change in endogenous GLP-1 levels occurs as early as 4 weeks after onset of HF diet maintenance. Rats were fasted overnight and then anesthetized briefly with isofluorane (2 to 4% in 1 liter oxygen/minute) for retro-orbital blood sampling. Approximately 1 ml of blood from each rat was collected into tubes containing a cocktail of 0.25 M EDTA, 200 U/ml Heparin, 283 uM aprotin, and 1.3 mM diprotin A (Sigma-Aldrich, St Louis, MO). Plasma was stored at −80°C until biologically active GLP-1 (7-36 amide) and GLP-1 (7-37) levels were measured via ELISA (Millipore, Billerica, MA) according to the manufacturer's instructions. This assay has a detection limit of 2 pM, and we obtained an average intra-assay CV of 3.5%, average inter-assay CV of 9.9%, and recovery rate of 93.9% on quality controls.
2.3.5. Experiment 5: Effect of HF diet on the feeding response to a GLP-1-R antagonist
After 1 week of recovery from drug treatments, the same rats from Experiment 1 were used in this study. We have previously shown that Ex9 has an orexigenic effect only at times when spontaneous food intake is minimal (3). Therefore, we tested the response to Ex9 during the light cycle. Food was removed 2 h before injections, and the injections took place approximately 6 h into the light phase. Each rat received either 1 ml saline or 100 μg/kg Ex9 ip, and pre-weighed food (maintenance diet) was returned immediately after injections. Food intake was measured 60 min later. Injection conditions were counterbalanced and separated by 48 h.
2.4. Statistical Analysis
Body weight was compared across the LF and HF groups via 2-way ANOVA with group and time as factors. The effects of HF diet maintenance, diet consumed during the test, and drug treatment on food intake were evaluated by mixed-design 2 or 3 factor ANOVA, with post hoc analysis by Tukey's HSD where appropriate. One-tailed Student's t-tests were used for between-group comparisons in Experiments 3 and 4. The pattern of results was the same whether food intake data were analyzed in the form of grams consumed or kcal consumed, but we present the data here as kcal consumed for ease of comparison between LF and HF intake. P values of < 0.05 were taken to be significant.
3. Results
3.1. Experiment 1: Effect of HF diet on the anorexic response to GLP-1
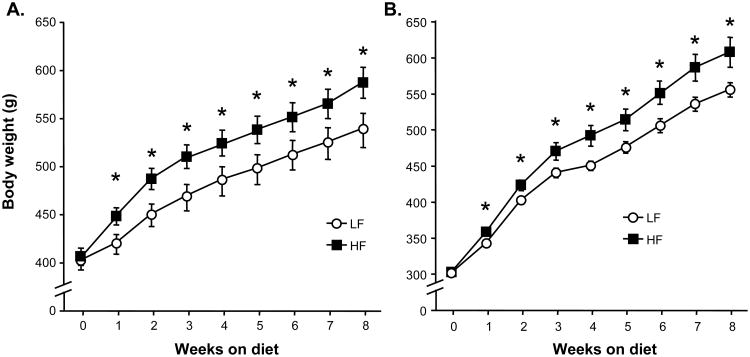
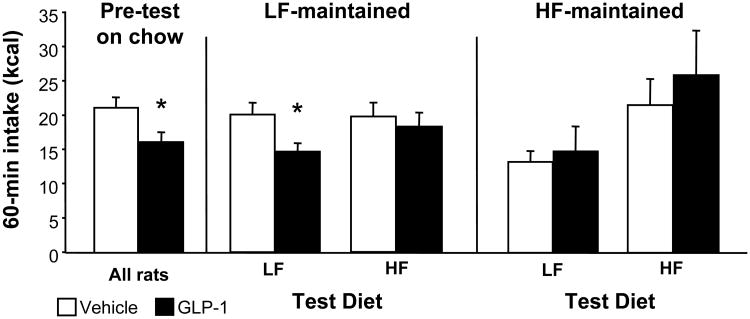
Maintenance on HF diet significantly increased body weight over time, with significant divergence starting after just 1 week on HF vs. LF diet (interaction between diet and time: F(1, 8) = 3.72, p < 0.001)(see Figure 1A). All subjects showed a robust anorexic response to GLP-1 during the pre-test on standard chow diet (p < 0.05), and LF-maintained controls tested on LF diet showed a decrease in food intake (p < 0.05) after GLP-1 treatment that was similar in magnitude to their pre-test response (see Figure 2). In contrast, rats maintained on HF diet for 4 weeks prior to the second GLP-1 test failed to respond to the drug regardless of whether they were tested on HF or LF food (see Figure 2). Acute ingestion of HF diet also had an impact on GLP-1 response; LF-maintained rats tested with HF diet for 60 min did not show a significant reduction in HF food intake after GLP-1 treatment (see Figure 2). ANOVA yielded significant interactions among maintenance diet and GLP-1 (F (1, 13) = 12.5, p < 0.01) and between test diet and GLP-1 (F (1, 13) = 4.6, p < 0.05).
Figure 1.
Body weight over time for rats in Experiments 1 and 4 (A) and Experiments 2 and 3 (B). Data are means ± SEM, *p < 0.05.
Figure 2.
Food intake (kcal) 60 min after ip injection of either saline vehicle (open bars) or 100 μg/kg GLP-1 (black bars). All subjects were tested on standard chow before they were placed on LF or HF diet, and were then tested after 4 weeks on LF or HF diet. Each rat was tested once while consuming LF diet and once while consuming HF diet during the 60-min test period. Data are means ± SEM, *p < 0.05 relative to vehicle.
3.2. Experiment 2: Effect of HF diet on the anorexic response to Ex4
Rats on HF diet gained weight at a faster rate than LF controls, with significant divergence beginning at 1 week after starting on HF or LF diet (interaction between diet and time: F (1, 20) = 5.19, p < 0.001)(see Figure 1B). Because rats in this experiment started on HF or LF diet at a lower mean body weight than rats in the previous experiment, we compared body weights across the 2 experiments from weeks 4-8 (the period during which drug responses were tested) with 3-way ANOVA in which week, diet, and experiment were factors. There were no significant main effects or interactions with experiment; only time and diet affected weight. Therefore, the effects of HF diet on body weight over time did not differ across the 2 experiments.
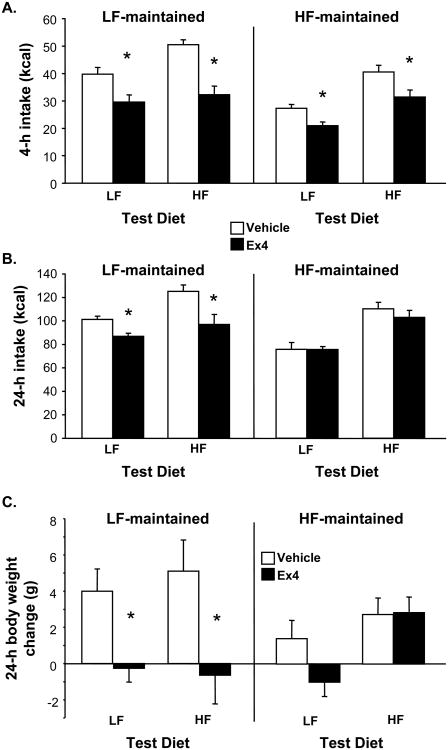
When rats were tested on their maintenance diet, Ex4 reduced food intake significantly at 4 h post-injections in both groups to a similar degree (p < 0.05)(see Figure 3A). However, we observed a different pattern at 24 h post-injections, where there was a significant interaction between maintenance diet and Ex4 (F(1, 20) = 10.6, p < 0.01). LF rats showed a significant anorexic effect of Ex4 at that time (p < 0.05), whereas HF rats no longer showed a response (See Figure 3B). Similarly, overnight body weight gain was reduced by Ex4 in LF rats (p < 0.05) but not affected in HF rats (interaction between maintenance diet and Ex4: F (1, 20) = 11.23. p < 0.01)(See Figure 3C).
Figure 3.
Food intake (kcal) 4 h (A) and 24 h (B) after ip injection of saline vehicle (open bars) or 1 μg/kg Ex4 (black bars). Panel C shows body weight change (g) over the 24 h following these injections. Rats were tested after 6 weeks on LF or HF diet. The results of 2 tests of Ex4 response are shown: the first test was conducted while rats consumed the diet on which they were maintained, whereas in the second test, they had access to their non-maintenance diet only. Data are means ± SEM, *p < 0.05 relative to vehicle.
In the second Ex4 trial, during which rats had access to the diet that they had not been maintained on (i.e., LF-maintained rats eating HF food, HF-maintained rats eating LF food), we obtained a similar pattern of results. LF-maintained rats showed a significant anorexic response to Ex4 at 4 and 24 h post-treatment (p's < 0.05) (see Figures 3A and 3B). Again, Ex4 suppressed intake in HF-maintained rats at 4 h (p < 0.05) but failed to do so at 24 h post-treatment. HF-maintained rats had lower baseline intakes when consuming LF food at both 4 and 24 h post-treatment relative to LF-maintained rats (p < 0.05). There were significant interactions between maintenance diet and drug at 4 h (F(1, 20) = 7.17, p < 0.05) and at 24 h (F(1, 20) = 7.26, p < 0.05). Ex4 also significantly limited body weight gain over the 24-h period in LF-maintained rats (p < 0.05), but had no significant effect on body weight change in HF-maintained rats (see Figure 3C).
3.3. Experiment 3: Effect of amylin on food intake in HF-maintained rats
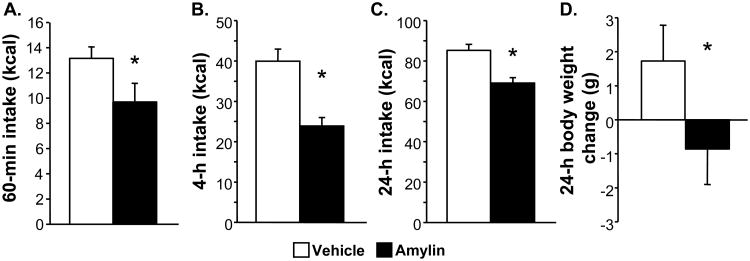
HF-maintained rats tested on LF food intake ingested the same amount within 60 min after ip saline as rats under the same conditions in Experiment 1. From that relatively low baseline, amylin significantly reduced intake by 26% (t (9) = 2.27, p < 0.05) (see Figure 4A). The same was true at 4 h (see Figure 4B) and 24 h post-treatment (see Figure 4C); amylin significantly reduced food intake compared with a relatively low vehicle baseline (4-h t (9) = 5.09, p < 0.001; 24-h t (9) = 4.44, p < 0.01). Body weight was also significantly reduced by amylin (see Figure 5D) relative to vehicle (t (9) = 2.29, p < 0.05).
Figure 4.
Food intake (kcal) 60 min (A), 4 h (B) and 24 h (C) after ip injection of saline vehicle (open bars) or 10 μg/kg amylin (black bars). Body weight change is shown in panel D. HF-maintained rats were tested while consuming LF diet, to rule out an effect of low baseline intakes as the explanation for lack of GLP-1 and Ex4 effects. Data are means ± SEM, *p < 0.05 relative to vehicle.
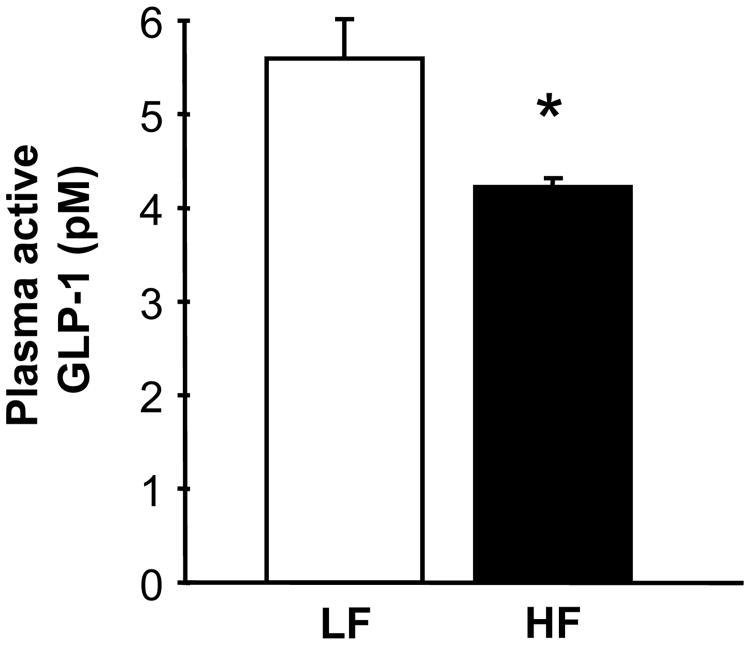
Figure 5.
Plasma levels of biologically active GLP-1 (pM) in rats maintained on LF or HF diet for 4 weeks. Data are means ± SEM, *p < 0.05.
3.4. Experiment 3: Effect of HF diet maintenance on plasma GLP-1 levels
Rats maintained on HF diet for 4 weeks had significantly lower active GLP-1 levels in plasma compared with rats maintained on LF diet for the same period (t (24) = 1.9, p < 0.05) (See Figure 5).
3.5. Experiment 4: Effect of HF diet on the feeding response to a GLP-1-R antagonist
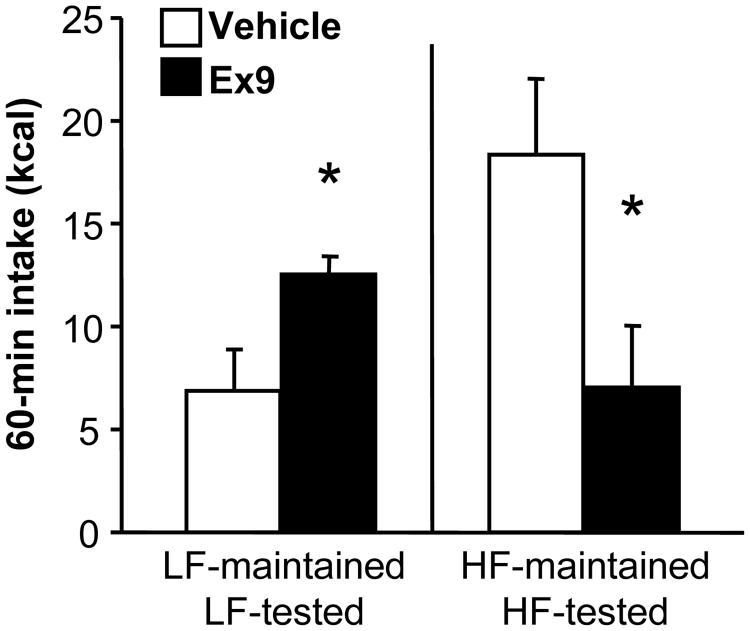
The GLP-1-R antagonist Ex9 significantly increased light-phase food intake 60 min after injections in LF-maintained rats (p < 0.05)(see Figure 6). Surprisingly, Ex9 had the opposite effect in HF-maintained rats, robustly decreasing their intake relative to vehicle (p < 0.05). ANOVA revealed a significant interaction between maintenance diet and Ex9 (F (1, 13) = 24.95, p < 0.001).
Figure 6.
Food intake (kcal) 60 min after ip injection of saline vehicle (open bars) or 100 μg/kg Ex9 (black bars). Rats were tested after 7 weeks on LF or HF diet. Data are means ± SEM, *p < 0.05 relative to vehicle.
4. Discussion
The studies presented here demonstrate that maintenance on HF diet for 4-6 weeks alters rats' ability to show anorexic responses to GLP-1-R activation. Food intake reduction induced by GLP-1, itself, as well as to the more potent GLP-1-R agonist Ex4, was significantly impaired by chronic HF diet consumption, regardless of what food the rats consumed during the test. We also observed that the decrease in plasma GLP-1 levels occurs as early as 4 weeks into HF diet maintenance. Thus, HF-maintained rats are producing less active GLP-1 (9) and are also less sensitive to its anorexic effects. This supports the suggestion that endogenous GLP-1 would be less relevant for food intake control in these animals, a hypothesis that we tested by examining the intake response to the GLP-1-R antagonist Ex9. Blockade of GLP-1-R elevated food intake as expected in LF-maintained rats, but we observed a decrease in intake when Ex9 was administered to HF-maintained rats. Taken together, our results suggest that HF diet maintenance alters the GLP-1 system in such a way that it no longer functions to promote satiation.
We observed that the 100 μg/kg dose of GLP-1, which was effective in chow-fed rats and LF-maintained rats tested on LF food, failed to significantly suppress food intake in LF-maintained rats when they were tested with HF food. This supports the idea that GLP-1 may be less effective at suppressing intake of HF diets even in lean subjects that have relatively little prior experience with HF food. At present, we can only speculate about the mechanism for this effect. It has been reported that peripheral GLP-1-R antagonism is less effective at increasing food intake under conditions that produce relatively high baseline spontaneous intake (intake of chow at the onset of the dark cycle, intake of vanilla Ensure during short-term tests in the light cycle) (3). One possible interpretation of those results is that GLP-1-R activation is less influential when rats are more highly motivated to consume. Here, LF rats received short-term access to highly palatable HF food, and this may have created a high-motivation situation. A number of researchers have proposed that factors such as palatability and reward can “override” gastrointestinal signals that would otherwise reduce food intake (12, 13), and this may explain the result observed here.
Chronic consumption of HF diet has an impact beyond that observed with a single HF meal, because HF-maintained rats failed to respond to GLP-1 whether they were tested on LF or HF food. When we examined the response to Ex4, we found that LF-maintained rats showed the typical anorexic response regardless of food consumed during the test. HF-maintained rats also responded to Ex4 during the first 4 h post-treatment, but their response was short-lived compared with LF-maintained rats that still showed significant anorexia at 24-h post-treatment. This is not the first time it has been observed that Ex4 produces more robust and longer-lasting effects than GLP-1, a difference that is at least in part due to the significantly shorter half-life of active GLP-1 (14). Peripherally administered GLP-1 has been shown to affect food intake through peripheral, but not central, GLP-1-R (3). Although the short-term (i.e., within several hours after treatment) effects of Ex4 are thought to be mediated through peripheral GLP-1-R and require an intact vagus nerve (15), Ex4 can freely cross the blood-brain barrier (16). Because of Ex4′s much longer half-life, it is plausible that at least the longer-duration effects of Ex4 observed here are mediated by central GLP-1-R. It is also worth noting that the differences between GLP-1 and Ex4 extend beyond half-life. Several studies have demonstrated effects of Ex4 that differ from those of GLP-1 and are not blocked by GLP-1-R antagonism, leading to speculation that there may be an as yet undiscovered receptor that is activated by Ex4 and not GLP-1 (17, 18). We suggest that these differences between the two treatments may account for the pattern of results observed across these experiments.
When HF-maintained rats were tested on LF food in Experiments 1 and 2, their baseline intakes were reduced compared with other conditions. This may be because the HF-maintained rats had adapted to the more calorically dense diet and require more than 24 h on LF food to adjust to the less dense diet. It is also possible that differences in taste, texture, or palatability contributed to the lower baseline intakes in this condition. Regardless of the reason for the reduced baseline, it raises the concern that we have a floor effect and cannot interpret the lack of GLP-1 or Ex4 effects in these tests as a meaningful result. We reject that possibility for several reasons. Most importantly, we demonstrated that low baseline intake in HF-maintained rats consuming LF food does not prevent amylin from effectively reducing food intake. Amylin significantly reduced 60-min food intake in HF-maintained rats consuming LF food during the test. Rats injected with saline in this control study ingested the same amount as rats under the same conditions in Experiment 1. Amylin also significantly reduced 4- and 24-h intake under these conditions, with a relatively low vehicle baseline at 24 h in particular. In addition, we saw in Experiment 2 that Ex4 significantly reduced food intake in HF-maintained rats tested on LF food at the 4 h measurement. Together, these data demonstrate that low baseline intake does not prevent the effects of anorexigenic treatments. This further supports our conclusion that HF-maintenance impairs rats' sensitivity to GLP-1-R activation independent of the food consumed during the test.
The orexigenic effect of GLP-1-R blockade has been taken as an indication that endogenous GLP-1 plays a role in the control of food intake. Others have reported that fasting plasma GLP-1 levels along with the nutrient-induced rise in plasma GLP-1 are reduced by HF diet maintenance (9), and we confirmed that the effect on fasting plasma GLP-1 level is present after as few as 4 weeks on HF diet. Thus, the HF-maintained rat has less endogenous GLP-1 available to suppress food intake and we hypothesize that it would be less sensitive to whatever GLP-1 it does produce. Under these circumstances, GLP-1-R antagonism should have little orexigenic effect compared with that observed in LF-maintained rats. When we tested this hypothesis directly, we were surprised to find that not only did Ex9 fail to increase food intake in HF-maintained rats, it decreased intake instead. HF-maintained rats showed a relatively high baseline level of intake in this test, but that cannot account for the anorexic effect of Ex9 observed here. We have previously shown that Ex9 simply has no effect in lean chow-fed rats in situations where baseline intakes are high (3). Thus, the present finding would seem to suggest that chronic consumption of a HF diet fundamentally changes the nature of the response to GLP-1-R activation. Although unexpected, the idea that such an effect may occur is not unprecedented. Knauf and colleagues (19) reported that CNS administration of Ex9 protects against the insulin resistance that normally occurs as a consequence of HF-DIO, a surprising finding given that pharmacologic activation of GLP-1-R via Ex4 improves HF-DIO-induced insulin resistance (20). Recently, it was reported that GLP-1-R knock-out mice are also protected against HF-induced insulin resistance, and moreover, female GLP-1-R knock-out mice were protected against weight gain on HF diet (21). These effects coupled with the data presented here strongly suggest that although pharmacologic activation of GLP-1-R in HF-DIO subjects can reduce feeding, body weight, and some of the undesirable metabolic effects of such diets (14, 22), the endogenous GLP-1 functions quite differently.
It was already established that HF diet maintenance modulates responsiveness to other satiation/satiety signals. For example, Covasa and colleagues have shown that the anorexic responses to cholecystokinin (CCK) and bombesin are impaired in rats maintained on HF diets (11). The reduction in sensitivity to CCK has been most well studied, and it is useful to compare that phenomenon with the current data focusing on GLP-1. The roles of GLP-1 and CCK in the control of food intake are similar in many respects. Both hormones contribute to nutrient-induced satiation, and both require an intact vagus nerve in order to affect food intake when delivered ip (23), although other routes of administration of GLP-1 successfully reduce food intake in a vagus-independent manner (24). In the case of CCK, the mechanism for the change in effectiveness when rats are maintained on HF diet may be related to CCK production and release by the intestine. Consumption of HF food promotes CCK release, and it has been suggested that these higher circulating levels of CCK cause its receptors to desensitize (25). By contrast, HF feeding causes a small but significant decrease circulating GLP-1 (present results and reference 9), so it seems unlikely that a change in plasma GLP-1 levels is the mechanism for altered sensitivity to exogenous GLP-1-R agonist administration.
We suggested above that the LF-maintained rats loss of response to GLP-1 when they were tested on HF food may be related to the palatability or hedonic value of the HF food. HF-maintained rats' reduction in response to GLP-1 and Ex4 was observed regardless of which food they were eating when tested. Therefore, we propose that the mechanism for this chronic HF feeding effect is distinct from the effect of acute HF ingestion. Although we cannot yet explain how HF diet alters responsiveness to GLP-1-R activation, there are several broad possibilities to consider. First, the change in sensitivity may occur in the periphery. For example, GLP-1-R expression by vagal afferent neurons could be reduced by HF maintenance. Alternatively, the change may occur at the level of the caudal brainstem neurons that are the first to receive the incoming vagal afferent signal. Another plausible scenario is that chronic HF diet consumption alters hypothalamic or other forebrain neurons that play a role in generating the anorexic response downstream of the initial GLP-1-R activation. If GLP-1 or Ex4 act on non-vagal receptor populations, then HF diet maintenance may be acting to alter sensitivity at those sites, central or peripheral. These potential mechanisms of action are not mutually exclusive.
We based our hypothesis that the anorexic response to GLP-1 would be impaired by HF diet maintenance in part on the finding that states of low leptin signaling or complete loss of leptin signaling blunted the effects of GLP-1 (8). It is well established that maintenance on HF diet leads to leptin resistance (7), and even just 3 days of voluntary overfeeding on a moderately high fat (33%) diet impairs the anorexic and metabolic effects of leptin (26). Given these previous results, there is little doubt that the rats in the present studies were leptin resistant after 4-8 weeks of maintenance on a 60% fat diet although we did not directly assess leptin resistance here. Even so, it remains possible that some other consequence of HF maintenance is responsible for the change in responsiveness to GLP-1-R activation. We have yet to determine whether overconsumption and weight gain is required for is change in sensitivity, or if we would see the same changes in response to GLP-1-R activation in rats maintained on a restricted HF diet so that do not gain weight. It is also possible that this effect is not dependent on the fat content of the diet, but rather on the increased caloric density or decreased carbohydrate content of the HF diet used here. All of these possibilities warrant further investigation.
There is no doubt that GLP-1-R activation via chronic Ex4 treatment, usually delivered at higher doses, is effective at reducing food intake and body weight in HF-DIO rat models (22, 27). Similarly, it has been documented that the synthetic form, exenatide, has intake- and body weight-reducing effects in obese humans (28). Here, we used low, near-threshold doses (8, 10) of GLP-1 and Ex4 to show that HF-maintenance changes sensitivity to these treatments. Our data suggests that an individual on HF diet requires greater GLP-1-R stimulation to produce an anorexic effect compared with an individual on LF diet. In addition, we show that HF diet alters the ability of endogenous GLP-1 to suppress intake. Taken together, the evidence suggests that impairment in the anorexic response to GLP-1 may contribute to overconsumption and weight gain when individuals chronically consume HF diets.
Acknowledgments
The authors wish to acknowledge the expert technical assistance of Kiersten Mullis, Nicole Hart, Alexis Pizzi, and Kathryn Samuel. This research was supported by NIH grant DK078779 to DLW and AMT is supported by NIH grant DC000044.
References
- 1.Moran TH. Gut peptides in the control of food intake. Int J Obes (Lond) 2009;33(1):S7–10. doi: 10.1038/ijo.2009.9. [DOI] [PubMed] [Google Scholar]
- 2.Hayes MR, De Jonghe BC, Kanoski SE. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010;100:503–10. doi: 10.1016/j.physbeh.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–7. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiol Behav. 2010;99:33–9. doi: 10.1016/j.physbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res. 2003;11:845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- 6.Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes (Lond) 2010;34:1427–33. doi: 10.1038/ijo.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison CD, Huypens P, Stewart LK, Gettys TW. Implications of crosstalk between leptin and insulin signaling during the development of diet-induced obesity. Biochim Biophys Acta. 2009;1792:409–16. doi: 10.1016/j.bbadis.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–93. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 9.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–9. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 10.Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, Dakin CL, Filipsson K, Wang F, Kent AS, Frost GS, Ghatei MA, Bloom SR. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146:5120–7. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 11.Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19:1407–15. doi: 10.1016/s0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes. 2009;33(2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117:77–88. doi: 10.1016/j.regpep.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–56. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 16.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–8. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Tilve D, González-Matías L, Alvarez-Crespo M, Leiras R, Tovar S, Diéguez C, Mallo F. Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes. 2007;56:143–51. doi: 10.2337/db05-0996. [DOI] [PubMed] [Google Scholar]
- 18.Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S. Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. J Biol Chem. 2009;284:33509–20. doi: 10.1074/jbc.M109.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, Colom A, Uldry M, Rastrelli S, Sabatier E, Godet N, Waget A, Penicaud L, Valet P, Burcelin R. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology. 2008;149:4768–77. doi: 10.1210/en.2008-0180. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Yang G, Li Q, Tan X, Liu H, Tang Y, Boden G. Exenatide prevents fat-induced insulin resistance and raises adiponectin expression and plasma levels. Diabetes Obes Metab. 2008;10:921–30. doi: 10.1111/j.1463-1326.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 21.Ayala JE, Bracy DP, James FD, Burmeister MA, Wasserman DH, Drucker DJ. Glucagon-like peptide-1 receptor knockout mice are protected from high-fat diet-induced insulin resistance. Endocrinology. 2010;151:4678–87. doi: 10.1210/en.2010-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006;30:1332–40. doi: 10.1038/sj.ijo.0803284. [DOI] [PubMed] [Google Scholar]
- 23.Neary MT, Batterham RL. Gut hormones: implications for the treatment of obesity. Pharmacol Ther. 2009;124:44–56. doi: 10.1016/j.pharmthera.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–81. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covasa M. Deficits in gastrointestinal responses controlling food intake and body weight. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1423–39. doi: 10.1152/ajpregu.00126.2010. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes. 2001;50:2786–91. doi: 10.2337/diabetes.50.12.2786. [DOI] [PubMed] [Google Scholar]
- 27.Reidelberger RD, Haver AC, Apenteng BA, Anders KL, Steenson SM. Effects of exendin-4 alone and with peptide YY(3-36) on food intake and body weight in diet-induced obese rats. Obesity (Silver Spring) 2011;19:121–7. doi: 10.1038/oby.2010.136. [DOI] [PubMed] [Google Scholar]
- 28.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]